Lycopersicon esculentum Extract Enhances Cognitive Function and Hippocampal Neurogenesis in Aged Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Preparation of Tomato Ethanolic Extract (TEE)
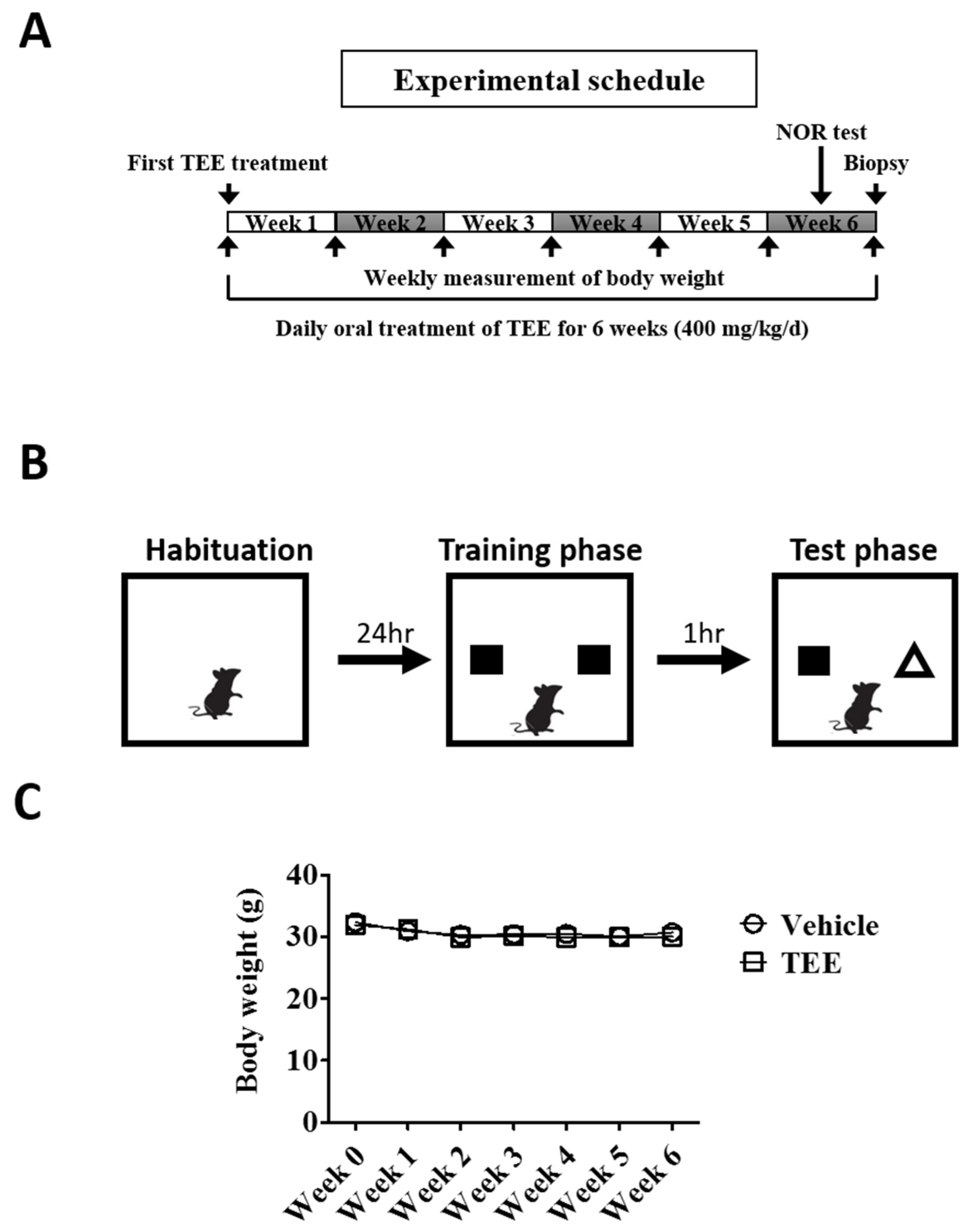
2.3. Animals and TEE Administration
2.4. Novel Object Recognition (NOR) Test
2.5. Sample Collection
2.6. DCX Immunohistochemistry
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Western Blot Analysis for ERK and PSD95
2.9. Statistical Analyses
3. Results
3.1. Oral TEE Supplement Did Not Affect Body Weight in Aged Mice
3.2. Oral TEE Supplement Improved Age-Related Memory Impairment
3.3. Oral TEE Supplement Increased DCX+ Cells and PSD95 Protein Expression in Aged Mice
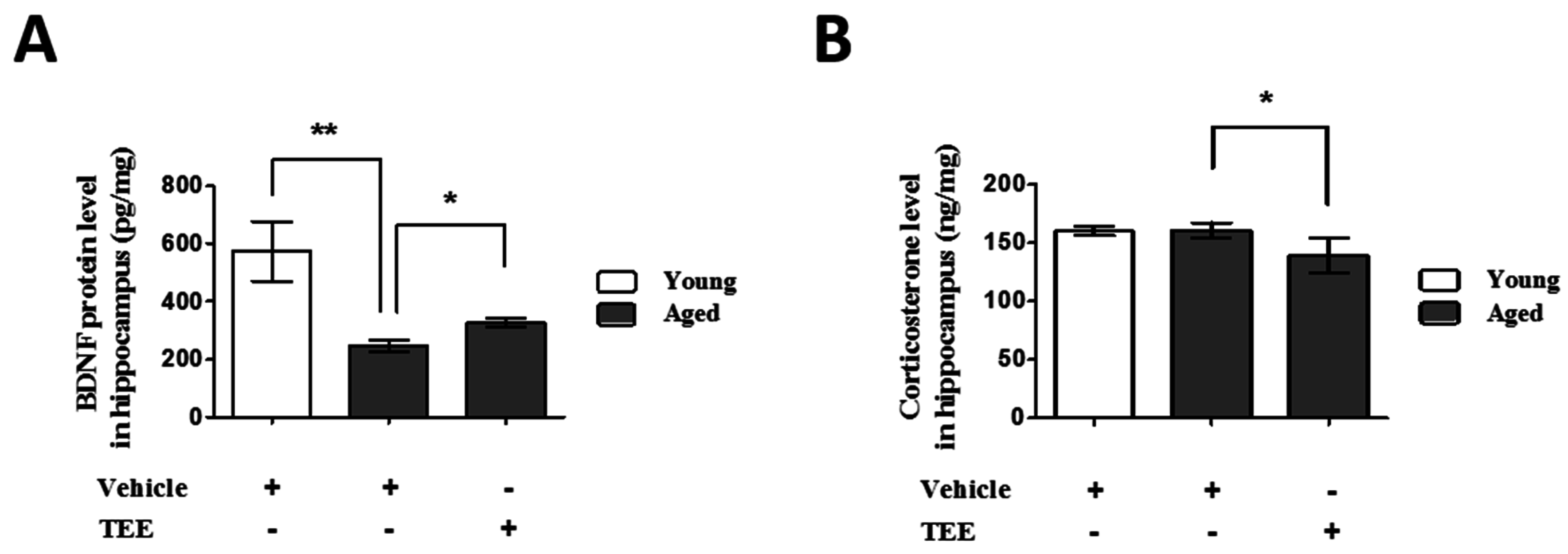
3.4. Oral TEE Supplement Decreased Corticosterone and Increased BDNF in Hippocampus
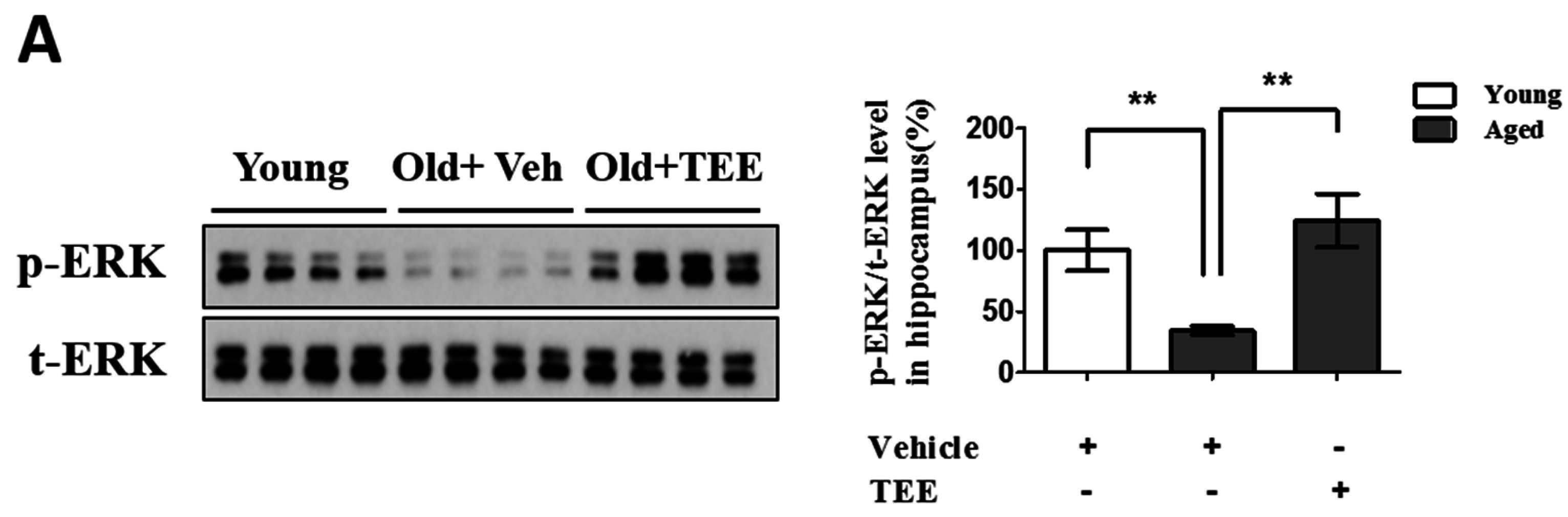
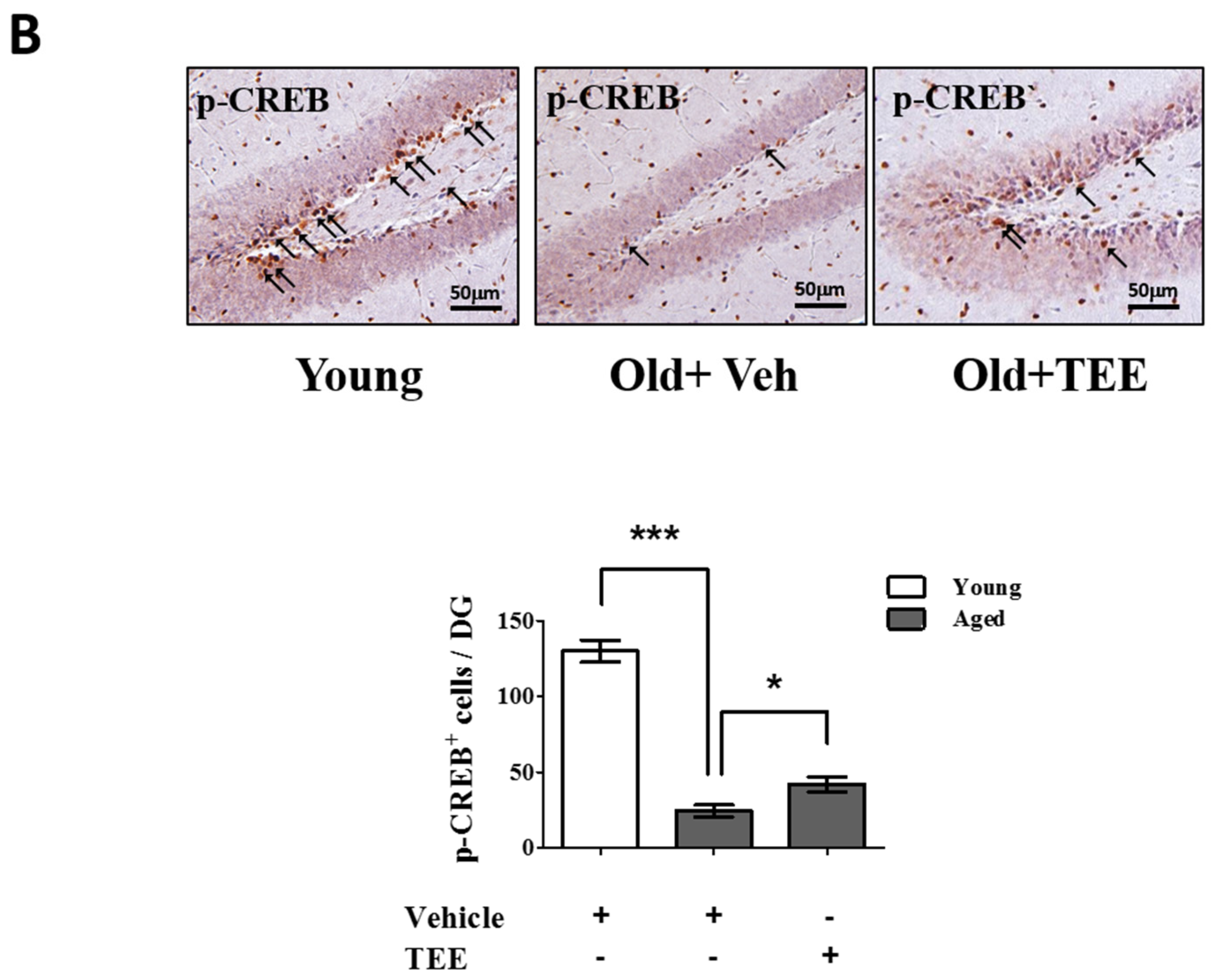
3.5. Oral TEE Supplement Activated ERK/CREB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lazarov, O.; Hollands, C. Hippocampal neurogenesis: Learning to remember. Prog. Neurobiol. 2016, 138–140, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.R.; Lichtenwalner, R.J. Neurogenesis in the adult and aging brain. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; Boca Raton (FL): Palm Beach County, FL, USA, 2007. [Google Scholar]
- Hung, C.W.; Chen, Y.C.; Hsieh, W.L.; Chiou, S.H.; Kao, C.L. Ageing and neurodegenerative diseases. Ageing Res. Rev. 2010, 9 (Suppl. 1), S36–S46. [Google Scholar] [CrossRef] [PubMed]
- Seib, D.R.; Martin-Villalba, A. Neurogenesis in the normal ageing hippocampus: A mini-review. Gerontology 2015, 61, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.; Hamilton, D.A.; Petropoulos, H.; Yeo, R.A.; Brooks, W.M.; Baumgartner, R.N.; Sutherland, R.J. The aging hippocampus: Cognitive, biochemical and structural findings. Cereb. Cortex 2003, 13, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Dabaghian, F.H.; Hashemi, M.; Entezari, M.; Movassaghi, S.; Goushegir, S.A.; Kalantari, S.; Movafagh, A.; Sharifi, Z.N. Effect of cyperus rotundus on ischemia-induced brain damage and memory dysfunction in rats. Iran. J. Basic Med. Sci. 2015, 18, 199–204. [Google Scholar] [PubMed]
