GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Operative Procedure, TPN Administration, and Collection of Tissue Sample
2.3. Morphology
2.3.1. Light Microscopy
2.3.2. Electron Microscopy
2.4. Intestinal Tissue Total Superoxide Dismutase (T-SOD) Activity Measurement
2.5. Intestinal Tissue Reduced-Glutathione (GSH) Measurement
2.6. Western Blot Analysis
2.7. Real-Time Fluorescent Quantitative Polymerase Chain Reaction Analysis (Real Time Q-PCR)
2.8. Statistical Analysis
3. Results
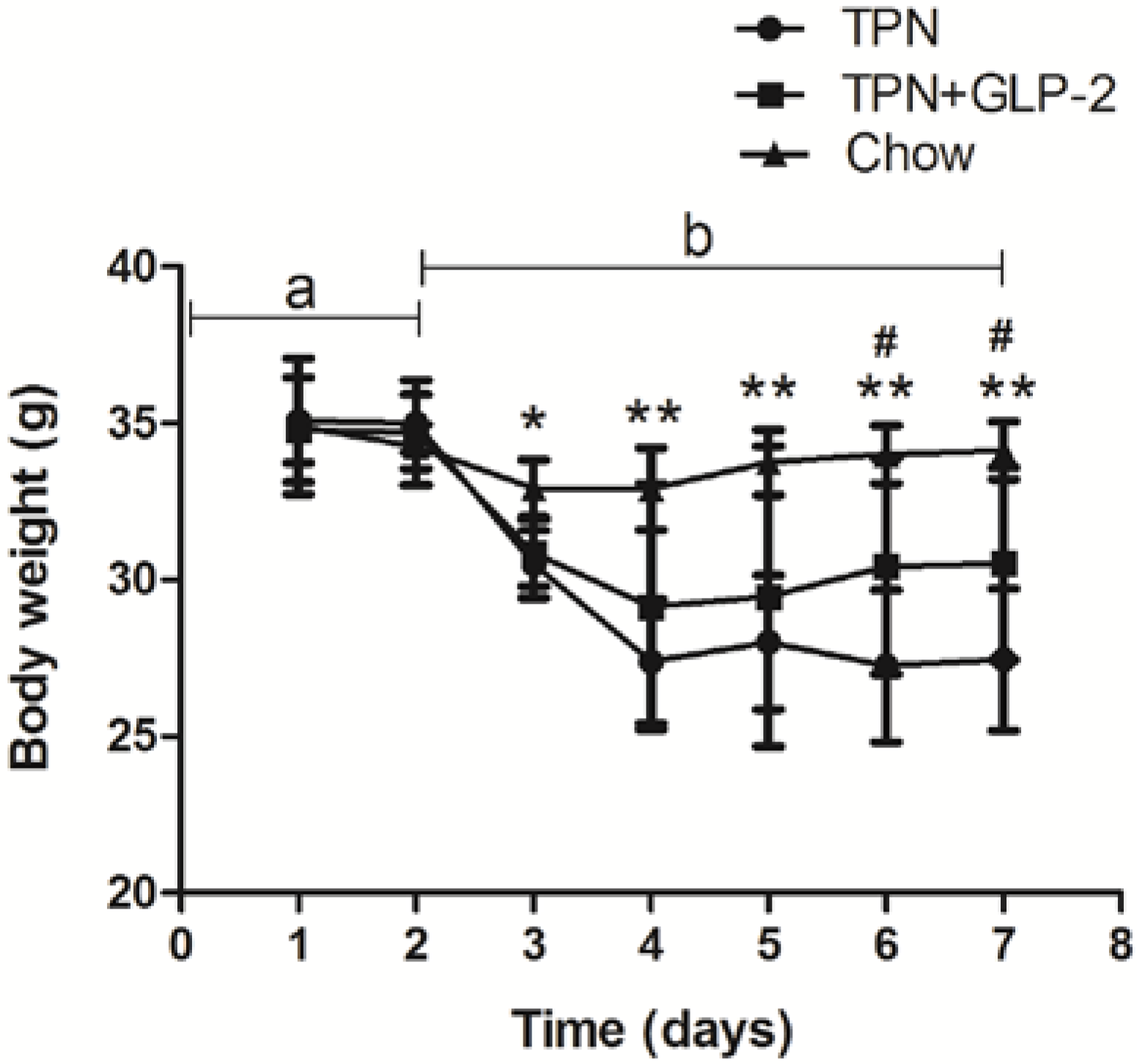
3.1. Body Weight
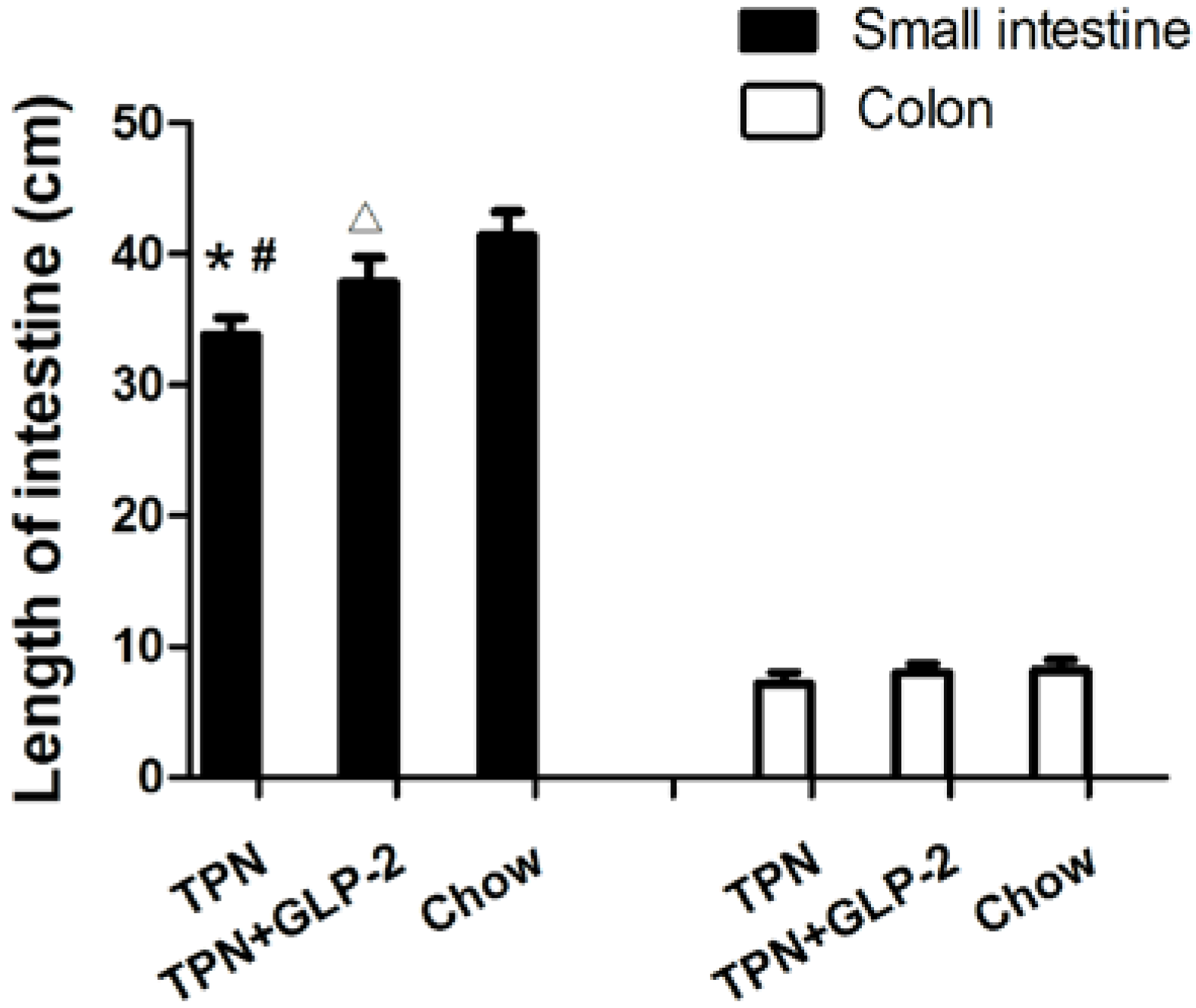
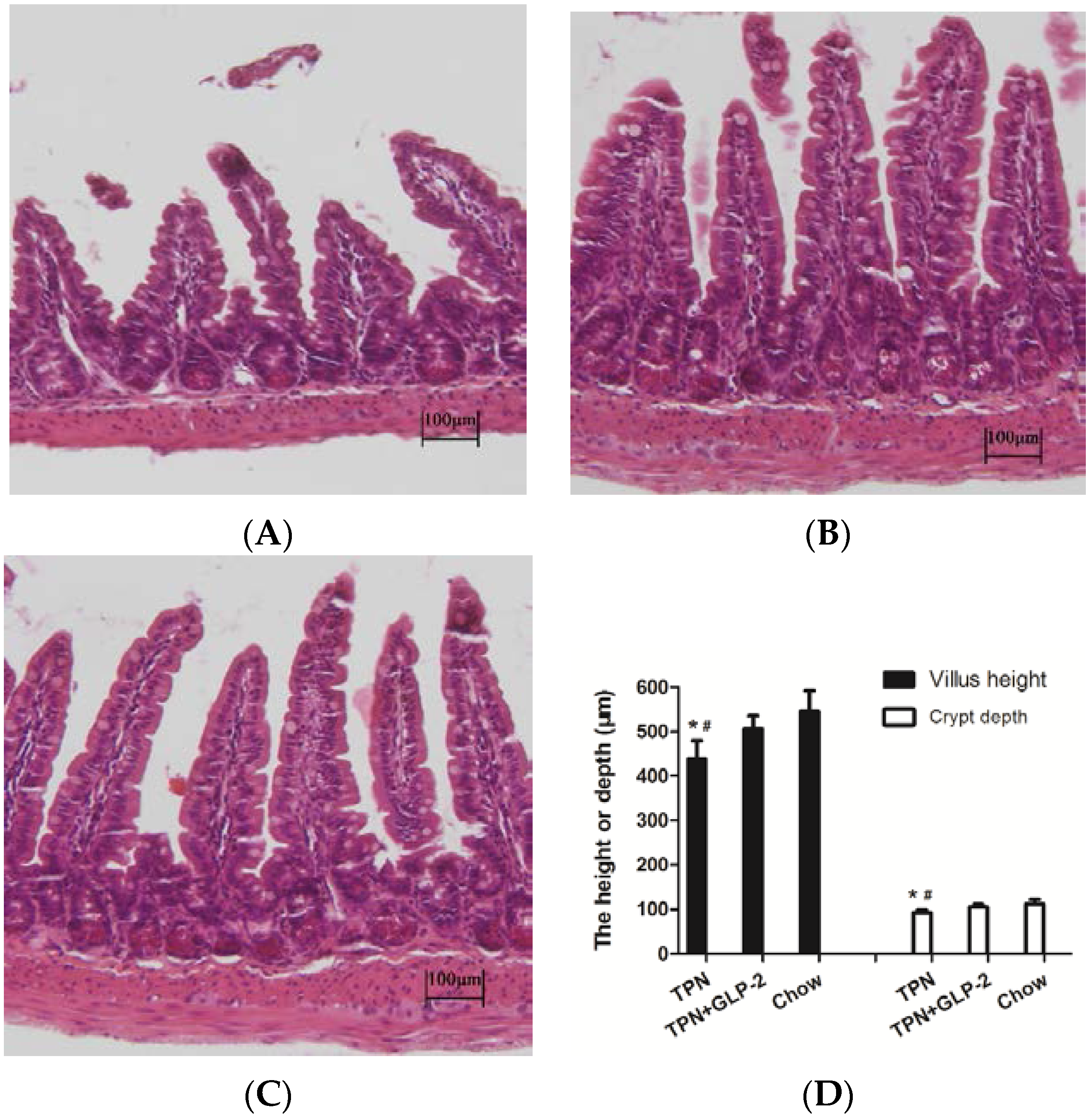
3.2. Small Intestinal Length and Morphology
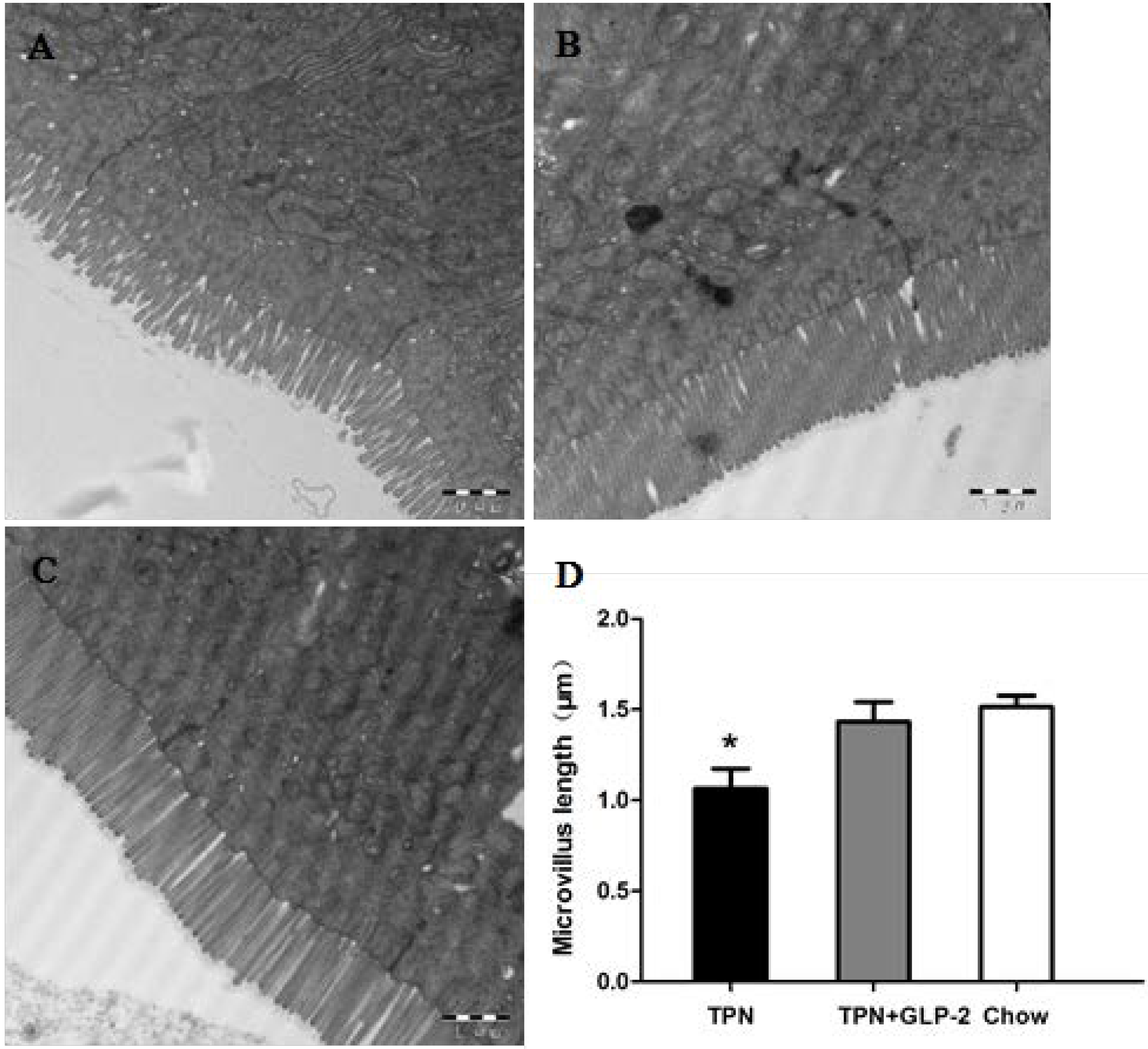
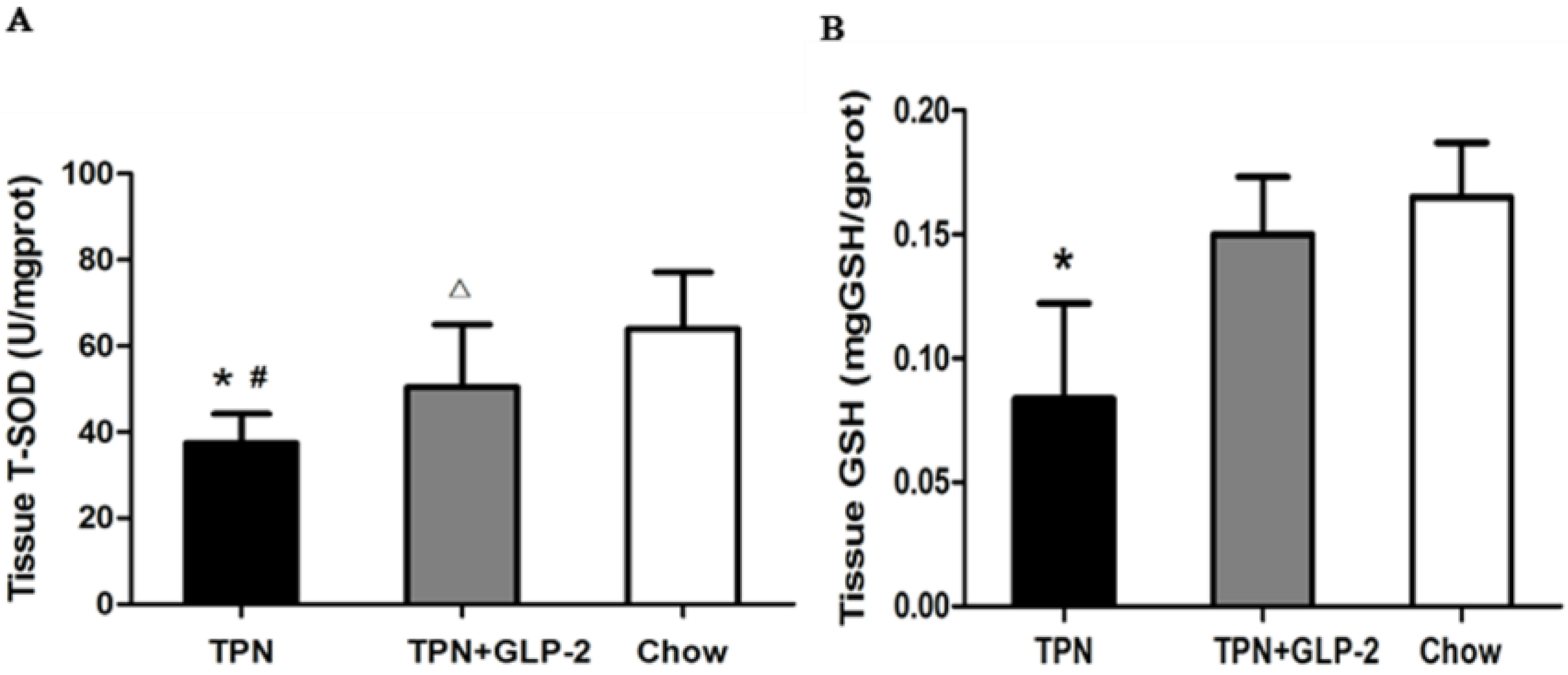
3.3. Changes in Intestinal Tissue T-SOD Activity and GSH Level
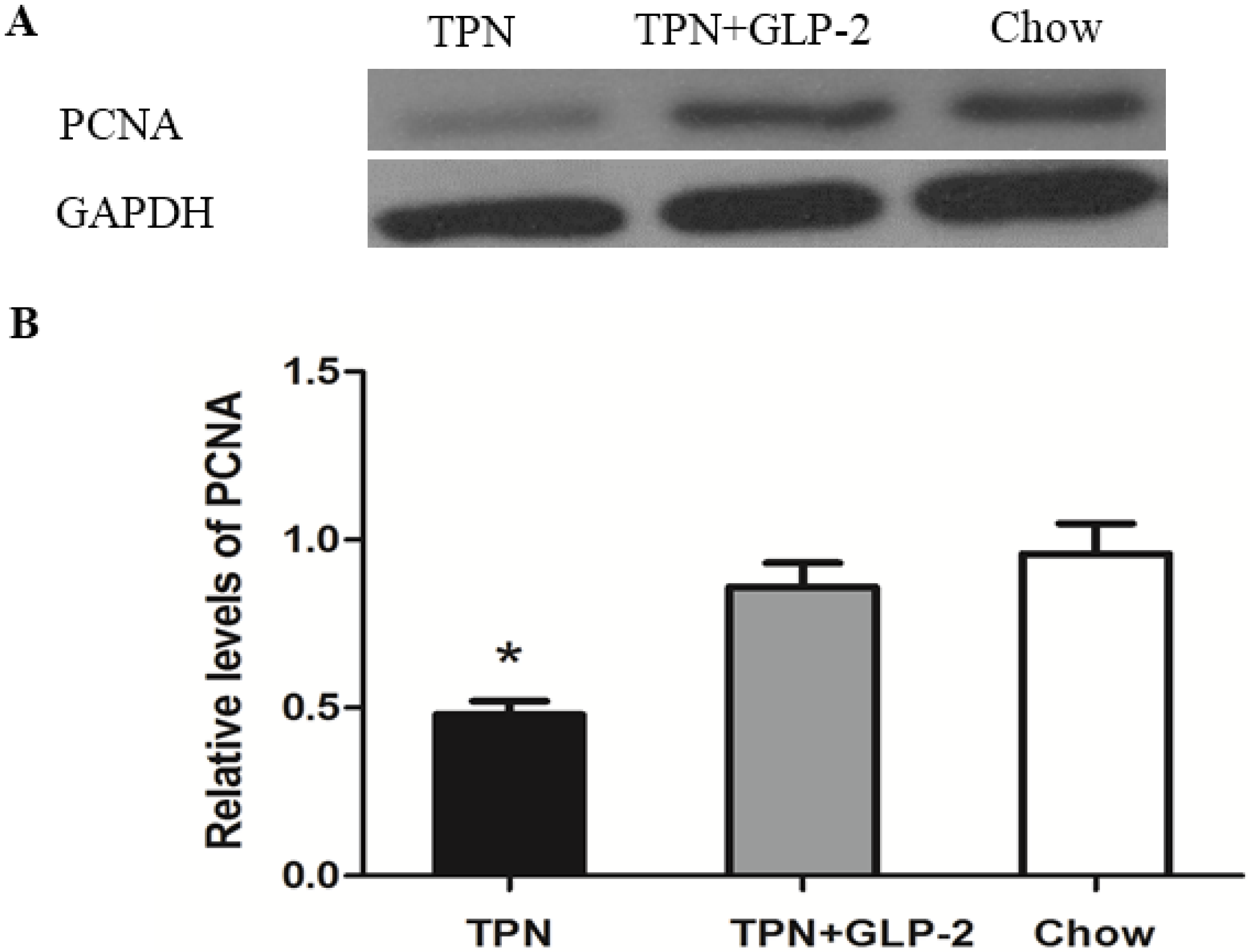
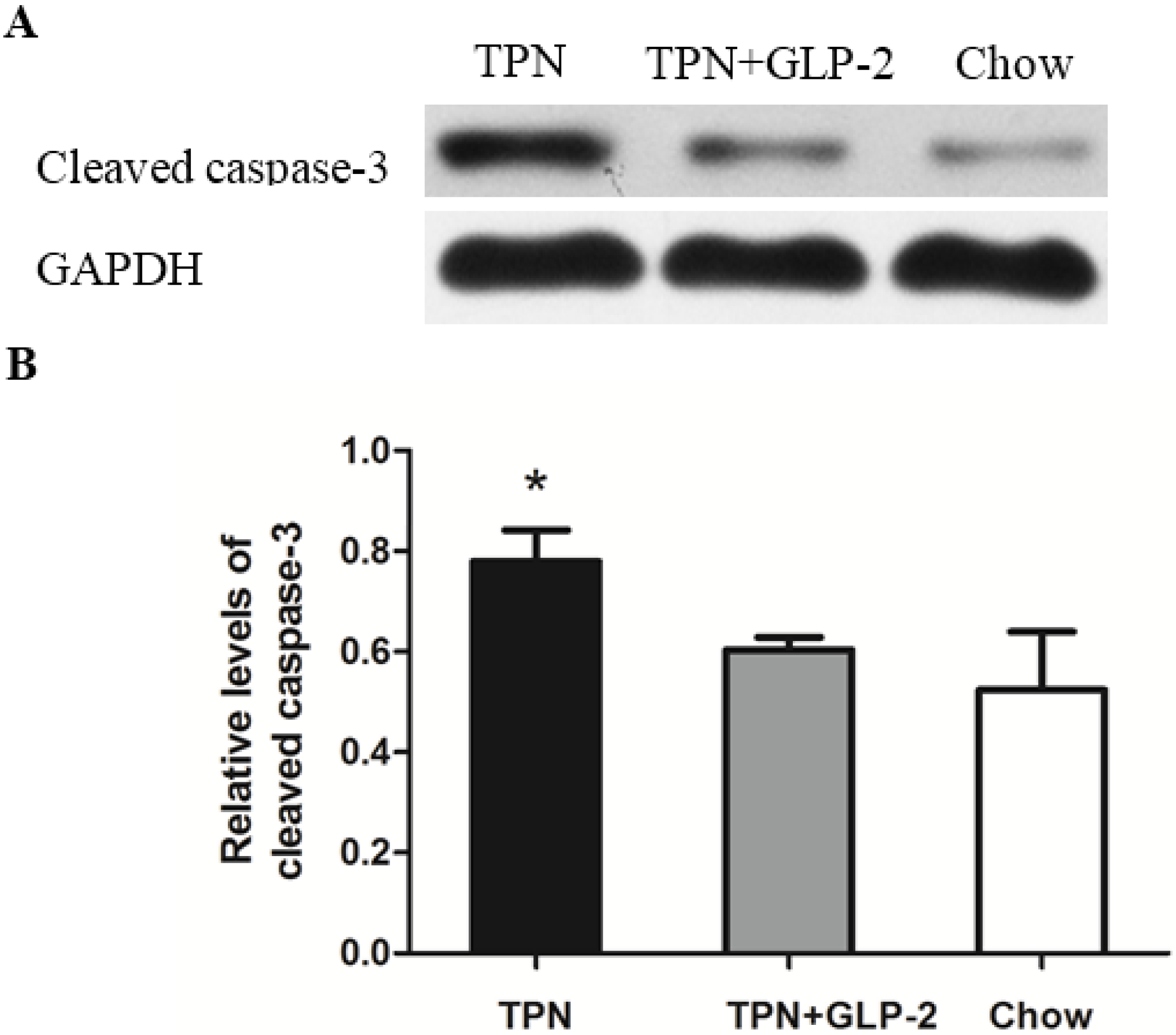
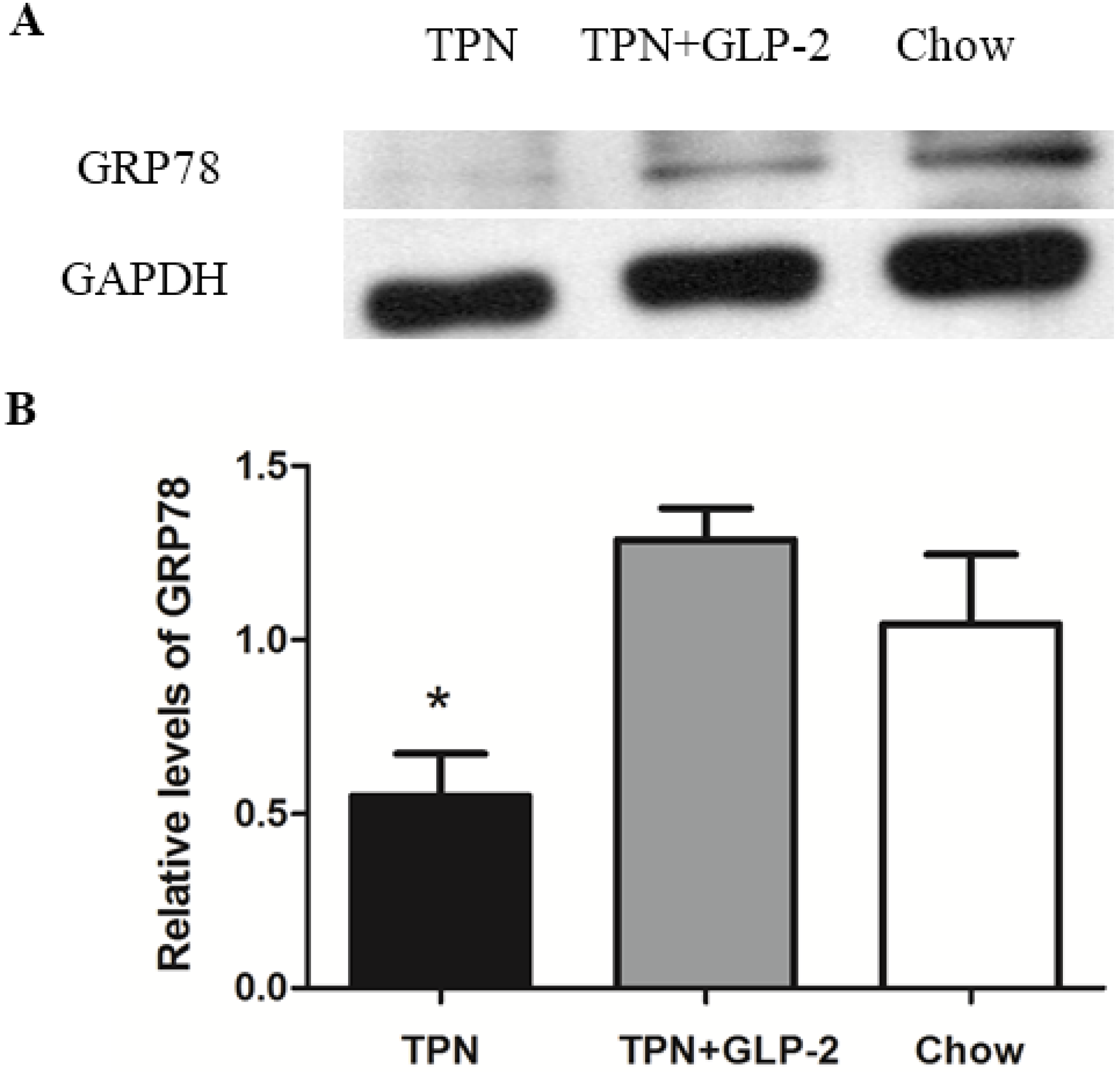
3.4. Western Blot Analysis for PCNA, Cleaved Caspase-3 and GRP78 Protein
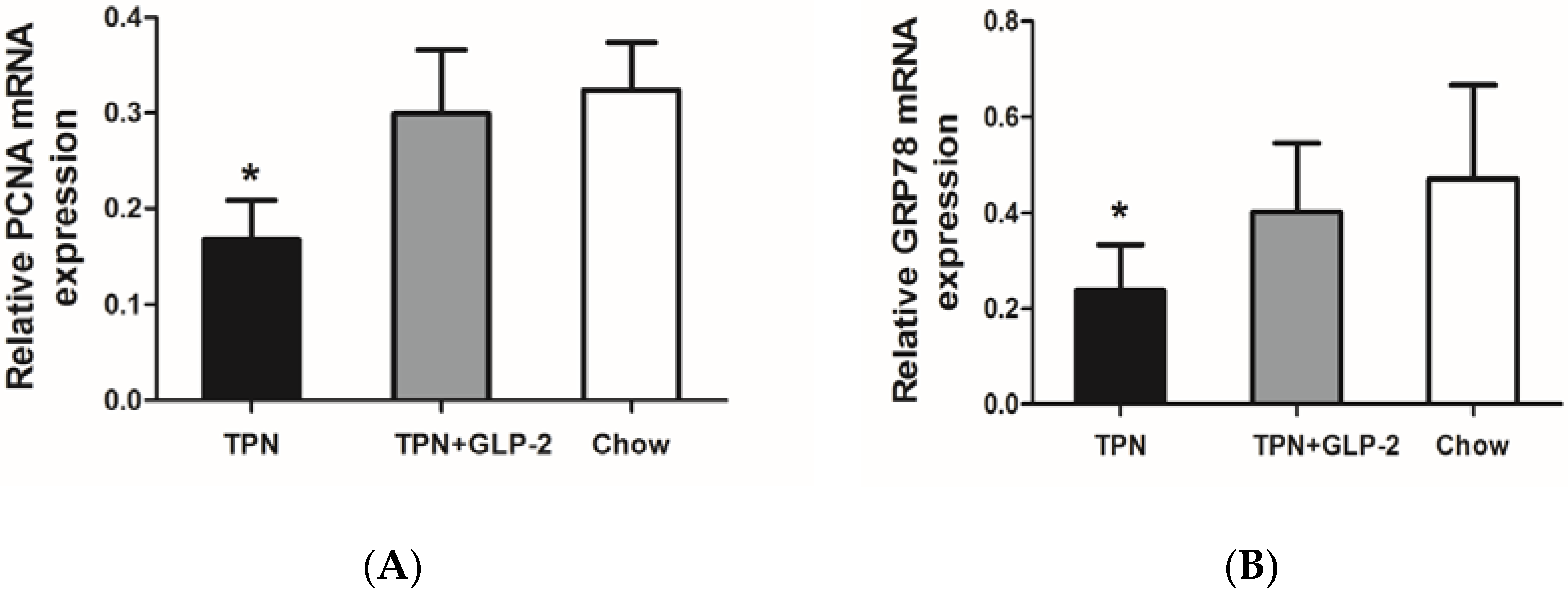
3.5. mRNA Expression of PCNA, GRP78
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ekema, G.; Milianti, S.; Boroni, G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr. 2009, 61, 283–291. [Google Scholar] [PubMed]
- Triantafillidis, J.K.; Papalois, A.E. The role of total parenteral nutrition in inflammatory bowel disease: Current aspects. Scand. J. Gastroenterol. 