Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus—A Systematic Review of Prospective Cohort Studies
Abstract
:1. Introduction
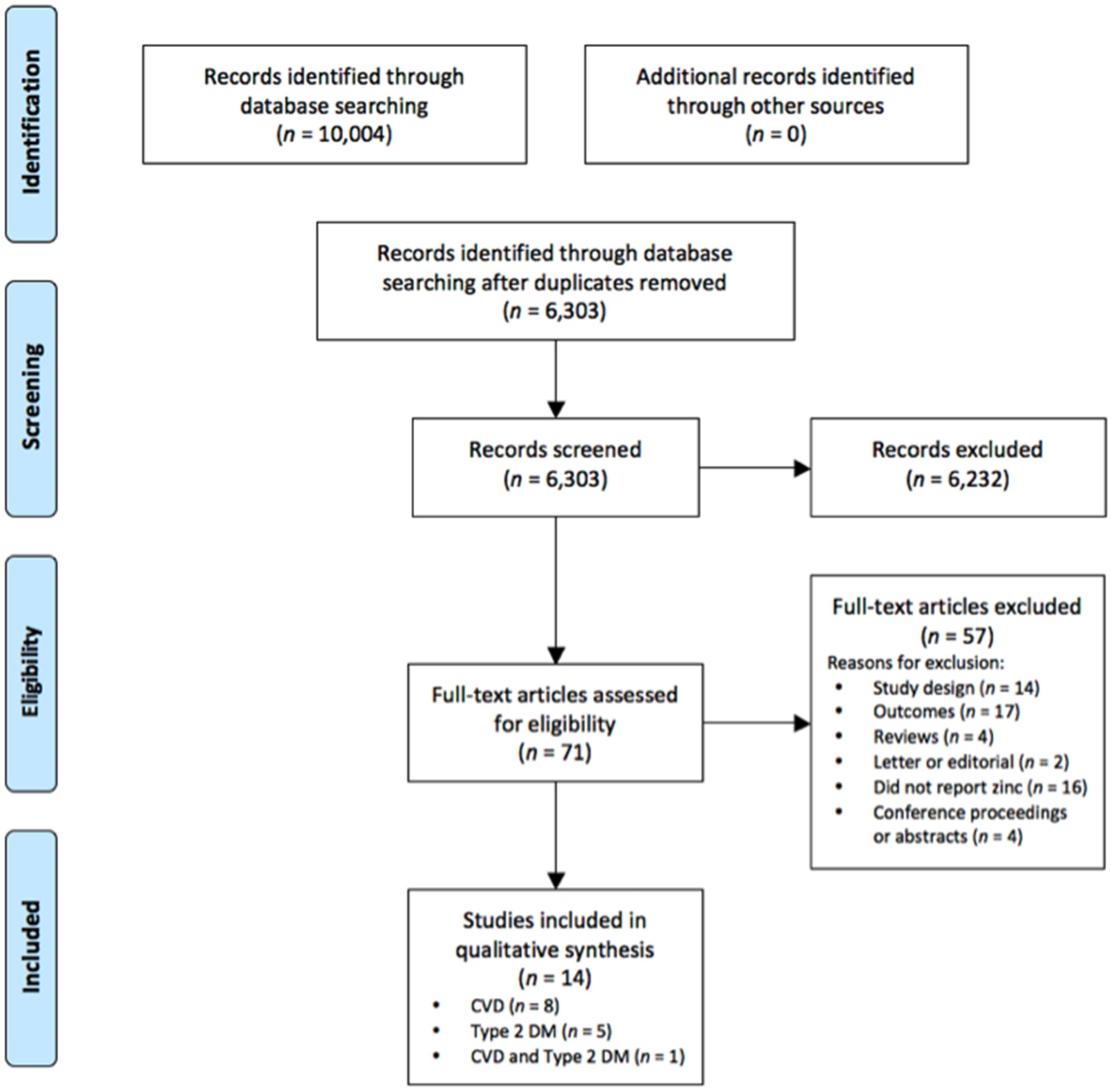
2. Methods
2.1. Search Strategy
2.2. Study Eligibilty Criteria
2.3. Data Extraction and Quality Assessment of Included Studies
2.4. Quality Assessment
3. Results
3.1. Zinc Status and CVD Outcomes
3.2. Zinc Status and Type 2 DM
3.3. Quality of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organisation. Global Status Report On Noncommunicable Diseases 2014; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of cardiology/American Heart Association task force on practice guidelines. Circulation 2014, 129, 76–99. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care 2015, 38, S1–S93. [Google Scholar]
- Samman, S. Zinc. Nutr. Diet. 2007, 64, S131–S134. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid. Redox Signal. 2010, 13, 1549–1573. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. Multiple impacts of zinc on immune function. Metallomics 2014, 6, 1175–1180. [Google Scholar] [CrossRef]
- Rosenkranz, E.; Maywald, M.; Hilgers, R.D.; Brieger, A.; Clarner, T.; Kipp, M.; Plümäkers, B.; Meyer, S.; Schwerdtle, T.; Rink, L. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J. Nutr. Biochem. 2016, 29, 116–123. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. Autoimmune mechanisms in type 1 diabetes. Autoimmun. Rev. 2008, 7, 550–557. [Google Scholar] [CrossRef]
- McCarthy, M.I. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 2010, 363, 2339–2350. [Google Scholar]
- Capdor, J.; Foster, M.; Petocz, P.; Samman, S. Zinc and glycemic control: A meta-analysis of randomised placebo controlled supplementation trials in humans. J. Trace Elem. Med. Biol. 2013, 27, 137–142. [Google Scholar] [CrossRef]
