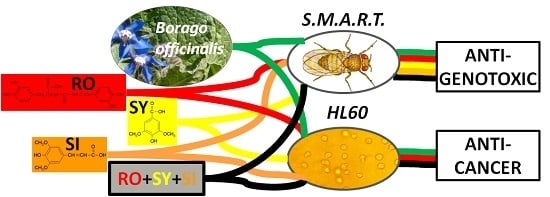
Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Drosophila Experiments
2.3.1. Fly Stocks and Crosses
2.3.2. Larvae Treatments
2.4. HL-60 Experiments
2.4.1. Cell Cultures
2.4.2. Cell Treatments
2.4.3. Trypan Blue Dye Exclusion Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. In Vivo Assays
| Survival 1 % Treatments | |||||
|---|---|---|---|---|---|
| Simple | Combined 2 | Simple | Combined 2 | ||
| H2O | 100 | H2O2 (0.12 M) | 37.87 * | ||
| BF (mg·mL−1) | WF (mg·mL−1) | ||||
| 1.25 | 100 | 52.44 *,‡ | 1.25 | 97.78 | 33.33 *,‡ |
| 2.5 | 100 | 54 *,‡ | 2.5 | 63.11 * | 27.56 *,‡ |
| 5 | 82 * | 86.89 * | 5 | 71.33 * | 17.33 *,‡ |
| RO (mM) | SY (mM) | ||||
| 0.35 | 48.44 * | 49.56 * | 0.16 | 39.78 * | 31.11 *,‡ |
| 0.7 | 22.22 * | 31.11 *,‡ | 0.32 | 42.67 * | 29.33 *,‡ |
| 1.39 | 33.33 * | 45.56 *,‡ | 0.63 | 31.11 * | 20.44 *,‡ |
| 2.78 | 21.33 * | 38.89 *,‡ | 1.26 | 58.22 * | 36.89 *,‡ |
| SI (mM) | RO + SY + SI (mM) | ||||
| 0.15 | 78.22 * | 64 *,‡ | a 3 | 48.67 * | 24.44 *,‡ |
| 0.29 | 60.22 * | 58.89 * | b | 55.11 * | 34.67 *,‡ |
| 0.58 | 69.33 * | 39.78 *,‡ | c | 74.44 * | 57.78 *,‡ |
| 1.16 | 55.11 * | 43.56 *,‡ | d | 44.89 * | 53.78 *,‡ |
| Mutation Rate (Spots/Wing) Diagnosis 1 | |||||
|---|---|---|---|---|---|
| N° of Wings | Small Single Spots 1–2 Cells m = 2 | Large Single Spots >2 Cells m = 5 | Twin Spots m = 5 | Total Spots m = 2 | |
| H2O | 212 | 0.26 (54) | 0.04 (8) | 0.03 (5) | 0.32 (67) |
| H2O2 (0.12 M) | 168 | 0.60 (94) + | 0.07 (11) − | 0.06 (4) − | 0.65 (109) + |
| BF (mg·mL−1) | |||||
| 1.25 | 40 | 0.13 (5) − | 0.03 (1) − | 0.05 (2) − | 0.20 (8) − |
| 2.5 | 54 | 0.22 (12) − | 0.06 (3) − | 0.02 (1) − | 0.30 (16) − |
| 5 | 66 | 0.29 (19) − | 0.03 (2) − | 0.05 (3) − | 0.36 (24) − |
| WF (mg·mL−1) | |||||
| 1.25 | 66 | 0.26 (17) − | 0.03 (2) − | 0.05 (3) − | 0.33 (22) − |
| 2.5 | 50 | 0.26 (13) − | 0.08 (4) − | 0.02 (1) − | 0.36 (18) − |
| 5 | 90 | 0.36 (32) − | 0.02 (2) − | 0.01 (1) − | 0.39 (35) − |
| RO (mM) | |||||
| 0.35 | 16 | 0.38 (6) − | 0 | 0 | 0.38 (6) − |
| 0.7 | 34 | 0.21 (7) − | 0 | 0.06 (2) − | 0.26 (9) − |
| 1.39 | 22 | 0.18 (4) − | 0 | 0.05 (1) − | 0.23 (5) − |
| 2.78 | 38 | 0.16 (6) − | 0.05 (2) − | 0 | 0.21 (8) − |
| SY (mM) | |||||
| 0.16 | 40 | 0.30 (12) − | 0.05 (2) − | 0.03 (1) − | 0.38 (15) − |
| 0.32 | 30 | 0.20 (6) − | 0.07 (2) − | 0 | 0.27 (8) − |
| 0.63 | 48 | 0.19 (9) − | 0.02 (1) − | 0 | 0.21 (10) − |