- Cho, N.; Lee, K.Y.; Huh, J.; Choi, J.H.; Yang, H.; Jeong, E.J.; Kim, H.P.; Sung, S.H. Cognitive-enhancing effects of rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem. Toxicol. 2013, 58, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Son, H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009, 42, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via ngf-induced erk/creb activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Pan, J.; Chen, F.; Zheng, L.; Chen, Y.; Zhang, S.; Feng, W. l-3-n-butylphthalide improves cognitive impairment of APP/PS1 mice by BDNF/TRKB/PI3K/AKT pathway. Int. J. Clin. Exper. Med. 2014, 7, 1706–1713. [Google Scholar]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhu, T.; Xia, Z.; Gao, F.; Gu, W.; Chen, X.; Yuan, T.; Yu, H. Inhibition of MAPK/ERK signaling blocks hippocampal neurogenesis and impairs cognitive performance in prenatally infected neonatal rats. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Forbes, K.M.; Hassed, C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst. Rev. 2011, 7, 899–905. [Google Scholar]
- Hung, C.F.; Huang, T.F.; Chen, B.H.; Shieh, J.M.; Wu, P.H.; Wu, W.B. Lycopene inhibits TNF-α-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur. J. Pharmacol. 2008, 586, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, V.; Pierucci, M.; di Giovanni, G.; Dragani, L.K.; Murzilli, S.; Poggi, A.; Esposito, E. Intake of tomato-enriched diet protects from 6-hydroxydopamine-induced degeneration of rat nigral dopaminergic neurons. J. Neural. Transm. Suppl. 2009, 73, 333–341. [Google Scholar]