- Foster, M.; Petocz, P.; Samman, S. Effects of zinc on plasma lipoprotein cholesterol concentrations in humans: A meta-analysis of randomised controlled trials. Atherosclerosis 2010, 210, 344–352. [Google Scholar] [CrossRef]
- Little, P.J.; Bhattacharya, R.; Moreyra, A.E.; Korichneva, I.L. Zinc and cardiovascular disease. Nutrition 2010, 26, 1050–1057. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kulakov, L.; Opländer, C.; Kolb-Bachofen, V.; Kröncke, K.-D.; Suschek, C.V. Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox Biol. 2014, 2, 945–954. [Google Scholar] [CrossRef]
- Jenner, A.; Ren, M.; Rajendran, R.; Ning, P.; Huat, B.T.K.; Watt, F.; Halliwell, B. Zinc supplementation inhibits lipid peroxidation and the development of atherosclerosis in rabbits fed a high cholesterol diet. Free Radic. Biol. Med. 2007, 42, 559–566. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef]
- Beattie, J.H.; Kwun, I.-S. Is zinc deficiency a risk factor for atherosclerosis? Br. J. Nutr. 2004, 91, 177–181. [Google Scholar] [CrossRef]
- Reunanen, A.; Knekt, P.; Marniemi, J.; Mäki, J.; Maatela, J.; Aromaa, A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur. J. Clin. Nutr. 1996, 50, 431–437. [Google Scholar]
- Singh, R.B.; Niaz, M.A.; Rastogi, S.S.; Bajaj, S.; Gaoli, Z.; Shoumin, Z. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J. Am. Coll. Nutr. 1998, 17, 564–570. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases (ICD-10); World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J. Clin. Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Review Manager (RevMan), version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Demark, 2014.