| 1.26 | 32 | 0.22 (7) − | 0.06 (2) − | 0 | 0.28 (9) − |
| SI (mM) | |||||
| 0.15 | 24 | 0.38 (9) − | 0.04 (1) − | 0.04 (1) − | 0.46 (11) − |
| 0.29 | 32 | 0.39 (12) − | 0.10 (3) − | 0 | 0.48 (15) − |
| 0.58 | 30 | 0.33 (10) − | 0.07 (2) − | 0 | 0.40 (12) − |
| 1.16 | 40 | 0.23 (9) − | 0.03 (1) − | 0.03 (1) − | 0.28 (11) − |
| RO + SY + SI (mM) | |||||
| a 2 | 26 | 0.15 (4) − | 0 | 0.04 (1) − | 0.19 (5) − |
| b | 34 | 0.12 (4) − | 0.03 (1) − | 0 | 0.15 (5) − |
| c | 32 | 0.22 (7) − | 0.13 (4) + | 0 | 0.34 (11) − |
| d | 22 | 0.41 (9) − | 0.05 (1) − | 0 | 0.45 (10) − |
| Mutation Rate (Spots/Wing) Diagnosis 1 | |||||
|---|---|---|---|---|---|
| N° of Wings | Small Single Spots 1–2 Cells m = 2 | Large Single Spots >2 Cells m = 5 | Twin Spots m = 5 | Total Spots m = 2 | |
| H2O | 212 | 0.26 (54) | 0.04 (8) | 0.03 (5) | 0.32 (67) |
| H2O2 (0.12 M) | 168 | 0.60 (94) + | 0.07 (11) – | 0.06 (4) – | 0.65 (109) + |
| BF (mg·mL−1) | |||||
| 1.25 | 30 | 0.13 (4) − | 0.03 (1) − | 0 | 0.17 (5) − |
| 2.5 | 34 | 0.24 (8) − | 0.03 (1) − | 0 | 0.26 (9) − |
| 5 | 18 | 0.17 (3) − | 0.06 (1) − | 0 | 0.23 (4) − |
| WF (mg·mL−1) | |||||
| 1.25 | 10 | 0.30 (3) − | 0.10 (1) − | 0 | 0.40 (4) − |
| 2.5 | 28 | 0.32 (9) − | 0 | 0 | 0.32 (9) − |
| 5 | 24 | 0.25 (6) − | 0.04 (1) − | 0 | 0.29 (7) − |
| RO (mM) | |||||
| 0.35 | 30 | 0.17 (5) − | 0 | 0 | 0.17 (5) − |
| 0.7 | 40 | 0.35 (14) − | 0.08 (3) − | 0.03 (1) − | 0.45 (18) − |
| 1.39 | 22 | 0.14 (3) − | 0.14 (3) − | 0 | 0.27 (6) − |
| 2.78 | 52 | 0.21 (11) − | 0 | 0.04 (2) − | 0.25 (13) − |
| SY (mM) | |||||
| 0.16 | 22 | 0.23 (5) − | 0 | 0 | 0.23 (5) − |
| 0.32 | 10 | 0.30 (3) − | 0 | 0 | 0.30 (3) − |
| 0.63 | 32 | 0.28 (9) − | 0 | 0 | 0.28 (9) − |
| 1.26 | 22 | 0.32 (7) − | 0 | 0 | 0.32 (7) − |
| SI (mM) | |||||
| 0.15 | 12 | 0.42 (5) − | 0 | 0 | 0.42 (5) − |
| 0.29 | 8 | 0.25 (2) − | 0 | 0 | 0.25 (2) − |
| 0.58 | 22 | 0.27 (6) − | 0.09 (2) − | 0.05 (1) − | 0.41 (9) − |
| 1.16 | 28 | 0.25 (7) − | 0.04 (1) − | 0 | 0.29 (8) − |
| RO + SY + SI (mM) | |||||
| a 2 | 38 | 0.29 (11) − | 0 | 0 | 0.29 (11) − |
| b | 26 | 0.27 (7) − | 0.15 (4) + | 0 | 0.42 (11) − |
| c | 17 | 0.18 (3) − | 0 | 0 | 0.18 (3) − |
| d | 12 | 0.25 (3) − | 0.08 (1) − | 0 | 0.33 (4) − |
3.1.1. Toxicity Assays
3.1.2. Genotoxicity Assays
3.1.3. Antigenotoxicity Assays
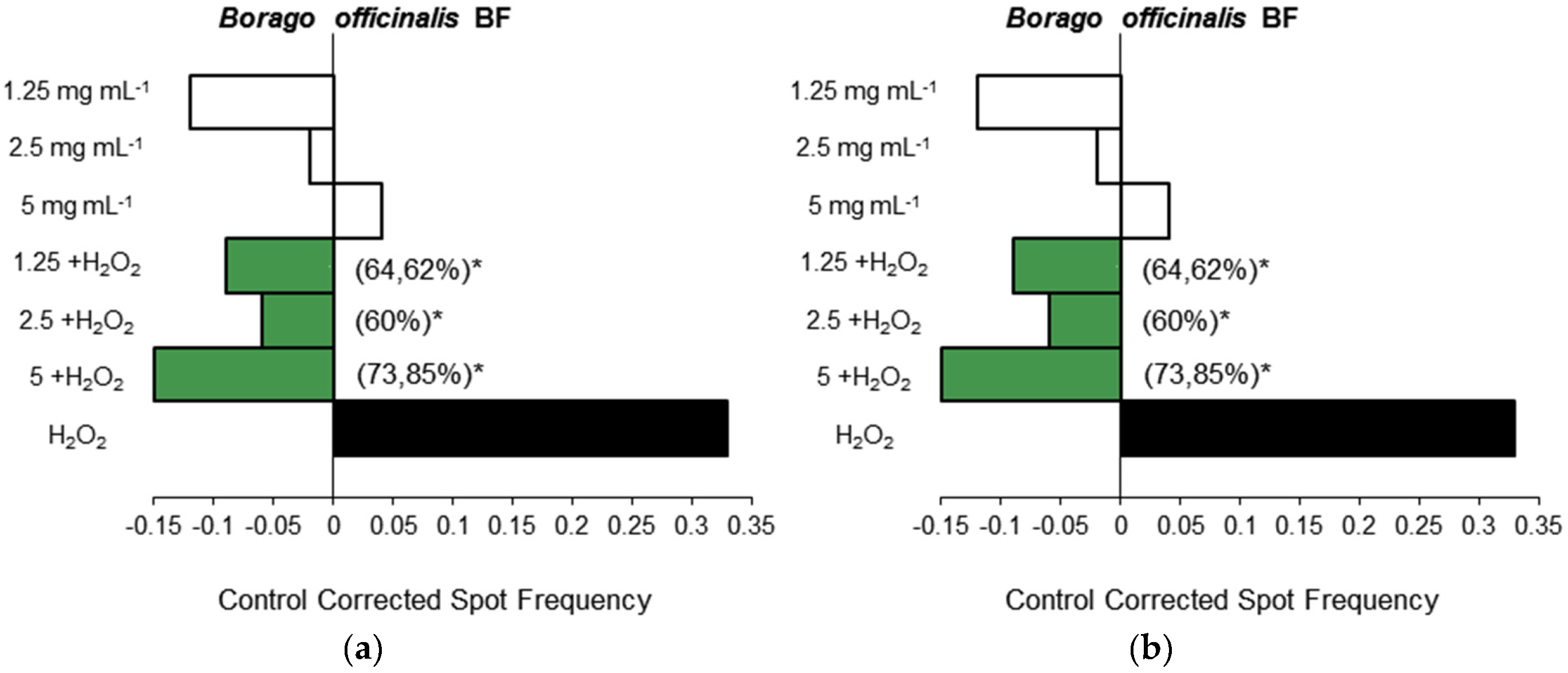
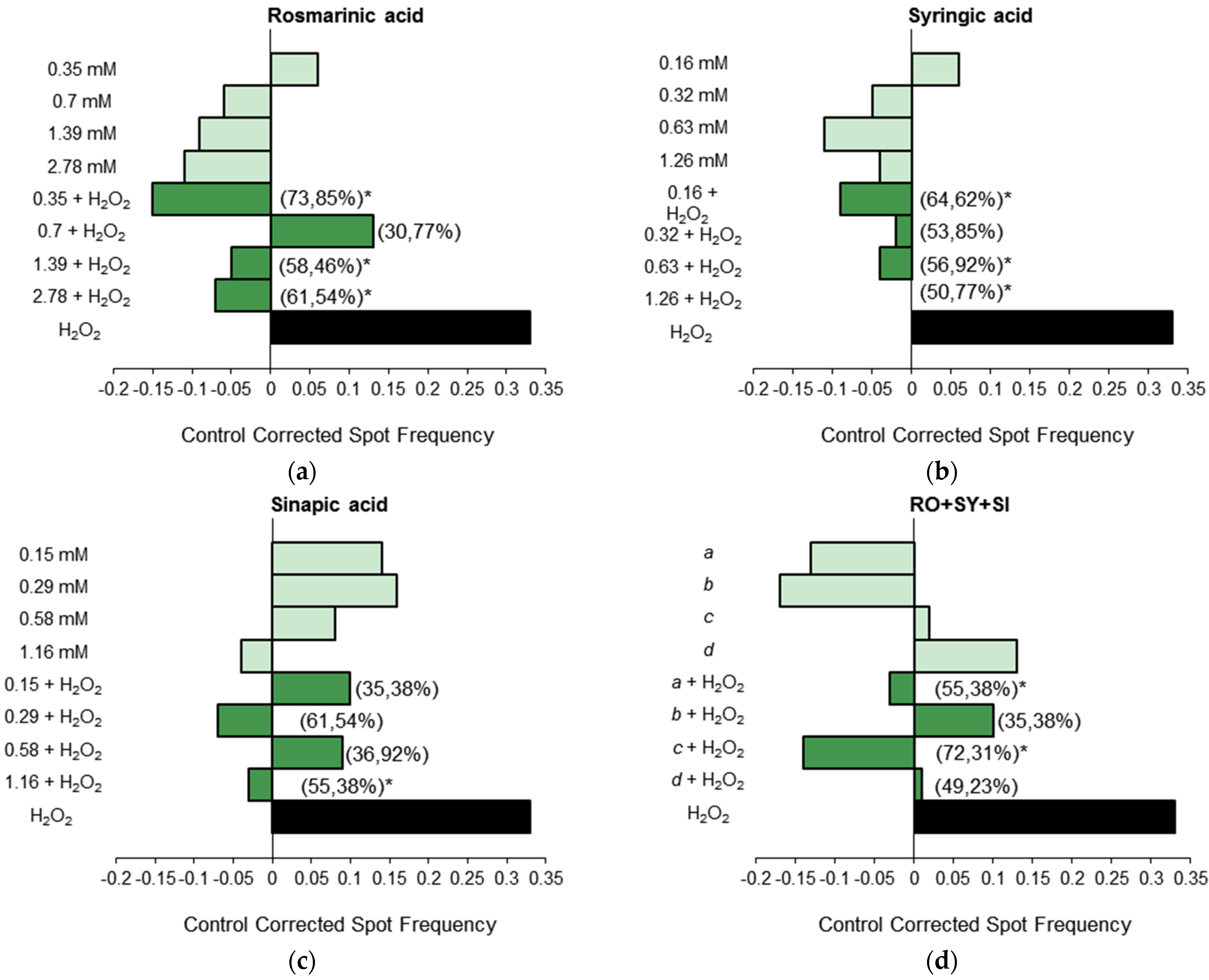
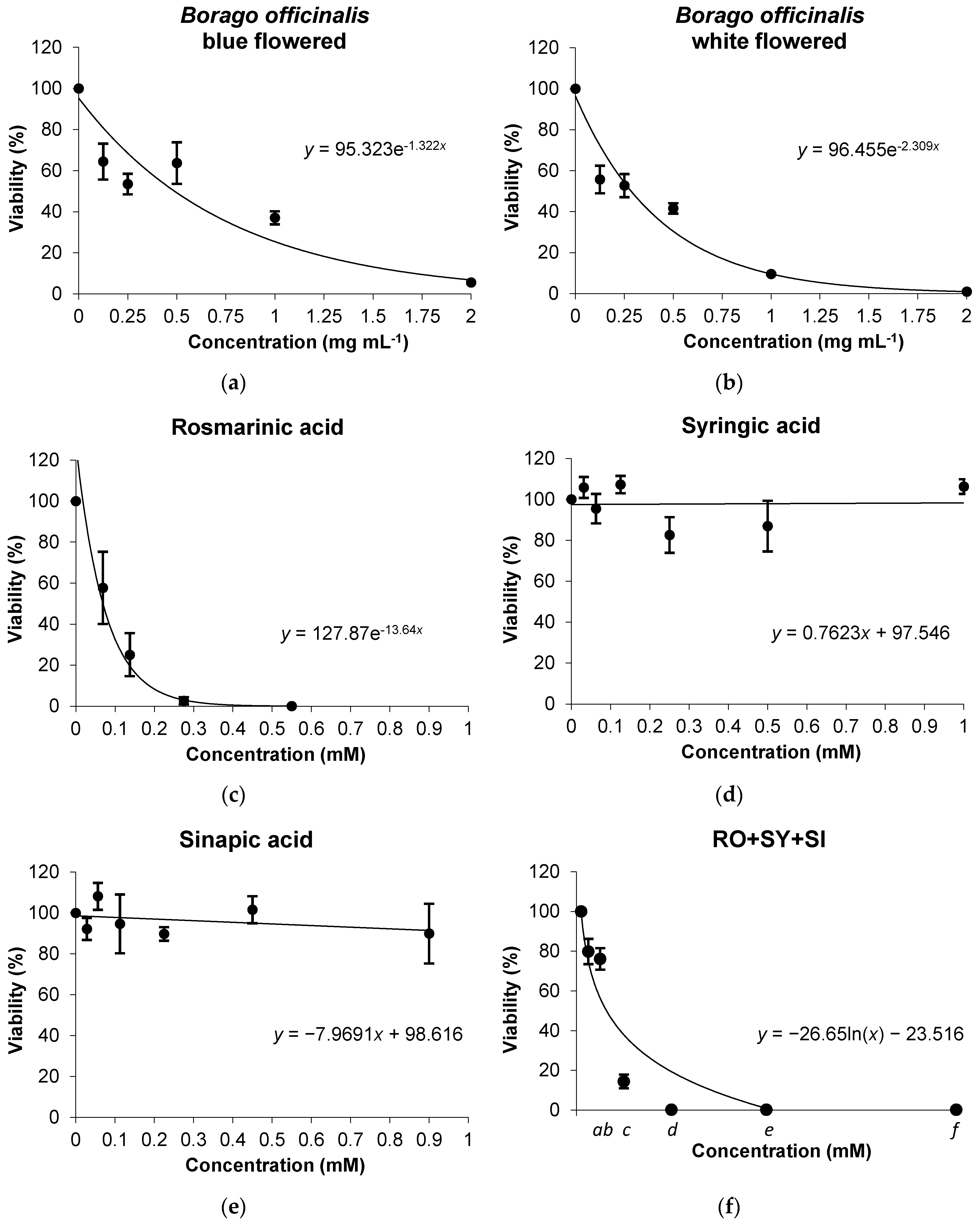
