Impact of Multi-Micronutrient Fortified Rice on Hemoglobin, Iron and Vitamin A Status of Cambodian Schoolchildren: a Double-Blind Cluster-Randomized Controlled Trial
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Site
2.2. Study Design
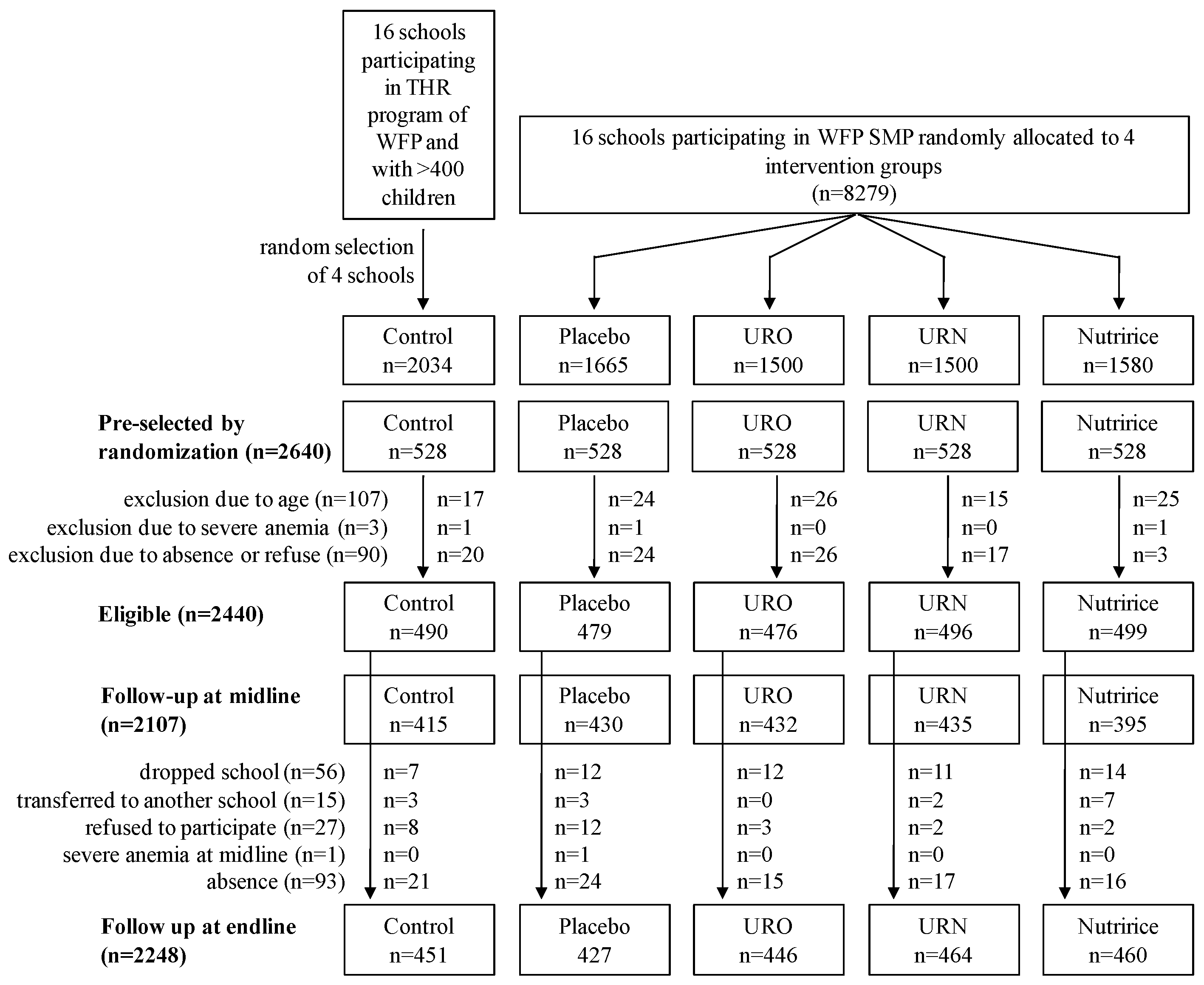
2.3. Intervention
| Micronutrients | URO | URN | NutriRice |
|---|---|---|---|
| Iron (mg) | 10.67 | 7.55 | 7.46 |
| Zinc (mg) | 3.04 | 2.02 | 3.68 |
| Vitamin B1 (mg) | 1.06 | 1.43 | 0.69 |
| Folic acid (mg) | 0.17 | 0.28 | 0.14 |
| Vitamin A (IU) | - | 2140 | 960 |
| Vitamin B3 (mg) | - | 12.57 | 7.98 |
| Vitamin B12 (μg) | - | 3.8 | 1.26 |
| Vitamin B6 (mg) | - | - | 0.92 |
2.4. Blood Samples Collection
2.5. Hemoglobin Concentration
2.6. Blood Samples Analysis: Markers of Iron, Vitamin A, and Inflammation Status
2.7. Parasite Infection
2.8. Data Management and Statistical Analysis
3. Results
| OUTCOMES | ALL | CONTROL | PLACEBO | URO | URN | NUTRIRICE | p-Value 5 |
|---|---|---|---|---|---|---|---|
| N | 2440 | 490 | 479 | 476 | 496 | 499 | |
| Age (year) | 9.65 ± 2.26 | 9.82 ± 2.30 | 9.61 ± 2.28 | 9.55 ± 2.14 | 9.64 ± 2.22 | 9.60 ± 2.35 | NS |
| % girls | 49.9 (n = 1218) | 50.6 (n = 248) | 49.9 (n = 239) | 49.6 (n = 236) | 50.6 (n = 251) | 48.9 (n = 244) | NS |
| % inflammation | 39.5 (n = 935) | 45.6 a (n = 215) | 43.5 a (n = 201) | 42.3 a,b (n = 199) | 32.3 c (n = 158) | 34.0 b,c (n = 162) | <0.05 |
| % parasite infection | 17.9 (n = 323) | 9.4 a (n = 33) | 22.9 b (n = 83) | 23.9 b (n = 89) | 19.5 b,c (n = 74) | 12.9 a,c (n = 44) | <0.05 |
| HEMOGLOBINOPATHY (% HbE) | |||||||
| Hb E ≤ 5% | 54.1 (n = 1130) | 57.4 a,b (n = 236) | 60.1 b (n = 256) | 52.7 a,b (n = 225) | 50.7 a,b (n = 218) | 49.5 a (n = 195) | <0.05 |
| Hb E 5%–80% | 37.8 (n = 789) | 36.0 a,b (n = 148) | 31.7 b (n = 135) | 41.2 a (n = 176) | 39.5 a,b (n = 170) | 40.6 a,b (n = 160) | <0.05 |
| HbE > 80% | 8.1 (n = 169) | 6.6 (n = 27) | 8.2 (n = 35) | 6.1 (n = 26) | 9.8 (n = 42) | 9.9 (n = 39) | NS |
| ANEMIA and IRON STATUS | |||||||
| Hb (g/L) | 124.2 ± 0.2 | 125.6 ± 0.4 a | 123.6 ± 0.4 b | 124.3 ± 0.4 a,b | 123.6 ± 0.4 b | 124.1 ± 0.4 a,b | <0.05 |
| % anemia | 15.6 (n = 376) | 9.8 a (n = 48) | 18.9 b (n = 89) | 15.3 a,b (n = 72) | 17.8 b (n = 88) | 16.4 b (n = 79) | <0.05 |
| FER 1 (μg/L) | 76.2 ± 36.9 (n = 2368) | 83.0 ± 35.8 a (n = 471) | 77.0 ± 36.5 a,b (n = 462) | 79.7 ±38.8 a (n = 470) | 69.7 ± 36.3 c (n = 489) | 71.9 ± 35.3 b,c (n = 476) | <0.05 |
| % FER 1 < 15 μg/L | 1.4 (n = 34) | 0.2 a (n = 1) | 0.4 a (n = 2) | 0.9 a (n = 4) | 3.7 b (n = 18) | 1.9 a,b ( n =9) | <0.05 |
| TFR (mg/L) | 8.8 ± 2.5 (n = 2368) | 8.5 ± 2.1 a (n = 471) | 9.0 ± 2.4 b (n = 462) | 9.1 ± 2.5 b (n = 470) | 8.4 ± 2.5 a (n = 489) | 8.9 ± 3.0 a,b (n = 476) | <0.05 |