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyörälä, K.; Lehto, S.; Rönnemaa, T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Magnesium intake and risk of coronary heart disease among men. J. Am. Coll. Nutr. 2004, 23, 63–70. [Google Scholar] [CrossRef]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef]
- Lee, D.-H.; Folsom, A.R.; Jacobs, D.R. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2005, 81, 787–791. [Google Scholar]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef]
- Marniemi, J.; Järvisalo, J.; Toikka, T.; Räihä, I.; Ahotupa, M.; Sourander, L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int. J. Epidemiol. 1998, 27, 799–807. [Google Scholar] [CrossRef]
- Mursu, J.; Robien, K.; Harnack, L.J.; Park, K.; Jacobs, D.R., Jr. Dietary supplements and mortality rate in older women. Arch. Intern. Med. 2011, 171, 1625–1633. [Google Scholar] [CrossRef]
- Otto, M.C.d.O.; Alonso, A.; Lee, D.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Winklhofer-Roob, B.M.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; März, W. Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br. J. Nutr. 2009, 101, 1534–1540. [Google Scholar] [CrossRef]
- Vashum, K.P.; McEvoy, M.; Shi, Z.; Milton, A.H.; Islam, M.R.; Sibbritt, D.; Patterson, A.; Byles, J.; Loxton, D.; Attia, J. Is dietary zinc protective for type 2 diabetes? Results from the Australian longitudinal study on women’s health. BMC Endocr. Disord. 2013, 13, 40. [Google Scholar] [CrossRef]
- Park, J.S.; Xun, P.; Li, J.; Morris, S.J.; Jacobs, D.R.; Liu, K.; He, K. Longitudinal association between toenail zinc levels and the incidence of diabetes among American young adults: The CARDIA Trace Element Study. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Sun, Q.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care 2009, 32, 629–634. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Q.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Chen, H. Multivitamins, individual vitamin and mineral supplements, and risk of diabetes among older U.S. adults. Diabetes Care 2011, 34, 108–114. [Google Scholar] [CrossRef]
- Yary, T.; Virtanen, J.K.; Ruusunen, A.; Tuomainen, T.-P.; Voutilainen, S. Serum zinc and risk of type 2 diabetes incidence in men: The Kuopio Ischaemic Heart Disease Risk Factor study. J. Trace Elem. Med. Biol. 2016, 33, 120–124. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C.; et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S94–S203. [Google Scholar]
- Ruz, M.; Carrasco, F.; Rojas, P.; Codoceo, J.; Inostroza, J.; Basfi-fer, K.; Valencia, A.; Vásquez, K.; Galgani, J.; Pérez, A.; et al. Zinc as a potential coadjuvant in therapy for type 2 diabetes. Food Nutr. Bull. 2013, 34, 215–221. [Google Scholar] [CrossRef]
- Luo, Y.-Y.; Zhao, J.; Han, X.-Y.; Zhou, X.-H.; Wu, J.; Ji, L.-N. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin. Med. J. (Engl.) 2015, 128, 3276–3282. [Google Scholar]
- Michas, G.; Micha, R.; Zampelas, A. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis 2014, 234, 320–328. [Google Scholar] [CrossRef]
- Mamdani, M.; Sykora, K.; Li, P.; Normand, S.-L.T.; Streiner, D.L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. Br. Med. J. 2005, 330, 960–962. [Google Scholar] [CrossRef]
- Normand, S.-L.T.; Sykora, K.; Li, P.; Mamdani, M.; Rochon, P.A.; Anderson, G.M. Readers guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. BMJ 2005, 330, 1021–1023. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Pfrimer, K.; Doreste-Alonso, J.; Ribas-Barba, L.; Sánchez-Villegas, A.; Ortiz-Andrellucchi, A.; Henríquez-Sánchez, P. Dietary assessment methods for intakes of iron, calcium, selenium, zinc and iodine. Br. J. Nutr. 2009, 102, S38–S55. [Google Scholar] [CrossRef]