3.2. In Vitro Assays
Cytotoxicity Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BF | Borago officinalis blue-flowered |
| CO2 | carbon dioxide |
| DNA | deoxyribonucleic acid |
| flr | flare |
| H2O2 | hydrogen peroxide |
| HL-60 | human acute promyelocytic leukemia cell line |
| IC50 | half maximal inhibitory concentration |
| IP | inhibition percentage |
| LD50 | median lethal dose |
| mwh | multiple wing-hair |
| RO | rosmarinic acid |
| SI | sinapic acid |
| SMART | somatic mutation and recombination test |
| SY | syringic acid |
| T | toxicity |
| WF | Borago officinalis white-flowered |
References
- Zhou, S.; Koh, H.L.; Gao, Y.; Gong, Z.Y.; Lee, E.J.D. Herbal bioactivation: The good, the bad and the ugly. Life Sci. 2004, 74, 935–968. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J. The History of Plants; Woodward, M., Ed.; Senate, Studio Editions Ltd.: London, UK, 1927; pp. 185–186. [Google Scholar]
- Basar, S.N.; Rani, S.; Farah, S.A.; Zaman, R. Review on Borago officinalis: A Wonder Herb. Int. J. Biol. Pharm. Res. 2013, 4, 582–587. [Google Scholar]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with alpha-amylase and pancreatic lipase inhibitory activities. J. Sci. Food Agric. 2014, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- De Ciriano, M.G.I.; García-Herreros, C.; Larequi, E.; Valencia, I.; Ansorena, D.; Astiasarán, I. Use of natural antioxidants from lyophilized water extracts of Borago officinalis in dry fermented sausages enriched in ω-3 PUFA. Meat Sci. 2009, 83, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Barre, D. Potential of evening primrose, borage, black currant, and fungal oils in human health. Ann. Nutr. Metab. 2001, 45, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A. The potential for dietary supplements to reduce premenstrual syndrome (PMS) symptoms. J. Am. Coll. Nutr. 2000, 19, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S. A Treasury of Persian Cuisine, 2nd ed.; Stamford House Publishing: Hong Kong, China, 2007. [Google Scholar]
- Del Río-Celestino, M.; Font, R.; de Haro-Bailón, A. Distribution of the fatty acids content in edible organs and seed fractions of borage (Borago officinalis L.). J. Sci. Food Agric. 2008, 88, 248–255. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; García-Maroto, F.; Vilches-Ferrón, M.A.; López-Alonso, D. Gamma-linolenic acid from fourteen Boraginaceae species. Ind. Crops Prod. 2003, 18, 85–89. [Google Scholar] [CrossRef]
