Australian and New Zealand Fish Oil Products in 2016 Meet Label Omega-3 Claims and Are Not Oxidized
Abstract
:1. Introduction
2. Materials and Methods
3. Results
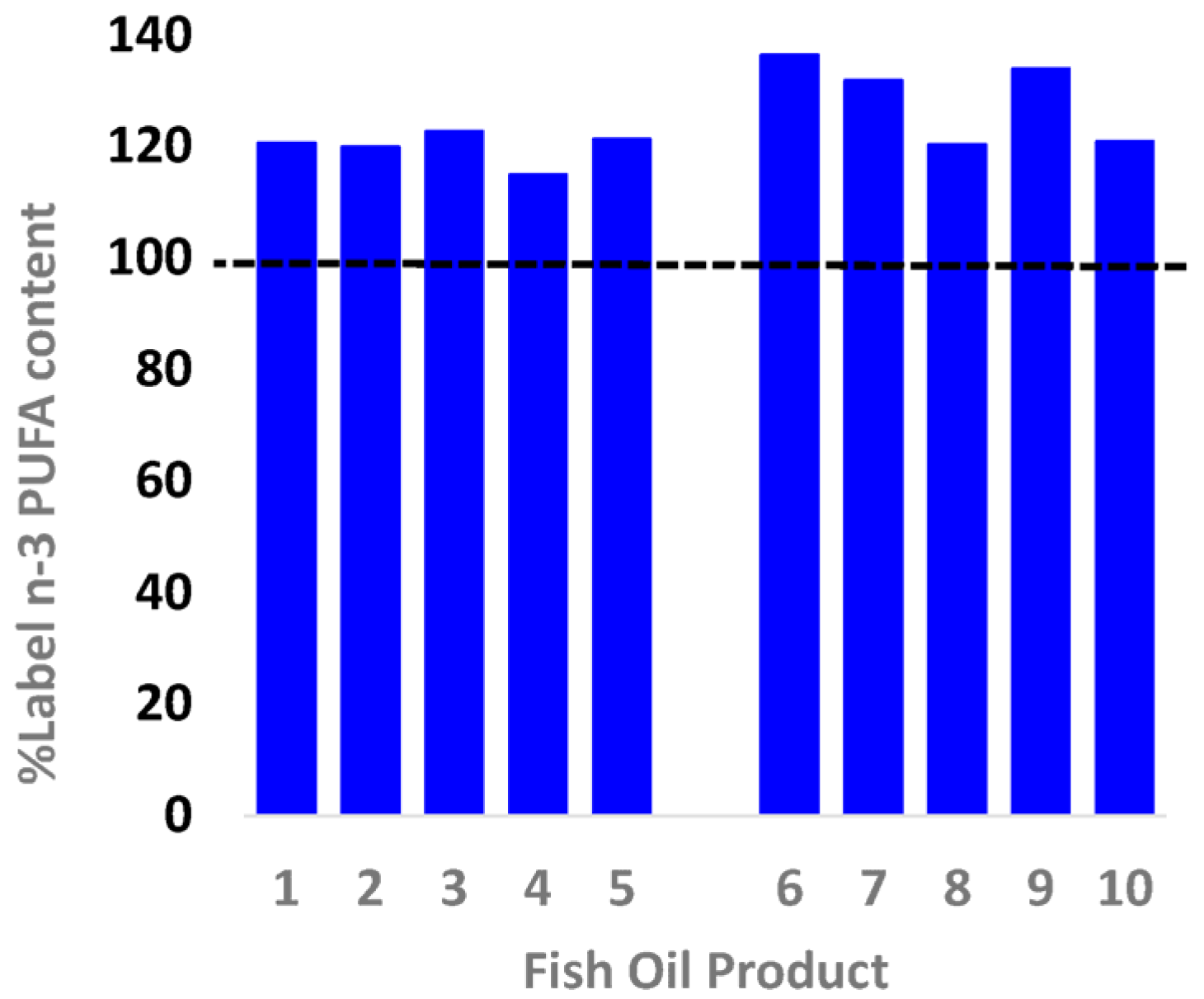
3.1. EPA + DHA Content
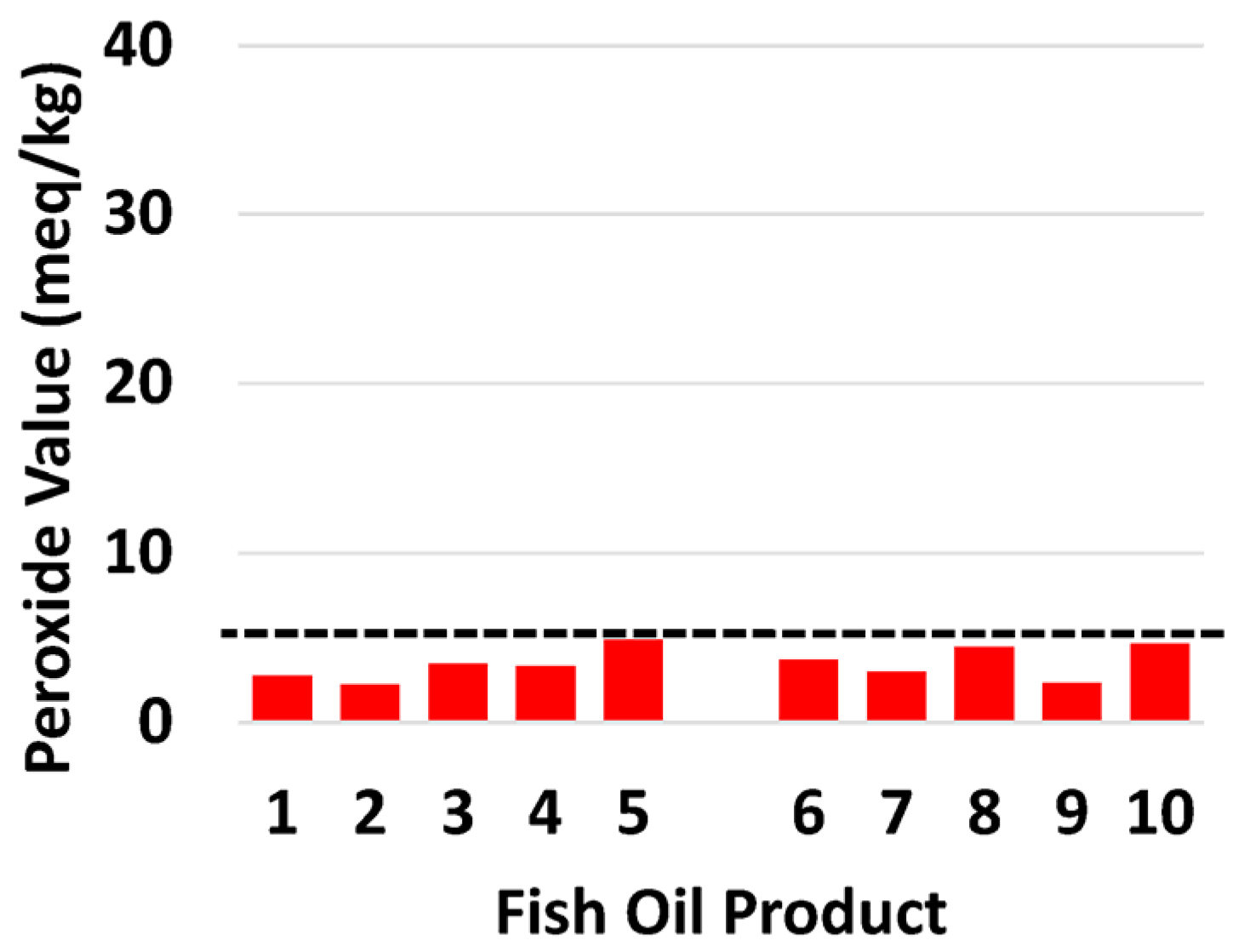
3.2. Oxidative Markers
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bang, H.O.; Dyerberg, J.E. Lipid metabolism and ischaemic heart disease in Greenland Eskimos. Adv. Nutr. Res. 1980, 3, 1–22. [Google Scholar]
- Nichols, P.D.; Petrie, J.; Singh, S. Long-chain omega-3 oils—An update on sustainable sources. Nutrients 2010, 2, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Schutt, E.; Global Organization for EPA and DHA Omega-3s, Salt Lake City, UT, USA. Personal communication, 2016.
