“A Vegetarian vs. Conventional Hypocaloric Diet: The Effect on Physical Fitness in Response to Aerobic Exercise in Patients with Type 2 Diabetes.” A Parallel Randomized Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Diet
2.2. Exercise
2.3. Medication
2.4. Adherence
2.5. Hunger and Depressive Symptoms
2.6. Statistical Analysis
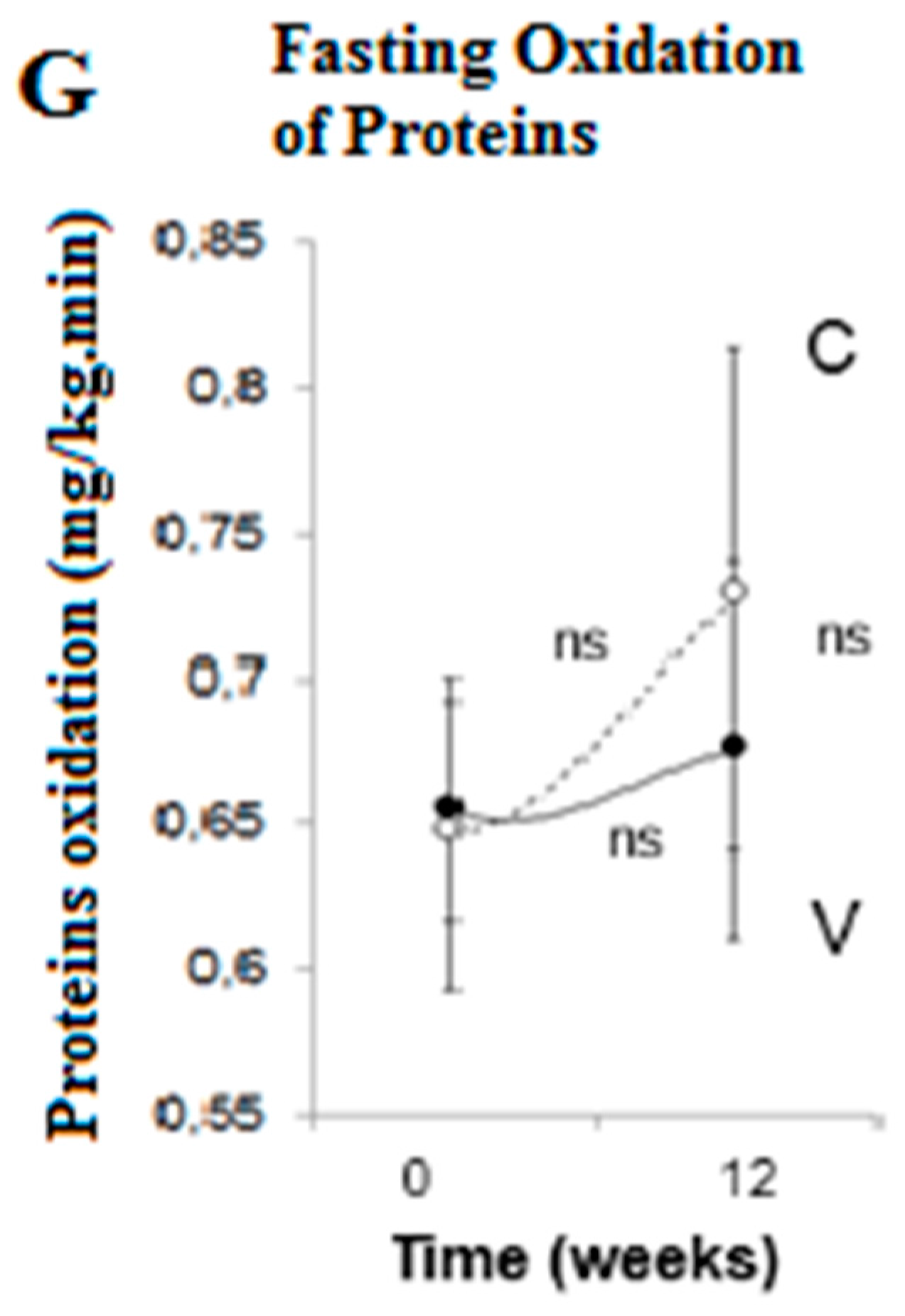
3. Results
Adherence
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31, S61–S78. [Google Scholar]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.S.; Sklar, M.; Barnard, N.D.; Gore, S.; Sullivan, R.; Browning, S. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev. Med. 1999, 29, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.M.; Bell, J.D.; So, P.W.; Dornhorst, A.; Frost, G.S. Veganism and its relationship with insulin resistance and intramyocellular lipid. Eur. J. Clin. Nutr. 2005, 59, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Steer, R.A.; Cavalieri, T.A.; Leonard, D.M.; Beck, A.T. Use of the Beck depression inventory for primary care to screen for major depression disorders. Gen. Hosp. Psychiatry 1999, 21, 106–111. [Google Scholar] [CrossRef]
- Nieman, D.C. Physical fitness and vegetarian diets: Is there a relation? Am. J. Clin. Nutr. 1999, 70, 570S–575S. [Google Scholar] [PubMed]
- Eisinger, M.; Plath, M.; Jung, K.; Leitymann, C. Nutrient intake of endurance runners with ovo-lacto-vegetarian diet and regular western diet. Z. Ernahrung. 1994, 33, 217–229. [Google Scholar] [CrossRef]
- Nieman, D.C.; Butler, J.V.; Pollett, L.M.; Dietrich, S.J.; Lutz, R.D. Nutrient intake of marathon runners. J. Am. Diet Assoc. 1989, 89, 1273–1278. [Google Scholar] [PubMed]
- Haub, M.D.; Wells, A.M.; Tarnopolsky, M.A.; Campbell, W.W. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 2002, 76, 511–517. [Google Scholar] [PubMed]