| % TFR > 8.3 mg/L | 51.0 (n = 1207) | 46.7 a (n = 220) | 55.6 a,b (n = 257) | 56.6 b (n = 266) | 47.0 a (n = 230) | 49.2 a,b (n = 234) | <0.05 |
| % ID 2 total | 51.2 (n = 1212) | 46.7 a (n = 220) | 55.6 a,b (n = 257) | 56.6 b (n = 266) | 47.6 a,b (n = 233) | 49.6 a,b (n = 236) | <0.05 |
| % ID 2 with anemia | 9.9 (n = 235) | 6.4 a (n = 30) | 12.1 b (n = 56) | 10.4 a,b (n = 49) | 10.6 a,b (n = 52) | 10.1 a,b (n = 48) | <0.05 |
| Body iron (mg/kg) | 6.0 ± 2.2 (n = 2368) | 6.5 ± 1.8 a (n = 471) | 6.0 ± 2.0 b (n = 462) | 6.0 ± 2.2 b (n = 470) | 5.8 ± 2.4 b (n = 489) | 5.8 ± 2.4 b (n = 476) | <0.05 |
| % BI ≥ 4 mg/kg | 86.2 (n = 2042) | 92.6% a (n = 436) | 87.2 a,b (n = 403) | 86.2 b (n = 405) | 83.8 b (n = 410) | 81.5 b (n = 388) | <0.05 |
| % BI 0–4 mg/kg | 11.9 (n = 281) | 6.8% a (n = 32) | 11.7 a,b (n = 54) | 12.6 b (n = 59) | 12.7 b (n = 62) | 15.5 b (n = 74) | <0.05 |
| % BI < 0 mg/kg | 1.9 (n = 45) | 0.6 a (n = 3) | 1.1 a,b (n = 5) | 1.3 a,b (n = 6) | 3.5 b (n = 17) | 2.9 a,b (n = 14) | <0.05 |
| VITAMIN A STATUS | |||||||
| RBP 1 (μmol/L) | 1.58 ± 0.43 (n = 2368) | 1.60 ± 0.39 a,d (n = 471) | 1.62 ± 0.43 a, b (n = 462) | 1.69 ± 0.43 b (n = 470) | 1.48 ± 0.43 c (n = 489) | 1.52 ± 0.44 c,d (n = 476) | <0.05 |
| % marginal VA status 3 | 7.9 (n = 188) | 5.3 a (n = 25) | 6.9 a,b (n = 32) | 3.2 a (n = 15) | 12.9 c (n = 63) | 11.1 b,c (n = 53) | <0.05 |
| % VAD 4 | 0.7 (n = 17) | 0.2 (n = 1) | 0.4 (n = 2) | 0.2 (n = 1) | 1.2 (n = 6) | 1.5 (n = 7) | |
| Factors | Adjusted OR 2 (95% CI) | p-Value |
|---|---|---|
| Gender (reference: male) | 0.86 (0.62; 1.19) | 0.355 |
| Age | 1.03 (0.96; 1.12) | 0.378 |
| Parasite infection (reference: no infection) | 1.63 (1.10; 2.42) | 0.016 |
| Inflammation (reference: no inflammation) | ||
| Incubation | 1.95 (0.26; 14.58) | 0.514 |
| Early convalescence | 2.20 (1.08; 4.48) | 0.029 |
| Late convalescence | 1.18 (0.82; 1.69) | 0.375 |
| Hemoglobinopathy (reference: HbE < 5%) | ||
| 5% ≤ HbE < 80% | 1.87 (1.30; 2.69) | 0.001 |
| HbE ≥ 80% | 24.10 (15.09; 38.49) | <0.001 |
| VA status (reference: normal VA status) | ||
| marginal VA status (0.7 < RBP 1 < 1.05 μmol/L) | 1.57 (0.91; 2.72) | 0.106 |
| VAD (RBP 1 < 0.7 μmol/L) | 8.56 (2.30; 31.89) | 0.001 |
| Depleted iron stores (FER 1 < 15 μg/L) | 52.97 (11.43; 245.55) | <0.001 |
| Tissue iron deficiency (TfR > 8.3 mg/L) | 0.99 (0.70; 1.42) | 0.979 |
| Time Point | Group | Hb (g/L) | FER 1 (μg/L) | ||||||||
| n | Mean | SE | Interaction Term 2 | n | Mean | SE | Interaction Term 2 | ||||
| β Coefficient (95% CI) | p-Value | β Coefficient (95% CI) | p-Value | ||||||||