- James, M.J.; Sullivan, T.R.; Metcalf, R.G.; Cleland, L.G. Pitfalls in the use of randomised controlled trials for fish oil studies with cardiac patients. Br. J. Nutr. 2014, 112, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Derraik, J.G.B.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.G.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Nat. Sci. Rep. 2015, 5, 7928. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Vickers, M.H.; Gray, C.; Reynolds, C.M.; Segovia, S.A.; Derraik, J.G.B.; Lewandowski, P.A.; Garg, M.L.; Cameron-Smith, D.; Hofman, P.L.; et al. Oxidised fish oil in rat pregnancy causes high newborn mortality and increases maternal insulin resistance. Am. J. Physiol. 2016, 311, R497–R504. [Google Scholar]
- Krail, K. What Can You Do in the Face of a ‘Flawed’ Omega-3 Research Study? Available online: http://www.foodnavigator-asia.com/Nutrition/What-can-you-do-in-the-face-of-a-flawed-omega-3-research-study (accessed on 15 September 2016).
- Omega-3 Fish Oil Products—An Update on the Quality of Australian and New Zealand Products. Australasian Section of the American Oils Chemists Society. December Newsletter. Available online: http://aocs.files.cms-plus.com/Membership/Sections/Omega-3%20Fish%20Oil%20Products.pdf (accessed on 15 September 2016).
- Nichols, P.D.; Glencross, B.; Petrie, J.; Singh, S.P. Readily available sources of long-chain omega-3 oils: Is farmed Australian seafood a better source of the good oil than wild-caught seafood? Nutrients 2014, 6, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Bengtson Nash, S.M.; Schlabach, M.; Nichols, P.D. A nutritional-toxicological assessment of Antarctic krill oil versus fish oil dietary supplements. Nutrients 2014, 6, 3382–3402. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; MacKay, D. Oxidation in EPA- and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Ellett, S.; Jesuthasan, A.; Marlow, G.; Zhu, S.; Agnew, M.; Greenwood, J.M.; Laing, B. New Zealand research—New omega-3 supplement—Lester’s oil. In Proceedings of the New Zealand Institute of Food Science and Technology 50th Anniversary Conference, Palmerston North, New Zealand, 30 June–2 July 2015; p. 5.
- Nichols, P.D. Long-chain omega-3 Oils: Sources, ingredient quality and methods of analysis. In Australian Section of AOCS and Omega-3 Centre, Proceedings of the Biennial Conference, Geelong, Australia, 9–11 September 2015; p. 29.
- Fagan, P.; Therapeutic Goods Administration, Symonston, Australia. Personal communication, 2015.
- Ismail, A.; Global Organization for EPA and DHA Omega-3s, Salt Lake City, UT, USA. Personal communication, 2015.