2014, 49, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Cogliandro, R.F.; de Giorgio, R.; Barbara, G.; Cremon, C.; Antonucci, A.; Fronzoni, L.; Cogliandro, L.; Naponelli, V.; Serra, M.; et al. Natural history of intestinal failure induced by chronic idiopathic intestinal pseudo-obstruction. Transplant. Proc. 2010, 42, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Costakos, D.T. Of lobsters, electronic medical records, and neonatal total parenteral nutrition. Pediatrics 2006, 117, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.I. Overview of the parenteral nutrition use process. JPEN J. Parenter. Enteral. Nutr. 2012, 36, 10S–13S. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Teitelbaum, D.H. Epidermal growth factor/TNF-αtransactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G236–G249. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.J.; Feng, Y.; Demehri, F.R.; Dempsey, P.J.; Teitelbaum, D.H. TPN-associated intestinal epithelial cell atrophy is modulated by TLR4/EGF signaling pathways. FASEB J. 2015, 29, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Kudsk, K.A.; Croce, M.A.; Fabian, T.C.; Minard, G.; Tolley, E.A.; Poret, H.A.; Kuhl, M.R.; Brown, R.O. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann. Surg. 1992, 215, 503–511. [Google Scholar] [CrossRef] [PubMed]
- The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N. Engl. J. Med. 1991, 325, 525–532. [Google Scholar]
- Feng, Y.; Ralls, M.W.; Xiao, W.; Miyasaka, E.; Herman, R.S.; Teitelbaum, D.H. Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann. N. Y. Acad. Sci. 2012, 1258, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alverdy, J.C.; Aoys, E.; Moss, G.S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988, 104, 185–190. [Google Scholar] [PubMed]