| B | Placebo | 470 | 123.7 | 1.3 | - | 462 | 77.5 | 3.5 | - | ||
| URO | 471 | 124.7 | 1.3 | - | 470 | 79.9 | 3.5 | - | |||
| URN | 494 | 123.7 | 1.3 | - | 489 | 70.0 | 3.5 | - | |||
| Nutririce | 482 | 124.4 | 1.3 | - | 476 | 72.1 | 3.5 | - | |||
| M | Placebo | 428 | 123.3 | 1.3 | - | 426 | 69.0 | 3.5 | - | ||
| URO | 428 | 123.5 | 1.3 | −0.74 (−1.54; 0.06) | 0.068 | 428 | 68.4 | 3.6 | −3.08 (−7.22; 1.06) | 0.144 | |
| URN | 434 | 124.1 | 1.3 | 0.80 (0.01; 1.59) | 0.048 | 347 | 64.2 | 3.6 | 2.72 (−1.57; 7.02) | 0.214 | |
| Nutririce | 394 | 124.4 | 1.3 | 0.50 (−0.31; 1.31) | 0.230 | 393 | 66.5 | 3.6 | 2.88 (−1.33; 7.09) | 0.180 | |
| E | Placebo | 425 | 122.6 | 1.3 | - | 421 | 71.6 | 3.6 | - | ||
| URO | 445 | 124.1 | 1.3 | 0.51 (−0.28; 1.30) | 0.207 | 443 | 72.6 | 3.5 | −1.46 (−5.58; 2.65) | 0.486 | |
| URN | 464 | 123.0 | 1.3 | 0.36 (−0.42; 1.14) | 0.368 | 463 | 74.8 | 3.5 | 10.70 (6.62; 14.78) | <0.001 | |
| Nutririce | 454 | 123.5 | 1.3 | 0.19 (−0.60; 0.98) | 0.633 | 450 | 74.5 | 3.5 | 8.32 (4.19; 12.44) | <0.001 | |
| Time Point | Group | TFR (mg/L) | Body Iron (mg/kg) | ||||||||
| n | Mean | SE | Interaction Term 2 | n | Mean | SE | Interaction Term 2 | ||||
| β Coefficient (95% CI) | p -Value | β Coefficient (95% CI) | p -Value | ||||||||
| B | Placebo | 462 | 8.98 | 0.24 | - | 462 | 6.01 | 0.27 | - | ||
| URO | 470 | 9.11 | 0.24 | - | 470 | 6.05 | 0.27 | - | |||
| URN | 489 | 8.42 | 0.24 | - | 489 | 5.79 | 0.27 | - | |||
| Nutririce | 476 | 8.87 | 0.24 | - | 476 | 5.78 | 0.27 | - | |||
| M | Placebo | 426 | 8.11 | 0.24 | - | 426 | 5.99 | 0.27 | - | ||
| URO | 428 | 7.98 | 0.24 | −0.26 (−0.53; 0.01) | 0.059 | 428 | 5.94 | 0.27 | −0.09 (−0.30; 0.13) | 0.427 | |
| URN | 347 | 7.90 | 0.24 | 0.34 (0.06; 0.62) | 0.017 | 347 | 5.75 | 0.27 | −0.01 (−0.23; 0.21) | 0.928 | |
| Nutririce | 393 | 8.49 | 0.24 | 0.49 (0.21; 0.76) | 0.001 | 393 | 5.62 | 0.27 | −0.13 (−0.35; 0.08) | 0.227 | |
| E | Placebo | 421 | 8.18 | 0.24 | - | 421 | 6.11 | 0.27 | - | ||
| URO | 443 | 8.08 | 0.24 | −0.24 (−0.50; 0.04) | 0.088 | 443 | 6.09 | 0.27 | −0.06 (−0.27; 0.15) | 0.582 | |
| URN | 463 | 8.51 | 0.24 | 0.89 (0.62; 1.15) | <0.001 | 463 | 6.00 | 0.27 | 0.11 (−0.09; 0.32) | 0.284 | |
| Nutririce | 450 | 8.74 | 0.24 | 0.66 (0.39; 0.93) | <0.001 | 450 | 5.87 | 0.27 | −0.01 (−0.22; 0.20) | 0.947 | |
| Time Point | Group | Hb (g/L) | FER 1 (μg/L) | TFR (mg/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | Interaction Term 2 | n | Mean | SE | Interaction Term 2 | n | Mean | SE | Interaction Term 2 | |||||
| β Coefficient (95% CI) | p-Value | β Coefficient (95% CI) | p-Value | β Coefficient (95% CI) | p-Value | |||||||||||