- Beckett, J.M.; Hartley, T.F.; Ball, M.J. Evaluation of the Randox colorimetric serum copper and zinc assays against atomic absorption spectroscopy. Ann. Clin. Biochem. 2009, 46, 322–326. [Google Scholar] [CrossRef]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. Br. Med. J. 2011, 342, d671. [Google Scholar] [CrossRef]
- Chu, A.; Foster, M.; Samman, S. Determinants of Zinc Transport in Humans: Zinc Status, Exercise, Inflammation and Chronic Diseases. In Human Health and Nutrition: New Research; Ostojic, S., Ed.; Nova Science: New York, NY, USA, 2015; pp. 17–48. [Google Scholar]
- Xi, P.; Liu, R.H. Whole food approach for type 2 diabetes prevention. Mol. Nutr. Food Res. 2016, 60, 1819–1836. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Anjana, R.M.; Mohan, V. Diabetes mellitus and its complications in India. Nat. Rev. Endocrinol. 2016, 12, 357–370. [Google Scholar] [CrossRef]
- Valera, P.; Zavattari, P.; Sanna, A.; Pretti, S.; Marcello, A.; Mannu, C.; Targhetta, C.; Bruno, G.; Songini, M. Zinc and other metals deficiencies and risk of type 1 diabetes: An ecological study in the high risk Sardinia Island. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef]
- Valera, P.; Zavattari, P.; Albanese, S.; Cicchella, D.; Dinelli, E.; Lima, A.; de Vivo, B. A correlation study between multiple sclerosis and type 1 diabetes incidences and geochemical data in Europe. Environ. Geochem. Health 2014, 36, 79–98. [Google Scholar] [CrossRef]
- Lowe, N.M.; Medina, M.W.; Stammers, A.-L.; Patel, S.; Souverein, O.W.; Dullemeijer, C.; Serra-Majem, L.; Nissensohn, M.; Moran, V.H. The relationship between zinc intake and serum/plasma zinc concentration in adults: a systematic review and dose-response meta-analysis by the EURRECA network. Br. J. Nutr. 2012, 108, 1962–1971. [Google Scholar] [CrossRef]
- Sandström, B. Bioavailability of zinc. Eur. J. Clin. Nutr. 1997, 51, S17–S19. [Google Scholar]
- Foster, M.; Samman, S. Vegetarian Diets Across the Lifecycle: Impact on Zinc Intake and Status. Adv. Food Nutr. Res. 2015, 74, 93–131. [Google Scholar]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014, 37, 120–143. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Freeland-Graves, J.H. Dietary phytate, zinc and hidden zinc deficiency. J. Trace Elem. Med. Biol. 2014, 28, 414–417. [Google Scholar] [CrossRef]
- Chu, A.; Petocz, P.; Samman, S. Immediate effects of aerobic exercise on plasma/serum zinc levels: A meta-analysis. Med. Sci. Sports Exerc. 2016, 48, 726–733. [Google Scholar] [CrossRef]
- Hawley, J.A.; Burke, L.M.; Phillips, S.M.; Spriet, L.L. Nutritional modulation of training-induced skeletal muscle adaptations. J. Appl. Physiol. 1985, 110, 834–845. [Google Scholar] [CrossRef]

| Authors, Country | Population | Baseline Age (Year) 1 | Sex | Follow up (Year) | Total n (No. of Cases) | Adjustments | Disease Outcome | Outcome Summary |
|---|---|---|---|---|---|---|---|---|
| Al-Delaimy et al. 2004 [24], USA | Health professionals, excluded participants with cancer, MI and CVD | 40–75 | Male | 12 | 39,633 (1449) | Age, energy | Trend (p = 0.06) towards increased risk of CHD with higher total Zn intake (dietary and supplemental), however no effect in the 5th quintile (median dietary Zn = 37 mg/day; RR 0.96, 95% CI 0.81, 1.14). | No association |
| Age, time period, energy intake, history of diabetes, history of high cholesterol, family history of MI, smoking history, aspirin intake, BMI, alcohol intake, physical activity, dietary trans fatty acid, Vitamin E, total protein intake, cereal fiber, folate, omega 3 fatty acids | No association between total Zn intake and risk of CHD (RR in the 5th quintile: 1.07; 95% CI 0.87, 1.3; p-trend = 0.93) or when separated into fatal CHD or non-fatal CHD. | No association | ||||||