- Krishnan, K.; Arnone, B.; Buchman, A. Intestinal growth factors: Potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm. Bowel. Dis. 2011, 17, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Estall, J.L.; Drucker, D.J. Glucagon-like peptide-2. Annu. Rev. Nutr. 2006, 26, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Vegge, A.; Thymann, T.; Lund, P.; Stoll, B.; Bering, S.B.; Hartmann, B.; Jelsing, J.; Qvist, N.; Burrin, D.G.; Jeppesen, P.B.; et al. Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G277–G285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusta, B.; Holland, D.; Koehler, J.A.; Maziarz, M.; Estall, J.L.; Higgins, R.; Drucker, D.J. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 2009, 137, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Guan, X.; Cui, L.; Chang, X.; Holst, J.J. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatalpiglets. Endocrinology 2005, 146, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bremholm, L.; Hornum, M.; Andersen, U.B.; Hartmann, B.; Holst, J.J.; Jeppesen, P.B. The effect of Glucagon-Like Peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul. Pept. 2011, 168, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ivory, C.P.; Wallace, L.E.; McCafferty, D.M.; Sigalet, D.L. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Hadjiyanni, I.; Li, K.K.; Drucker, D.J. Glucagon-like peptide-2 reduces intestinal permeability but does not modify the onset of type 1 diabetes in the nonobese diabetic mouse. Endocrinology 2009, 150, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Arda-Pirincci, P.; Bolkent, S. The role of glucagon-like peptide-2 on apoptosis, cell proliferation, and oxidant-antioxidant system at a mouse model of intestinal injury induced by tumor necrosis factor-alpha/actinomycin, D. Mol. Cell. Biochem. 