| B | Placebo | 125 | 124.7 | 1.6 | - | 125 | 84.5 | 5.0 | - | 125 | 8.3 | 0.3 | - | |||
| URO | 142 | 125.3 | 1.6 | - | 142 | 81.8 | 4.8 | - | 142 | 8.9 | 0.3 | - | ||||
| URN | 136 | 124.7 | 1.7 | - | 136 | 68.5 | 5.0 | - | 136 | 7.9 | 0.3 | - | ||||
| Nutririce | 125 | 124.8 | 1.6 | - | 125 | 71.6 | 4.9 | - | 125 | 8.3 | 0.3 | - | ||||
| M | Placebo | 125 | 123.9 | 1.6 | - | 125 | 78.0 | 5.0 | - | 125 | 7.8 | 0.3 | - | |||
| URO | 142 | 124.2 | 1.6 | –0.3 (–1.7; 1.1) | 0.688 | 142 | 73.1 | 4.8 | –2.2 (–9.3; 5.0) | 0.550 | 142 | 7.8 | 0.3 | –0.6 (–1.1; –0.2) | 0.004 | |
| URN | 135 | 126.0 | 1.7 | 2.1 (0.7; 3.5) | 0.004 | 136 | 63.0 | 5.0 | 1.0 (–6.2; 8.3) | 0.781 | 136 | 7.5 | 0.3 | 0.1 (–0.4; 0.5) | 0.747 | |
| Nutririce | 125 | 125.1 | 1.6 | 1.1 (–0.4; 2.5) | 0.147 | 125 | 66.6 | 4.9 | 1.6 (–5.8; 9.0) | 0.669 | 125 | 7.8 | 0.3 | 0.0 (–0.4; 0.5) | 0.936 | |
| E | Placebo | 125 | 123.0 | 1.6 | - | 125 | 79.2 | 5.0 | - | 125 | 7.5 | 0.3 | - | |||
| URO | 142 | 124.9 | 1.6 | 1.4 (–0.0; 2.8) | 0.054 | 142 | 77.9 | 4.8 | 1.4 (–5.7; 8.6) | 0.697 | 142 | 8.0 | 0.3 | –0.1 (–0.5; 0.3) | 0.646 | |
| URN | 136 | 124.7 | 1.7 | 1.8 (0.3; 3.2) | 0.015 | 136 | 75.9 | 5.0 | 12.7 (5.5; 20.0) | 0.001 | 136 | 8.2 | 0.3 | 1.1 (0.6; 1.5) | <0.001 | |
| Nutririce | 125 | 124.3 | 1.6 | 1.2 (–0.2; 2.7) | 0.095 | 125 | 73.1 | 4.9 | 6.8 (–0.5; 14.2) | 0.070 | 125 | 8.2 | 0.3 | 0.7 (0.3; 1.2) | 0.001 | |
| All Children | |||||
|---|---|---|---|---|---|
| Time Point | Group | n | % (95% CI) | Interaction Term | |
| Adjusted OR 1 (95% CI) | p-Value | ||||
| B | Placebo | 462 | 5.4 (2.9; 9.9) | - | |
| URO | 470 | 2.6 (1.3; 5.4) | - | ||
| URN | 489 | 12.3 (7.2; 20.3) | - | ||
| Nutririce | 476 | 11.0 (6.4; 18.4) | - | ||
| M | Placebo | 426 | 11.0 (6.3; 18.6) | - | |
| URO | 428 | 13.0 (7.6; 21.4) | 2.55 (1.22; 5.33) | 0.012 | |
| URN | 347 | 15.4 (9.0; 25.1) | 0.60 (0.33; 1.10) | 0.101 | |
| Nutririce | 393 | 12.2 (7.0; 20.4) | 0.52 (0.28; 0.96) | 0.036 | |
| E | Placebo | 421 | 12.4 (7.1; 20.6) | - | |
| URO | 443 | 8.3 (4.7; 14.5) | 1.37 (0.65; 2.91) | 0.410 | |
| URN | 463 | 6.3 (3.4; 11.4) | 0.20 (0.10; 0.37) | <0.001 | |
| Nutririce | 450 | 6.8 (3.7; 12.1) | 0.24 (0.13; 0.45) | <0.001 | |
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- UNC Fund. Vitamin and Mineral Deficiency: A Global Damage Assessment Report; UNICEF: New York, NY, USA; The Micronutrient Initiative: Ottawa, ON, Canada, 2004. [Google Scholar]
- De Benoist, B.; McLean, E.; Egli, I.; Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- World Health Organization. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- World Health Organization. Human Vitamin and Mineral Requirements. Report of a Joint FAO/WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Marengo-Rowe, A.J. The thalassemias and related disorders. Proc. Bayl. Univ. Med. Cent. 2007, 20, 27–31. [Google Scholar] [PubMed]