- Gunstone, F.D. Gamma-linolenic acid—Occurrence and physical and chemical properties. Prog. Lipid Res. 1992, 31, 145–161. [Google Scholar] [CrossRef]
- Janick, J.; Simon, J.E.; Quinn, J.; Beaubaire, N. Borage: A source of gamma-linolenic acid. In Herbs, Spices and Medicinal Plants; Cracker, L.E., Simon, J.E., Eds.; Oryx Press: Phoenix, Arizona, 1989; Volume 4, pp. 145–168. [Google Scholar]
- Jaramillo, G.; Vilela, A. Critical size for reproduction and ontogenetic changes in the allocation patterns of wild and domesticated species of evening primrose (Oenothera L.). Ind. Crop. Prod. 2015, 65, 324–327. [Google Scholar] [CrossRef]
- El Hafid, R.; Blade, S.F.; Hoyano, Y. Seeding date and nitrogen fertilization effects on the performance of borage (Borago officinalis L.). Ind. Crop. Prod. 2002, 16, 193–199. [Google Scholar] [CrossRef]
- Gilbertson, P.K.; Berti, M.T.; Johnson, B.L. Borage cardinal germination temperatures and seed development. Ind. Crop. Prod. 2014, 59, 202–209. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop. Med. 2014, 7, S22–S28. [Google Scholar] [CrossRef]
- Segovia, F.; Lupo, B.; Peiró, S.; Gordon, M.H.; Almajano, M.P. Extraction of antioxidants from borage (Borago officinalis L.) leaves—Optimization by response surface method and application in oil-in-water emulsions. Antioxidants 2015, 3, 339–357. [Google Scholar] [CrossRef] [Green Version]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; el Feki, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arabian J. Chem. 2014. [Google Scholar] [CrossRef]
- Mhamdi, W.; Aidi, W.W.; Chahed, T.; Ksouri, R.; Marzouk, B. Phenolic compounds and antiradical scavenging activity changes during Borago officinalis stalk leaf development. Asian J. Chem. 2010, 22, 6397–6402. [Google Scholar]
- Chlopcíková, S.; Psotová, J.; Miketová, P.; Sousek, J.; Lichnovský, V.; Simánek, V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part II. Caffeic, chlorogenic and rosmarinic acids. Phytother. Res. 2004, 18, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Gamaniel, K.; Samuel, B.B.; Kapu, D.S.; Samson, A.; Wagner, H.; Okogun, J.I.; Wambebe, C. Anti-sickling, analgesic and anti-inflammatory properties of 3,5-dimethoxy-4-hydroxy benzoic acid and 2,3,4-trihydroxyacetophenone. Phytomedicine 2000, 7, 105–110. [Google Scholar] [CrossRef]