- Gokul, K.; Muralidhara. Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: Relevance to parkinson’s disease. Neurochem. Res. 2014, 39, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against mptp induced experimental parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Park, H.S.; Park, J.W.; Li, S.H.; Chun, Y.S. Red ginseng and 20(s)-rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J. Nat. Med. 2012, 66, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.P.; Mork, A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mrna and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006, 1110, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.S.; Holmes, P.V. An overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. Int. J. Pept. 2011, 2011, 654085. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Kim, M. Aging and neurodegeneration. Molecular mechanisms of neuronal loss in huntington’s disease. Mech. Ageing Dev. 2006, 127, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Von Bohlen und Halbach, O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front. Aging Neurosci. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Moore, S.J.; Deshpande, K.; Stinnett, G.S.; Seasholtz, A.F.; Murphy, G.G. Conversion of short-term to long-term memory in the novel object recognition paradigm. Neurobiol. Learn. Mem. 2013, 105, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal. Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, S.D.; Schroeder, J.E.; Trentani, A. Changes in hippocampal neurogenesis throughout early development. Neurobiol. Aging 2015, 36, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Brummelte, S.; Galea, L.A. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 2010, 168, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Tronche, C.; Pierard, C.; Coutan, M.; Chauveau, F.; Liscia, P.; Beracochea, D. Increased stress-induced intra-hippocampus corticosterone rise associated with memory impairments in middle-aged mice. Neurobiol. Learn. Mem. 2010, 93, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Wang, Z. Chronic corticosterone exposure reduces hippocampal astrocyte structural plasticity and induces hippocampal atrophy in mice. Neurosci. Lett. 2015, 592, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Sommer, W.H.; Metsis, M.; Stromberg, I.; Agnati, L.F.; Fuxe, K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by Exon IV promoter. J. Neuroendocrinol. 