- Fucharoen, S.; Winichagoon, P. Haemoglobinopathies in southeast Asia. Indian J. Med. Res. 2011, 134, 498–506. [Google Scholar] [PubMed]
- National Institute of Statistics, Directorate General for Health, and ICF Macro. Cambodia Demographic and Health Survey 2010; National Institute of Statistics, Directorate General for Health and ICF Macro: Phnom Penh, Cambodia and Calverton, MD, USA, 2011. [Google Scholar]
- Best, C.; Neufingerl, N.; van Geel, L.; van den Briel, T.; Osendarp, S. The nutritional status of school-aged children: Why should we care? Food Nutr. Bull. 2010, 31, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Neufingerl, N.; del Rosso, J.M.; Transler, C.; van den Briel, T.; Osendarp, S. Can multi-micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review. Nutr. Rev. 2011, 69, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Bruner, A.B.; Joffe, A.; Duggan, A.K.; Casella, J.F.; Brandt, J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet 1996, 348, 992–996. [Google Scholar] [CrossRef]
- Shrestha, R.M. Effect of Iodine and Iron Supplementation on Physical, Psychomotor and Mental Development in Primary School Children in Malawi; Thesis Wageningen University: Wageningen, The Netherlands, 1994. [Google Scholar]
- Copenhagen Consensus 2008. Available online: http://www.copenhagenconsensus.com/copenhagen-consensus-ii/outcomes (accessed on 13 August 2015).
- Semba, R.D.; Bloem, M.W. Nutrition and Health in Developing Countries; Humana Press: New York, NY, USA, 2008. [Google Scholar]
- Nga, T.T.; Winichagoon, P.; Dijkhuizen, M.A.; Khan, N.C.; Wasantwisut, E.; Furr, H.; Wieringa, F.T. Multi-micronutrient-fortified biscuits decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children. J. Nutr. 2009, 139, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Van Khanh, T.; Burja, K.; Thuy Nga, T.; Kong, K.; Berger, J.; Gardner, M.; Dijkhuizen, M.A.; Hop, L.T.; Tuyen, L.D.; Wieringa, F.T. Organoleptic qualities and acceptability of fortified rice in two Southeast Asian countries. Ann. N. Y. Acad. Sci. 2014, 1324, 48–54. [Google Scholar] [CrossRef] [PubMed]
- WHO. Iron Deficiency Anaemia Assessment, Prevention and Control. A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [PubMed]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Molinari, L.; Staubli-Asobayire, F.; Hess, S.Y.; Chaouki, N.; Adou, P.; Hurrell, R.F. Serum transferrin receptor and zinc protoporphyrin as indicators of iron status in African children. Am. J. Clin. Nutr. 2005, 81, 615–623. [Google Scholar] [PubMed]