2011, 350, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Arda-Pirincci, P.; Oztay, F.; Bayrak, B.B.; Yanardag, R.; Bolkent, S. Teduglutide, a glucagon-like peptide 2 analogue: A novel protective agent with anti-apoptotic and anti-oxidant properties in mice with lung injury. Peptides 2012, 38, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Topaloğlu, N.; Memi, G.; Kaner, T.; Deniz, M.; Şahin, Ö.; Güven, M.; Çoşar, M. Does Glp-2 have a protective effect on cerebral ischemia/reperfusion model? Turk. J. Med. Sci. 2015, 45, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Denno, R.; Rounds, J.D.; Faris, R.; Holejko, L.B.; Wilmore, D.W. Glutamine-enriched total parenteral nutrition enhances plasma glutathione in the resting state. J. Surg. Res. 1996, 15, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Eizaguirre, I.; Aldámiz, L.; Aldazábal, P.; García Urkia, N.; Asensio, A.B.; Bachiller, P.; García Arenzana, J.M.; Ruiz, J.L.; Sanjurjo, P.; Perez Nanclares, G. Tissue antioxidant capacity and bacterial translocation under total parenteral nutrition. Pediatr. Surg. Int. 2001, 17, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wu, J.; Hong, L.; Xu, Y.; Tang, Q.; Shi, C. Oxidative injury and hepatocyte apoptosis in total parenteral nutrition-associated liver dysfunction. J. Pediatr. Surg. 2006, 41, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Stoll, B.; Burrin, D.G.; Holst, J.J.; Moore, D.D. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G218–G224. [Google Scholar] [CrossRef] [PubMed]
- Ivaylo, S.; Thomas, H. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar]