- Niwa, T.; Doi, U.; Kato, Y.; Osawa, T. Inhibitory mechanism of sinapinic acid against peroxynitrite-mediated tyrosine nitration of protein in vitro. FEBS Lett. 1999, 459, 43–46. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crop. Prod. 2014, 65, 390–396. [Google Scholar] [CrossRef]
- Galwey, N.W.; Shirlin, A.J. Selection of borage (Borago officinalis) as a seed crop for pharmaceutical uses. Heredity 1990, 65, 249–257. [Google Scholar] [CrossRef]
- Kelber, O.; Wegener, T.; Steinhoff, B.; Staiger, C.; Wiesner, J.; Knöss, W.; Kraft, K. Assessment of genotoxicity of herbal medicinal products: Application of the “bracketing and matrixing” concept using the example of Valerianae radix (valerian root). Phytomedicine 2014, 2, 1124–1129. [Google Scholar] [CrossRef]
- Prasad, B.R.; Hegde, S.N. Use of Drosophila as a model organism in medicine. J. Med. Med. Sci. 2010, 1, 589–593. [Google Scholar]
- Romero-Jiménez, M.; Campos-Sánchez, J.; Analla, M.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Genotoxicity and antigenotoxicity of some traditional medicinal herbs. Mutat. Res. 2005, 585, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Baena, M.D.; Tasset, I.; Obregón-Cano, S.; de Haro-Bailon, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Antigenotoxicity and tumor growing inhibition by leafy Brassica carinata and sinigrin. Molecules 2015, 20, 15748–15765. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Campos-Sánchez, J.; el Hamss, R.; Rojas-Molina, M.; Muñoz-Serrano, A.; Analla, M.; Alonso-Moraga, Á. Modulation of genotoxicity by extra-virgin olive oil and some of its distinctive components assessed by use of the Drosophila wing-spot test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 703, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Molina, M.; Campos-Sánchez, J.; Analla, M.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Genotoxicity of vegetable cooking oils in the Drosophila wing spot test. Environ. Mol. Mutagen. 2005, 45, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mol. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Ren, N.; Charlton, J.; Adler, P.N. The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics 2007, 176, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huen, D.; Morely, T.; Johnson, G.; Gubb, D.; Roote, J.; Adler, P.N. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 2008, 180, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.J.; Ruscetti, F.W.; Gallagher, R.E.; Gallo, R.C. Terminal differentiation of human promyelocytic leukaemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA 1978, 75, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Cheung, S.; Chan, E.; Hasman, D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J. Ethnopharmacol. 2004, 93, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Y.; Loft, S. Effect of chemopreventive compounds from Brassica vegetables on NAD(P)H: Quinine reductase and induction of DNA strand breaks in murine hepa1c1c7 cells. Food Chem. Toxicol. 2003, 41, 455–462. [Google Scholar] [CrossRef]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective effect of borage seed oil and gamma linolenic acid on DNA: in vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, H.; Würgler, F.E. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Frei, H.; Würgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat. Res. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis 1994, 9, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Moraga, Á.; Graf, U. Genotoxicity testing of antiparasitic nitrofurans in the Drosophila wing somatic mutation and recombination test. Mutagenesis 1989, 4, 105–110. [Google Scholar] [CrossRef]
- Graf, U.; Alonso-Moraga, Á.; Castro, R.; Díaz-Carrillo, E. Genotoxicity testing of different types of beverages in the wing somatic mutation and recombination test. Food Chem. Toxicol. 1994, 32, 423–430. [Google Scholar] [CrossRef]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef]
- Larson, K.M.; Roby, M.R.; Stermitz, F.R. Unsaturated pyrrolizidines from Borage (Borago officinalis) a common garden herb. J. Nat. Prod. 1984, 47, 747–748. [Google Scholar] [CrossRef]
- McGuffin, M.; Hobbs, C.; Upton, R.; Goldberg, A. American Herbal Products Association’s Botanical Safety Handbook, 2nd ed.; Boca Raton, CRC Press: Florida, CA, USA, 1997. [Google Scholar]
- Zenk, M.; Etschenberg, E.; Graf, E. Use of Rosmarinic Acid in the Treatment of Inflammations and Pharmaceutical Products Used Therein. U.S. Patent 4329361, 11 May 1982. [Google Scholar]
- Mhamdi, B.; Wannes, W.A.; Sriti, J.; Jellali, I.; Ksouri, R.; Marzouk, B. Effect of harvesting time on phenolic compounds and antiradical scavenging activity of Borago officinalis seed extracts. Ind. Crop. Prod. 2010, 31, e1–e4. [Google Scholar] [CrossRef]