| Age, energy | No association between Zn supplement dose and risk of CHD (RR in the 5th quintile: 0.91; 95% CI: 0.69, 1.2) or when separated into fatal CHD or non-fatal CHD. | No association | ||||||
| Age, time period, energy intake, history of diabetes, history of high cholesterol, family history of MI, smoking history, aspirin intake, BMI, alcohol intake, physical activity, dietary trans fatty acid, Vitamin E, total protein intake, cereal fiber, folate, omega 3 fatty acids | No association between Zn supplement dose and risk of CHD (RR in the 5th quintile: 1.06; 95% CI 0.79, 1.43) or when separated into fatal CHD or non-fatal CHD. | No association | ||||||
| Bates et al. 2011 [25], UK | Community-living age ≥ 65 year | 76.6 ± 7.4 | 49% Female | 14 | 1054 (189) | Age, sex | Higher dietary Zn intake was associated with decreased risk of vascular disease mortality 3 (HR 0.84 per SD increase, 95% CI 0.71, 0.99; p = 0.04). Mean Zn intakes were 8.81 ± 2.86 mg/day for males and 6.96 ± 2.56 mg/day for females (mean ± SD). | Reduced risk |
| Lee et al. 2005 [26] 2, USA | Postmenopausal women and no report of angina, heart disease or heart attack at baseline | 55–69 | Female | 15 | 34,492 (1767) | Age, total energy intake, history of hypertension, BMI, waist-hip ratio, physical activity, cigarette smoking, alcohol consumption, hormone replacement therapy, intakes of saturated fat, trans fat, PUFA, folate, β-carotene, Vitamin C and Vitamin E | No association between dietary Zn intake and risk of CVD mortality, when stratified into alcohol consumption or in combined analysis. In participants with alcohol consumption of 10–29 g/day, trend for a reduction in CVD risk with higher dietary Zn intake in participants (5th quintile RR 0.37, 95% CI 0.13,1.06; p-trend = 0.07). | No association |
| Mursu et al. 2011 [29] 2, USA | Mostly postmenopausal women | 61.6 ± 4.2 | Female | 22 (mean 19) | 37,033 (5454) | Age, energy intake | No association between Zn supplement use and CVD mortality (HR for users 0.97; 95% CI 0.91, 1.03). | No association |
| 37,033 (5454) | Age, energy intake, education, place of residence, diabetes, hypertension, BMI, waist-hip ratio, hormone replacement therapy, physical activity, smoking, intake of alcohol, saturated fat, whole grain products, fruits and vegetables | No association between Zn supplement use and CVD mortality (HR for users 1.08; 95% CI 1.01, 1.15). | No association | |||||
| Otto et al. 2012 [30], USA | Population-based sample, free of clinical CVD | 61.8 ± 10.3 (SE) | 53% Female | 6.2 | 5285 (8.5 new cases per 1000 person-years) | Energy intake, age, sex, race-ethnicity, education, study center, alcohol intake, physical activity, BMI, fiber intake, cigarette smoking, dietary supplement use | No association between dietary Zn intake and risk of CVD (p-trend = 0.46, 5th quintile Zn intake ≥ 9.8 mg/day). Higher Zn intake from red meat was associated with increased risk of CVD (5th quintile HR 1.51, 95% CI 1.02, 2.24; p-trend = 0.01). No association between Zn intake from other sources and risk of CVD. | No association |
| 5285 (8.5 new cases per 1000 person-years) | Energy intake, age, sex, race-ethnicity, education, study center, alcohol intake, physical activity, BMI, fiber intake, cigarette smoking, dietary supplement use, PUFA:SFA, intake of Mg, nonheme iron, heme iron, β-carotene, Vitamin E and Vitamin C | No association between dietary Zn intake and risk of CVD (p-trend = 0.66). Higher Zn intake from red meat was associated with increased risk of CVD (5th quintile HR 1.66, 95% CI 1.10, 2.49; p-trend < 0.01). No association between Zn intake from other sources and risk of CVD. | No association |