- Muskhelishvili, L.; Latendresse, J.R.; Kodell, R.L.; Henderson, E.B. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J. Histochem. Cytochem. 2003, 51, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell. Death. Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kozutsumi, Y.; Segal, M.; Normington, K.; Gething, M.J.; Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 1988, 332, 462–464. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yaung, J.; Kim, Y.H.; Barron, E.; Ryan, S.J.; Hinton, D.R. Endoplasmic reticulum stress induced by oxidative stress in retinal pigment epithelial cells. Graefes. Arch. Clin. Exp. Ophthalmol. 2008, 246, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Luo, H.; Fu, W.; Mattson, M.P. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: Suppression of oxidative stress and stabilization of calcium homeostasis. Exp. Neurol. 1999, 155, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Bi, J.; Gao, X.; Tian, F.; Wang, X.; Li, N.; Li, J. Partial enteral nutrition preserves elements of gut barrier function, including innate immunity, intestinal alkaline phosphatase (IAP) level, and intestinal microbiota in mice. Nutrients 2015, 7, 6294–6312. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, M.; Kristensson, E.; Ekelund, M.; Ekblad, E. Total parenteral nutrition causes circumferential intestinal atrophy, remodeling of the intestinal wall, and redistribution of eosinophils in the rat gastrointestinal tract. Dig. Dis. Sci. 2007, 52, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Wildhaber, B.; Lynn, K.; Yang, H.; Teitelbaum, D.H. Total parenteral nutrition-induced apoptosis in mouse intestinal epithelium: Regulation by the Bcl-2 protein family. Pediatr. Surg. Int. 2002, 18, 570–575. [Google Scholar] [PubMed]