- The Global Organization for EPA and DHA Omega-3s; The Council for Responsible Nutrition. GOED and CRN Oxidation in Omega-3 Oils: An Overview; GOED and CRN: Salt Lake City, UT, USA, 2015. [Google Scholar]
- Jackowski, S.A.; Alvi, A.Z.; Mirajkar, A.; Imani, Z.; Gamalevych, Y.; Shaikh, N.A.; Jackowski, G. Oxidation levels of North American over the counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative strategy. J. Nutr. Sci. 2015, 4, e30. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Miller, M.R. (Eds.) Recent Advances in Omega-3: Health Benefits, Sources, Products and Bioavailability. MDPI, 2014; p. 201. Available online: http://books.mdpi.com/pdfview/book/94 (accessed on 15 September 2016).
| Product | Sample | Batch | Number | EPA | DHA | Total | Capsule | n-3 | % Label | Cost | Cost | Cost per | Cost per |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Number | Capsules | n-3 | Size | Content | n-3 PUFA | per | per | 500 mg | Annum for | |||
| Claim | Content | Bottle | Capsule | EPA + DHA | 500 mg | ||||||||
| EPA + DHA | |||||||||||||
| mg/cap | mg/cap | mg/cap | mg | mg | $ | $ | $ | $ | |||||
| Standard Fish Oil Triacylglycerols | |||||||||||||
| Blackmore Fish Oil 1000 | 1 | 275257 | 200 | 178 | 133 | 362 | 1000 | 300 | 121 | 15.99 | 0.08 | 0.11 | 40.35 |
| Healthy Care Fish Oil 1000 | 2 | 677168 | 400 | 186 | 127 | 360 | 1000 | 300 | 120 | 12.99 | 0.03 | 0.05 | 16.48 |
| Natures Own Fish Oil 1000 | 3 | 14530058 | 400 | 190 | 132 | 368 | 1000 | 300 | 123 | 17.99 | 0.04 | 0.06 | 22.30 |
| Natures Way Fish Oil 1000 | 4 | 151967 | 200 | 176 | 122 | 345 | 1000 | 300 | 115 | 16.99 | 0.08 | 0.12 | 44.96 |
| Swisse Wild Fish Oil 1500 | 5 | 52112A | 200 | 279 | 193 | 546 | 1500 | 450 | 121 | 17.99 | 0.09 | 0.08 | 30.08 |
| Fish Oil Concentrates | |||||||||||||
| Blackmores Omega Triple | 6 | 276923 | 150 | 651 | 423 | 1228 | 1500 | 900 | 136 | 31.99 | 0.21 | 0.09 | 77.84 |
| Bioglan Super Fish Oil | 7 | 20674A | 200 | 413 | 287 | 791 | 1000 | 600 | 132 | 17.99 | 0.09 | 0.06 | 32.83 |
| Natures Own Triple Concentrated | 8 | 1469561 | 70 | 566 | 392 | 1083 | 1500 | 900 | 120 | 16.99 | 0.24 | 0.11 | 88.59 |
| Natures way Triple Strength | 9 | 160061 | 60 | 522 | 379 | 1045 | 1500 | 780 | 134 | 16.99 | 0.28 | 0.14 | 103.36 |
| Swisse 4x Strength Concentrate | 10 | 160116 | 90 | 757 | 539 | 1450 | 1800 | 1200 | 121 | 24.99 | 0.28 | 0.10 | 101.35 |
| Product | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid | ||||||||||
| 14:0 | 7.5 | 6.7 | 7.6 | 6.4 | 6.7 | 0.4 | 0.3 | 0.4 | 0.1 | 0.4 |
| 15:0 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 16:0 | 15.7 | 15.3 | 15.4 | 15.6 | 14.5 | 0.5 | 0.5 | 1.0 | 0.2 | 0.6 |
| 16:1n-7c | 8.7 | 8.2 | 9.5 | 7.8 | 8.5 | 0.3 | 0.3 | 0.5 | 0.3 | 0.3 |