| Authors, Country | Population | Baseline Age (Year) 1 | Sex | Follow up (Year) | Total n (No. of Cases) | Adjustments | Disease Outcome | Outcome Summary |
|---|---|---|---|---|---|---|---|---|
| Bates et al. 2011 [25], UK | Community-living age ≥ 65 year | 76.6 ± 7.4 | 49% Female | 14 | 741 (189) | Age, sex | Higher plasma Zn concentration was associated with reduced risk of vascular disease mortality 2 (HR 0.73 per SD increase, 95% CI 0.61, 0.88; p = 0.001). Mean plasma Zn levels were 14.2 ± 2.1 μmol/L for males and 14.2 ± 2.4 μmol/L for females (mean ± SD). | Reduced risk |
| 629 (105) | Age, sex, vitamin and mineral predictors, α1-antichymotrypsin, creatinine, total and HDL-cholesterol, albumin, BMI, SBP, smoking, No. of prescribed drugs, self-reported health, physical activity, poverty | No association between plasma Zn concentration and risk of vascular disease mortality (HR 0.83 per SD increase, 95% CI 0.65, 1.07; p = 0.15). | No association | |||||
| Leone et al. 2006 [27], France | Men aged ≥ 30 year at CVD screening | 30–60 | Male | 18 | 4035 (56) | Age, BMI, smoking, alcohol consumption, physical activity, hypertension, serum LDL, HDL and triglycerides, diabetes, and CVD history | No association between serum Zn level and CVD death (RR in the 4th quartile 0.7; 95% CI 0.3, 1.5). Mean serum Zn levels were 14.6 ± 1.8 μmol/L for survivors and 14.5 ± 2.1 μmol/L for deceased. | No association |
| Marniemi et al. 1998 [28], Finland | Community-living age ≥ 65 year | ≥65 | 47% Female | 13 | 344 (142) | Age, sex, smoking, alcohol use, BMI, coronary heart diseases, hypertension, diabetes, serum total and HDL-cholesterol, TAG | No association between serum Zn level and vascular mortality (RR for 3rd tertile 1.17; 95% CI 0.74, 1.84). Mean serum Zn levels were 12.9 ± 1.7 μmol/L for survivors and 12.9 ± 1.9 μmol/L for deceased by vascular death. | No association |
| Pilz et al. 2009 [31], Germany | Clinically stable patients of German ancestry who were referred to coronary angiography | >60 (median) | 30% Female | 7.75 (median) | 3274 (484) | Unadjusted | Lower serum Zn level was associated with increased CVD mortality (per quartile decrease HR 1.30, 95% CI 1.19, 1.41; p < 0.001). Higher risk of CVD mortality for 1st quartile (serum Zn < 11.93 μmol/L; HR 2.12, 95% CI 1.63, 2.77; p < 0.001). | Reduced risk |
| 3274 (484) | Age, sex, BMI, HbA1c, systemic hypertension, smoking, HDL and LDL-cholesterol, TAG, GFR, CRP, N-terminal pro-B-type natriuretic peptide, copper, albumin, Hb, homocysteine, ACE inhibitors, diuretics | Lower serum Zn level was associated with increased CVD mortality (per quartile decrease HR 1.10, 95% CI 1.01, 1.21; p = 0.038). No association in CVD mortality for 1st quartile (serum Zn < 11.93 μmol/L; HR 1.24, 95% CI 0.92, 1.66; p = 0.162). | Reduced risk | |||||
| Soinio et al. 2007 [23], Finland | Patients with Type 2 DM | 45–64 | 45% Female | 7 | 1050 (156 CHD deaths) | Unadjusted | Higher baseline serum Zn level was associated with reduction in risk of CHD death (p = 0.015). Participants in the lowest quartile (≤14.1 μmol/L) have increased risk of CHD death than those in the upper 3 quartiles (RR 1.80, 95% CI 1.30, 2.49, p < 0.001). | Reduced risk |
| 1050 (156 CHD deaths) | Age, sex, duration of diabetes, cholesterol (total and HDL), TAG, HbA1c, eGFR, hypertension, smoking, BMI, area of residence, type of diabetes therapy | Participants in the lowest quartile of serum Zn level (≤14.1 μmol/L) have increased risk of CHD death than those in the upper 3 quartiles (RR 1.70, 95% CI 1.21, 2.38, p = 0.002) 2. | Reduced risk | |||||