- Hua, Y.; Irfan, K.; Yongyi, F.; Forbush, B.; Bishop, D.K.; Antony, P.A.; Zhou, H.; Teitelbaum, D.H. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann. Surg. 2002, 236, 226–234. [Google Scholar]
- Rohallah, M.; Fatemeh, N.; Hossein, N.; Hassanzadeh, G. Effect of different doses of GLP-2 (Teduglutide) on acute esophageal lesion due to acid-pepsin perfusion in male rats. Peptides 2011, 32, 2086–2090. [Google Scholar]
- Wallis, K.; Walters, J.R.; Forbes, A. Glucagon-like peptide-2-current applications and future directions. Aliment. Pharmacol. Ther. 2007, 25, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.A.; McKay, D.M.; Yang, P.C.; Cameron, H.; Perdue, M.H. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular andparacellular pathways in the mouse. Gut 2000, 47, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chance, W.T.; Foley-Nelson, T.; Thomas, I.; Balasubramaniam, A. Prevention of parenteral nutrition-induced gut hypoplasia by coinfusion of glucagon-like peptide-2. Am. J. Physiol. 1997, 273, G559–G563. [Google Scholar] [PubMed]
- Burrin, D.G.; Stoll, B.; Jiang, R.; Petersen, Y.; Elnif, J.; Buddington, R.K.; Schmidt, M.; Holst, J.J.; Hartmann, B.; Sangild, P.T. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1249–G1256. [Google Scholar] [PubMed]
- Martin, G.R.; Wallace, L.E.; Sigalet, D.L. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G964–G972. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Hill, M.; Asa, S.L.; Brubaker, P.L.; Drucker, D.J. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am. J. Physiol. 1997, 273, E77–E84. [Google Scholar] [PubMed]