| 16:3 + 16:4 | 1.3 | 1.1 | 1.3 | 1.1 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 17:0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 18:0 | 3.1 | 3.0 | 3.0 | 3.3 | 3.1 | 3.0 | 2.4 | 4.6 | 1.8 | 3.7 |
| 18:1n-9c | 8.5 | 9.9 | 7.6 | 8.1 | 9.8 | 3.4 | 2.9 | 7.2 | 3.1 | 4.5 |
| 18:1n-7c | 3.1 | 3.0 | 3.1 | 3.0 | 3.4 | 1.5 | 1.3 | 2.7 | 1.3 | 1.7 |
| 18:2n-6 | 3.5 | 3.2 | 3.6 | 3.2 | 3.5 | 0.7 | 0.6 | 1.2 | 0.5 | 0.9 |
| 18:3n-3 | 1.0 | 0.9 | 0.9 | 0.9 | 1.1 | 1.2 | 1.0 | 1.3 | 0.8 | 1.2 |
| 18:3n-6 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 |
| 18:4n-3 | 2.9 | 2.5 | 2.6 | 3.1 | 3.0 | 0.8 | 0.8 | 1.7 | 0.7 | 0.9 |
| 20:1n-11c/n-9c | 1.07 | 1.5 | 0.9 | 1.8 | 1.2 | 3.6 | 3.4 | 2.5 | 3.9 | 3.0 |
| 20:2n-6 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 0.7 | 0.6 | 0.6 | 0.5 | 0.6 |
| 20:3n-6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.5 | 0.4 | 0.3 | 0.4 | 0.4 |
| 20:4n-6 | 1.1 | 1.4 | 1.1 | 1.4 | 1.2 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
| 20:4n-3 | 0.7 | 0.7 | 0.7 | 0.9 | 0.8 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 |
| 20:5n-3 (EPA) | 17.5 | 18.1 | 18.4 | 17.6 | 18.4 | 40.5 | 39.3 | 35.9 | 36.2 | 39.2 |
| 21:5n-3 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 1.8 | 2.0 | 1.2 | 2.1 | 1.4 |
| 22:1n-11c/n-9c | 0.52 | 1.2 | 0.5 | 1.2 | 0.6 | 0.6 | 2.0 | 1.3 | 4.5 | 1.6 |
| 22:5n-6 (DPA6) | 0.1 | 0.0 | 0.5 | 0.1 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 |
| 22:5n-3 (DPA3) | 1.9 | 1.8 | 2.0 | 1.9 | 1.9 | 3.4 | 4.6 | 3.1 | 5.2 | 3.2 |
| 22:6n-3 (DHA) | 12.4 | 11.7 | 12.1 | 11.7 | 11.8 | 25.0 | 25.6 | 23.4 | 23.9 | 21.3 |
| Product | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| PV (meq/kg) | 2.77 | 2.24 | 3.45 | 3.32 | 4.87 | 3.68 | 2.97 | 4.45 | 2.3 | 4.63 |
| pAV | 13 | 10.4 | 108 | 25 | 29.9 | 20.2 | 4.44 | 14 | 109 | 6.23 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichols, P.D.; Dogan, L.; Sinclair, A. Australian and New Zealand Fish Oil Products in 2016 Meet Label Omega-3 Claims and Are Not Oxidized. Nutrients 2016, 8, 703. https://doi.org/10.3390/nu8110703
Nichols PD, Dogan L, Sinclair A. Australian and New Zealand Fish Oil Products in 2016 Meet Label Omega-3 Claims and Are Not Oxidized. Nutrients. 2016; 8(11):703. https://doi.org/10.3390/nu8110703
Chicago/Turabian StyleNichols, Peter D., Lalen Dogan, and Andrew Sinclair. 2016. "Australian and New Zealand Fish Oil Products in 2016 Meet Label Omega-3 Claims and Are Not Oxidized" Nutrients 8, no. 11: 703. https://doi.org/10.3390/nu8110703
APA StyleNichols, P. D., Dogan, L., & Sinclair, A. (2016). Australian and New Zealand Fish Oil Products in 2016 Meet Label Omega-3 Claims and Are Not Oxidized. Nutrients, 8(11), 703. https://doi.org/10.3390/nu8110703