| 1050 (254 fatal or non-fatal MI) | Unadjusted | Higher baseline serum Zn level was associated with reduction in risk of MI (p = 0.014). Participants in the lowest quartile (≤14.1 μmol/L) have increased risk of CHD death or nonfatal MI than those in the upper 3 quartiles (RR 1.40, 95% CI 1.06, 1.84, p = 0.019). | Reduced risk | |||||
| 1050 (254 fatal or non-fatal MI) | Age, sex, duration of diabetes, cholesterol (total and HDL), TAG, HbA1c, eGFR, hypertension, smoking, BMI, area of residence, type of diabetes therapy | Participants in the lowest quartile of serum Zn level (≤14.1 μmol/L) have increased risk of MI than those in the upper 3 quartiles (RR 1.37, 95% CI 1.03, 1.82, p = 0.033) 2. | Reduced risk |
| Authors, Country | Population | Baseline Age (Year) 1 | Sex | Follow up (Year) | Total n (No. of Cases) | Marker of Zn Status | Adjustments | Disease Outcome | Outcome Summary |
|---|---|---|---|---|---|---|---|---|---|
| Otto et al. 2012 [30], USA | Population-based sample, free of clinical CVD and Type 2 DM at baseline | 45–84 | 53% | 4.8 | 4982 (16.7 new cases per 1000 person-years) | Dietary Zn | Energy intake, age, sex, race-ethnicity, education, study center, alcohol intake, physical activity, BMI, fiber intake, cigarette smoking, dietary supplement use | No association between dietary Zn intake and risk of Type 2 DM (5th quintile HR 1.15; 95% CI 0.8, 1.63; p = 0.71). No association between Zn intake from red meat and risk of Type 2 DM. | No association |
| Energy intake, age, sex, race-ethnicity, education, study center, alcohol intake, physical activity, BMI, fiber intake, cigarette smoking, dietary supplement use, PUFA:SFA, intake of Mg, nonheme iron, heme iron, β-carotene, vitamin E and vitamin C | No association between dietary Zn intake and risk of Type 2 DM (5th quintile HR 1.41; 95% CI 0.88, 2.27; p = 0.33). No association between Zn intake from red meat and risk of Type 2 DM. | No association | |||||||
| Park et al. 2016 [33], USA | African American and Caucasian men and women | 27.03 ± 3.61 | 52.5% Female | 23 | 3960 (418) | Total Zn intake | Age, gender, ethnicity, study center, BMI, baseline HOMA-IR | No association between total zinc intake (dietary + supplement) and risk of Type 2 DM (4th quartile HR 0.98; 95% CI 0.75, 1.27; p = 0.97). | No association |
| Age, gender, ethnicity, study center, BMI, baseline HOMA-IR, education, smoking, alcohol consumption, physical activity, family history of diabetes, intakes of long-chain omega 3 PUFA, Mg, iron and total energy | No association between total zinc intake (dietary + supplemental) and risk of Type 2 DM (4th quartile HR 1.27; 95% CI 0.81, 2.01; p = 0.23). | No association | |||||||
| Song et al. 2011 [35], USA | AARP 2 members free of diabetes in the initial 4–5 years of follow up (2000) | 50–71 | 42% Female | 8–11 | 232,007 (14,130) | Zn supplement use | Age, sex, race, BMI, education, marital status, physical activity, smoking, coffee consumption, alcohol, general health, total energy intake, multivitamin use, individual vitamin and minerals use and frequency | No association between Zn supplement use with risk of Type 2 DM (users OR 0.94; 95% CI 0.86, 1.03; p = 0.16). | No association |
| Sun et al. 2009 [34], USA | Nurses free of diabetes, cancer or CVD at baseline | 33–60 | Female | 24 | 82,297 (6030) | Dietary Zn | Age | Higher total Zn intake (dietary + supplement) was associated with reduced risk of Type 2 DM (5th quintile RR 0.83; 95% CI 0.77, 0.9; p-trend < 0.0001). No association between dietary Zn intake and risk of Type 2 DM (5th quintile RR 1.00; 95% CI 0.92, 1.08; p-trend = 0.04). | Reduced risk |