- Thulesen, J. Glucagon-like peptide 2 (GLP-2), an intestinotrophic mediator. Curr. Protein. Pept. Sci. 2004, 5, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Erlich, P.; Asa, S.L.; Brubaker, P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA 1996, 93, 7911–7916. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Guan, X.; Cui, L.; Chang, X.; Hadsell, D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E281–E291. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, H.X.; Fu, X.S.; Li, C.P.; Zhong, X.L. Glucagonlike peptide 2 protects intestinal barrier in severe acute pancreatitis through regulatingintestinal epithelial cell proliferation and apoptosis. Pancreas 2012, 41, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Szpetnar, M.; Matras, P.; Kiełczykowska, M.; Horecka, A.; Bartoszewska, L.; Pasternak, K.; Rudzki, S. Antioxidants in patients receiving total parenteral nutrition after gastrointestinal cancer surgery. Cell. Biochem. Funct. 2012, 30, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hasanoğlu, A.; Dalgiç, N.; Tümer, L.; Atalay, Y.; Cinasal, G.; Biberoğlu, G.; Bukan, N.; Aybar, C. Free oxygen radical-induced lipid peroxidation and antioxidant in infants receiving total parenteral nutrition. Prostaglandins Leukot. Essent Fatty Acids 2005, 73, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Wesley, J.R.; Coran, A.G. Intravenous nutrition for the pediatric patient. Semin. Pediatr. Surg. 1992, 1, 212–230. [Google Scholar] [PubMed]