- Skikne, B.S. Serum transferrin receptor. Am. J. Hematol. 2008, 83, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- De Pee, S.; Dary, O. Biochemical indicators of vitamin A deficiency: Serum retinol and serum retinol binding protein. J. Nutr. 2002, 132 (Suppl. 9), 2895s–2901s. [Google Scholar] [PubMed]
- Thurnham, D.I.; McCabe, G.P.; Northrop-Clewes, C.A.; Nestel, P. Effects of subclinical infection on plasma retinol concentrations and assessment. Lancet 2003, 362, 2052–2058. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Ash, L.R.; Savioli, L.; Orihel, T.C. Bench Aids for the Diagnosis of Intestinal Parasites; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Harlow, L.L. The Essence of Multivariate Thinking: Basic Themes and Methods; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2005. [Google Scholar]
- Hix, J.; Rasca, P.; Morgan, J.; Denna, S.; Panagides, D.; Tam, M.; Shankar, A.H. Validation of a rapid enzyme immunoassay for the quantitation of retinol-binding protein to assess vitamin A status within populations. Eur. J. Clin. Nutr. 2006, 60, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Fucharoen, S.; Winichagoon, P.; Sirankapracha, P.; Zeder, C.; Gowachirapant, S.; Judprasong, K.; Tanno, T.; Miller, J.L.; Hurrell, R.F. Iron metabolism in heterozygotes for hemoglobin E (HbE), alpha-thalassemia 1, or beta-thalassemia and in compound heterozygotes for HbE/beta-thalassemia. Am. J. Clin. Nutr. 2008, 88, 1026–1031. [Google Scholar] [PubMed]
- Andrews, N.C. Anemia of inflammation: The cytokine-hepcidin link. J. Clin. Investig. 2004, 113, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Van Stuijvenberg, M.E.; Smuts, C.M.; Wolmarans, P.; Lombard, C.J.; Dhansay, M.A. The efficacy of ferrous bisglycinate and electrolytic iron as fortificants in bread in iron-deficient school children. Br. J. Nutr. 2006, 95, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Beinner, M.A.; Velasquez-Meléndez, G.; Pessoa, M.C.; Greiner, T. Iron-Fortified Rice Is As Efficacious As Supplemental Iron Drops in Infants and Young Children. J. Nutr. 2010, 140, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Porcayo, M.; Onofre, G.; Garcia-Guerra, A.; Elliott, T.; Jankowski, S.; Greiner, T. Efficacy of iron-fortified Ultra Rice in improving the iron status of women in Mexico. Food Nutr. Bull. 2008, 29, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Bastard, G.; Sembène, M.; Fortin, S.; Traissac, P.; Landais, E.; Icard-Vernière, C.; Wieringa, F.T.; Berger, J. Anthropometric and micronutrient status of school-children in