| Age, BMI, family history of diabetes, smoking, alcohol intake, menopausal status, postmenopausal hormone use, multivitamin use, physical activity, total energy intake, glycaemic load, PUFA:SFA, intakes of red meat, heme iron, whole grains, trans fat, Mg and caffeine (Zn intake from supplement use in tertiles was further adjusted when modeling the associations for dietary Zn intake) | Higher total Zn intake (dietary + supplement) was associated with reduced risk of Type 2 DM (5th quintile RR 0.9; 95% CI 0.82, 0.99; p-trend = 0.04). Higher dietary Zn intake was associated with reduced risk of Type 2 DM (5th quintile RR 0.92; 95% CI 0.84, 1.00; p-trend = 0.009). | Reduced risk | |||||||
| Vashum et al. 2013 [32], Australia | Women | 45–50 | Female | 6 | 8921 (333) | Dietary Zn | Energy, age | No association between dietary Zn intake and risk of Type 2 DM (5th quintile OR 0.75; 95% CI 0.53, 1.05; p-trend = 0.319). | No association |
| Energy, age, BMI, smoking, hormone replacement therapy, exercise, medical history of arthritis, CHD, hypertension, asthma and depression, energy adjusted fiber, iron and fat intake, alcohol and supplement use | Higher levels of dietary Zn intake was associated with reduced risk of Type 2 DM (5th quintile OR 0.50, 95% CI 0.32, 0.77, p-trend = 0.006). | Reduced risk | |||||||
| Yary et al. 2016 [36], Finland | Finnish men | 42–60 | Male | 20 | 2220 (416) | Serum Zn | Age, examination year | Positive association between serum Zn quartiles and incidence of Type 2 DM (4th quartile HR 1.52; 95% CI 1.15, 2.01; p-trend = 0.001). | Increased risk |
| Age, examination year, family history of DM, smoking, education years, leisure-time physical activity, intake of alcohol, fiber, sum of fruits, berries and vegetables | Positive association between serum zinc quartiles and incidence of Type 2 DM (4th quartile HR 1.60; 95% CI 1.20, 2.13; p-trend < 0.001). Adjustments for BMI, fasting blood glucose, serum insulin, HOMA-IR, HOMA-IS, HOMA-β or CRP individually, did not affect the statistical significant p-trend. | Increased risk |
| Study ID | Prospective Risk of CVD | Prospective Risk of Type 2 DM | |
|---|---|---|---|
| Zinc intake | Al-Delaimy et al. 2004 [24] | No association | N/A |
| Bates et al. 2011 [25] | ↓ risk | N/A | |
| Lee et al. 2005 [26] | No association | N/A | |
| Leone et al. 2006 [27] | No association | N/A | |
| Park et al. 2016 [33] | N/A | No association | |
| Otto et al. 2012 [30] | No association | No association | |
| Sun et al. 2009 [34] | N/A | ↓ risk | |
| Vashum et al. 2013 [32] | N/A | ↓ risk | |
| Zinc supplement | Al-Delaimy et al. 2004 [24] | No association | N/A |
| Mursu et al. 2011 [29] | No association | N/A | |
| Song et al. 2011 [35] | N/A | No association | |
| Plasma/serum zinc | Bates et al. 2011 [25] | ↓ risk/no association | N/A |
| Leone et al. 2006 [27] | No association | N/A | |
| Marniemi et al. 1998 [28] | No association | N/A | |
| Pilz et al. 2009 [31] | ↓ risk | N/A | |
| Soinio et al. 2007 [23] | ↓ risk | N/A | |
| Yary et al. 2016 [36] | N/A | ↑ risk |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, A.; Foster, M.; Samman, S. Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus—A Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 707. https://doi.org/10.3390/nu8110707
Chu A, Foster M, Samman S. Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus—A Systematic Review of Prospective Cohort Studies. Nutrients. 2016; 8(11):707. https://doi.org/10.3390/nu8110707
Chicago/Turabian StyleChu, Anna, Meika Foster, and Samir Samman. 2016. "Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus—A Systematic Review of Prospective Cohort Studies" Nutrients 8, no. 11: 707. https://doi.org/10.3390/nu8110707
APA StyleChu, A., Foster, M., & Samman, S. (2016). Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus—A Systematic Review of Prospective Cohort Studies. Nutrients, 8(11), 707. https://doi.org/10.3390/nu8110707