- Bast, A.; Chandler, R.F.; Choy, P.C.; Delmulle, L.M.; Gruenwald, J.; Halkes, S.B.A.; Keller, K.; Koeman, J.H.; Peters, P.; Przyrembel, H.; et al. Botanical health products, positioning and requirements for effective and safe use. Environ. Toxicol. Pharmacol. 2002, 12, 195–211. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolic compounds in plant foods: Chemistry and health benefits. J. Food Sci. Nutr. 2003, 8, 200–218. [Google Scholar] [CrossRef]
- Frei, H.; Luthy, J.; Brauchli, J.; Zweifel, U.; Wurgler, F.E.; Schlatter, C. Structure/activity relationships of the genotoxic potencies of sixteen pyrrolizidine alkaloids assayed for the induction of somatic mutation and recombination in wing cells of Drosophila melanogaster. Chem. Biol. Interact. 1992, 83, 1–22. [Google Scholar] [CrossRef]
- Pereira, P.; Tysca, D.; Oliveira, P.; Brum, L.F.S.; Picada, J.N.; Ardenghi, P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol. Res. 2005, 52, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kada, T.; Inoue, T.; Namiki, M. Environmental desmutagens and antimutagens. In Environmental Mutagenesis, Carcinogenesis and Plant Biology; Klekowski, E.J., Ed.; Praeger: New York, NY, USA, 1982; pp. 133–152. [Google Scholar]
- Wettasinghe, M.; Shahidi, F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem. 2000, 70, 17–26. [Google Scholar] [CrossRef]
- De Oliveira, N.C.; Sarmento, M.S.; Nunes, E.A.; Porto, C.M.; Rosa, D.P.; Bona, S.R.; da Silva, J. Rosmarinic acid as a protective agent against genotoxicity of ethanol in mice. Food Chem. Toxicol. 2012, 50, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, H.S.; Park, E.; Kim, S.; Lee, S.Y.; Kim, C.S.; Kim do, K.; Kim, S.J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, M.; Matić, S.; Stanić, S.; Solujić, S.; Mihailović, V.; Stanković, N.; Katanić, J. Combining molecular docking and 3-D pharmacophore generation to enclose the in vivo antigenotoxic activity of naturally occurring aromatic compounds: Myricetin, quercetin, rutin, and rosmarinic acid. Biochem. Pharmacol. 2013, 86, 1376–1396. [Google Scholar] [CrossRef] [PubMed]
- Hameed, H.; Aydin, S.; Başaran, A.; Basaran, N. Effects of sinapic acid on oxidative DNA damage in V79 cell line. In Proceedings of the 11th International Comet Assay Workshop, Conference Abstract: ICAW 2015, Antwerpen, Belgium, 1–4 September 2015. [CrossRef]