2006, 18, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.T.; Lee, S.H.; Lee, Y.S.; Son, H. Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro. Biochem. Biophys. Res. Commun. 2004, 317, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Harrisberger, F.; Smieskova, R.; Schmidt, A.; Lenz, C.; Walter, A.; Wittfeld, K.; Grabe, H.J.; Lang, U.E.; Fusar-Poli, P.; Borgwardt, S. BDNF VAL66MET polymorphism and hippocampal volume in neuropsychiatric disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2015, 55, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Tolwani, R.J.; Buckmaster, P.S.; Varma, S.; Cosgaya, J.M.; Wu, Y.; Suri, C.; Shooter, E.M. Bdnf overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 2002, 114, 795–805. [Google Scholar] [CrossRef]
- Gorski, J.A.; Balogh, S.A.; Wehner, J.M.; Jones, K.R. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience 2003, 121, 341–354. [Google Scholar] [CrossRef]
- Katoh-Semba, R.; Asano, T.; Ueda, H.; Morishita, R.; Takeuchi, I.K.; Inaguma, Y.; Kato, K. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002, 16, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, M.R. BDNF alters ERK/P38 mapk activity ratios to promote differentiation in growth plate chondrocytes. Mol. Endocrinol. 2012, 26, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Winder, D.G.; Martin, K.C.; Muzzio, I.A.; Rohrer, D.; Chruscinski, A.; Kobilka, B.; Kandel, E.R. ERK plays a regulatory role in induction of ltp by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron 1999, 24, 715–726. [Google Scholar] [CrossRef]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-β1–42-induced rat model of alzheimer’s disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Wang, C.H.; Wu, W.T.; Chen, H.L. Fructo-oligosaccharide improved brain β-amyloid, β-secretase, cognitive function, and plasma antioxidant levels in d-galactose-treated Balb/Cj mice. Nutr. Neurosci. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Buccafusco, J.J. Animal Models of Cognitive Impairment; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kloskowska, E.; Pham, T.M.; Nilsson, T.; Zhu, S.; Oberg, J.; Codita, A.; Pedersen, L.A.; Pedersen, J.T.; Malkiewicz, K.; Winblad, B.; et al. Cognitive impairment in the TG6590 transgenic rat model of alzheimer’s disease. J. Cell. Mol. Med. 2010, 14, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kaundal, R.K.; More, S.; Sharma, S.S. Beneficial effects of pioglitazone on cognitive impairment in MPTP model of parkinson’s disease. Behav. Brain Res. 2009, 197, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-S.; Han, M.; Shin, H.S.; Shon, D.-H.; Lee, S.-T.; Shin, C.-Y.; Lee, Y.; Lee, D.H.; Chung, J.H. Lycopersicon esculentum Extract Enhances Cognitive Function and Hippocampal Neurogenesis in Aged Mice. Nutrients 2016, 8, 679. https://doi.org/10.3390/nu8110679
Bae J-S, Han M, Shin HS, Shon D-H, Lee S-T, Shin C-Y, Lee Y, Lee DH, Chung JH. Lycopersicon esculentum Extract Enhances Cognitive Function and Hippocampal Neurogenesis in Aged Mice. Nutrients. 2016; 8(11):679. https://doi.org/10.3390/nu8110679
Chicago/Turabian StyleBae, Jung-Soo, Mira Han, Hee Soon Shin, Dong-Hwa Shon, Soon-Tae Lee, Chang-Yup Shin, Yuri Lee, Dong Hun Lee, and Jin Ho Chung. 2016. "Lycopersicon esculentum Extract Enhances Cognitive Function and Hippocampal Neurogenesis in Aged Mice" Nutrients 8, no. 11: 679. https://doi.org/10.3390/nu8110679
APA StyleBae, J.-S., Han, M., Shin, H. S., Shon, D.-H., Lee, S.-T., Shin, C.-Y., Lee, Y., Lee, D. H., & Chung, J. H. (2016). Lycopersicon esculentum Extract Enhances Cognitive Function and Hippocampal Neurogenesis in Aged Mice. Nutrients, 8(11), 679. https://doi.org/10.3390/nu8110679