- Jia, Z.; Zhu, H.; Misra, H.P.; Li, Y. Potent induction of total cellular GSH and NQO1 as well as mitochondrial GSH by 3H-1,2-dithiole-3-thione in SH-SY5Y neuroblastoma cells and primary human neurons: Protection against neurocytotoxicity elicited by dopamine, 6-hydroxydopamine, 4-hydroxy-2-nonenal, or hydrogen peroxide. Brain Res. 2008, 1197, 159–169. [Google Scholar] [PubMed]
- Haddad, J.J. Oxygen sensing and oxidant/redox-related pathways. Biochem. Biophys. Res. Commun. 2004, 316, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Suyama, K.; Watanabe, M.; Sakabe, K.; Otomo, A.; Okada, Y.; Terayama, H.; Imai, T.; Mochida, J. GRP78 Suppresses Lipid Peroxidation and Promotes Cellular Antioxidant Levels in Glial Cells following Hydrogen Peroxide Exposure. PLoS ONE 2014, 9, e86951. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Jiang, R.; Li, X.; Liu, Y.; Guo, F. Different Roles of GRP78 on Cell Proliferation and Apoptosis in Cartilage Development. Int. J. Mol. Sci. 2015, 16, 21153–21176. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Q.; Bi, J.; Wang, X.; Jiang, T.; Wu, C.; Tian, F.; Gao, X.; Wan, X.; Zheng, H. GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition. Nutrients 2016, 8, 33. https://doi.org/10.3390/nu8010033
Lei Q, Bi J, Wang X, Jiang T, Wu C, Tian F, Gao X, Wan X, Zheng H. GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition. Nutrients. 2016; 8(1):33. https://doi.org/10.3390/nu8010033
Chicago/Turabian StyleLei, Qiucheng, Jingcheng Bi, Xinying Wang, Tingting Jiang, Chao Wu, Feng Tian, Xuejin Gao, Xiao Wan, and Huijun Zheng. 2016. "GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition" Nutrients 8, no. 1: 33. https://doi.org/10.3390/nu8010033