- Gojda, J.; Patková, J.; Jaček, M.; Potočková, J.; Trnka, J.; Kraml, P.; Anděl, M. Higher insulin sensitivity in vegans is not associated with higher mitochondrial density. Eur. J. Clin. Nutr. 2013, 67, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Parra, D.; Martinez, J.A. Energy-restricted diets based on a distinct food selection affecting the glycemic index induce different weight loss and oxidative response. Clin. Nutr. 2008, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Pelikanova, T. Vegetarian diets in the prevention and treatment of type 2 diabetes. J. Am. Coll. Nutr. 2015, 34, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Hrachovinova, T.; Hill, M.; Pelikanova, T. Vegetarian diet in type 2 diabetes—Improvement in quality of life, mood and eating behaviour. Diabet. Med. 2013, 30, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Hsuchou, H.; Wang, Y.; Cornelissen-Guillaume, G.G.; Kastin, A.J.; Jang, E.; Halberg, F.; Pan, W. Diminished leptin signaling can alter circadian rhythm of metabolic activity and feeding. J. Appl. Physiol. 2013, 115, 995–1003. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veleba, J.; Matoulek, M.; Hill, M.; Pelikanova, T.; Kahleova, H. “A Vegetarian vs. Conventional Hypocaloric Diet: The Effect on Physical Fitness in Response to Aerobic Exercise in Patients with Type 2 Diabetes.” A Parallel Randomized Study. Nutrients 2016, 8, 671. https://doi.org/10.3390/nu8110671
Veleba J, Matoulek M, Hill M, Pelikanova T, Kahleova H. “A Vegetarian vs. Conventional Hypocaloric Diet: The Effect on Physical Fitness in Response to Aerobic Exercise in Patients with Type 2 Diabetes.” A Parallel Randomized Study. Nutrients. 2016; 8(11):671. https://doi.org/10.3390/nu8110671
Chicago/Turabian StyleVeleba, Jiri, Martin Matoulek, Martin Hill, Terezie Pelikanova, and Hana Kahleova. 2016. "“A Vegetarian vs. Conventional Hypocaloric Diet: The Effect on Physical Fitness in Response to Aerobic Exercise in Patients with Type 2 Diabetes.” A Parallel Randomized Study" Nutrients 8, no. 11: 671. https://doi.org/10.3390/nu8110671
APA StyleVeleba, J., Matoulek, M., Hill, M., Pelikanova, T., & Kahleova, H. (2016). “A Vegetarian vs. Conventional Hypocaloric Diet: The Effect on Physical Fitness in Response to Aerobic Exercise in Patients with Type 2 Diabetes.” A Parallel Randomized Study. Nutrients, 8(11), 671. https://doi.org/10.3390/nu8110671