- Fernández-Bedmar, Z.; Anter, J.; de la Cruz-Ares, S.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; Pérez-Guisado, J. Role of citrus juices and distinctive components in the modulation of degenerative processes: Genotoxicity, antigenotoxicity, cytotoxicity, and longevity in Drosophila. J. Toxicol. Environ. Health A 2011, 74, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Leos-Rivas, C.; Verde-Star, M.J.; Torres, L.O.; Oranday-Cardenas, A.; Rivas-Morales, C.; Barron-Gonzalez, M.P.; Morales-Vallarta, M.R.; Cruz-Veja, D.E. In vitro amoebicidal activity of borage (Borago officinalis) extract on Entamoeba histolytica. J. Med. Food 2011, 14, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gao, Q.; Cui, C.; Zhao, H.; Fu, L.; Chen, L.; Yang, B.; Luo, W.; Zhao, M. Isolation and identification of ent-kaurane-type diterpenoids from Rabdosia serra (MAXIM.) HARA leaf and their inhibitory activities against HepG-2, MCF-7, and HL-60 cell lines. Food Chem. 2012, 131, 1009–1014. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Stanikunaite, R.; Khan, S.I.; Trappe, J.M.; Ross, S.A. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phytother. Res. 2009, 23, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Rosignoli, P.; Fuccelli, R.; Pieravanti, F.; de Bartolomeo, A.; Morozzi, G. Involvement of hydrogen peroxide formation on apoptosis induction by olive oil phenolic compounds. Czech J. Food Sci. 2009, 27, S197–S199. [Google Scholar]
- Liu, H.L.; Wan, X.; Huang, X.F.; Kong, L.Y. Biotransformation of sinapic acid catalyzed by Momordica charantia peroxidase. J. Agric. Food Chem. 2007, 55, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Baena, M.-D.; Tasset, I.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; De Haro-Bailón, A. Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants. Nutrients 2016, 8, 48. https://doi.org/10.3390/nu8010048
Lozano-Baena M-D, Tasset I, Muñoz-Serrano A, Alonso-Moraga Á, De Haro-Bailón A. Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants. Nutrients. 2016; 8(1):48. https://doi.org/10.3390/nu8010048
Chicago/Turabian StyleLozano-Baena, María-Dolores, Inmaculada Tasset, Andrés Muñoz-Serrano, Ángeles Alonso-Moraga, and Antonio De Haro-Bailón. 2016. "Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants" Nutrients 8, no. 1: 48. https://doi.org/10.3390/nu8010048
APA StyleLozano-Baena, M.-D., Tasset, I., Muñoz-Serrano, A., Alonso-Moraga, Á., & De Haro-Bailón, A. (2016). Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants. Nutrients, 8(1), 48. https://doi.org/10.3390/nu8010048