an urban West Africa setting: A cross-sectional study in Dakar (Senegal). PLoS ONE 2013, 8, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Grant, F.K.E.; Martorell, R.; Flores-Ayala, R.; Cole, C.R.; Ruth, L.J.; Ramakrishnan, U.; Suchdev, P.S. Comparison of indicators of iron deficiency in Kenyan children. Am. J. Clin. Nutr. 2012, 95, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.; Moraleda, C.; Quintó, L.; Renom, M.; Mussacate, L.; Macete, E.; Aguilar, J.L.; Alonso, P.L.; Menéndez, C. Challenges in the diagnosis of iron deficiency in children exposed to high prevalence of infections. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.J.; Christian, P.; Wu, L.S.F.; Arguello, M.; Cui, H.; Nanayakkara-Bind, A.; Stewart, C.P.; Khatry, S.K.; LeClerq, S.; West, K.P. Micronutrient deficiencies are common in 6- to 8-year-old children of rural Nepal, with prevalence estimates modestly affected by inflammation. J. Nutr. 2014, 144, 979–987. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization/Centers for Disease Control and Prevention. Assessing the Iron Status of Populations: Including Literature Reviews: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level (Geneva, 2004); World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Zimmermann, M.B. Methods to assess iron and iodine status. Br. J. Nutr. 2008, 99, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Beguin, Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin. Chim. Acta 2003, 329, 9–22. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Biebinger, R.; Rohner, F.; Dib, A.; Zeder, C.; Hurrell, R.F.; Chaouki, N. Vitamin A supplementation in children with poor vitamin A and iron status increases erythropoietin and hemoglobin concentrations without changing total body iron. Am. J. Clin. Nutr. 2006, 84, 580–586. [Google Scholar] [PubMed]
- Cusick, S.E.; Tielsch, J.M.; Ramsan, M.; Jape, J.K.; Sazawal, S.; Black, R.E.; Stoltzfus, R.J. Short-term effects of vitamin A and antimalarial treatment on erythropoiesis in severely anemic Zanzibari preschool children. Am. J. Clin. Nutr. 2005, 82, 406–412. [Google Scholar] [PubMed]
- Roodenburg, A.J.; West, C.E.; Hovenier, R.; Beynen, A.C. Supplemental vitamin A enhances the recovery from iron deficiency in rats with chronic vitamin A deficiency. Br. J. Nutr. 1996, 75, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casal, M.N.; Layrisse, M.; Solano, L.; Baron, M.A.; Arguello, F.; Llovera, D.; Ramirez, J.; Leets, I.; Tropper, E. Vitamin A and beta-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J. Nutr. 1998, 128, 646–650. [Google Scholar] [PubMed]
- Layrisse, M.; García-Casal, M.N.; Solano, L.; Baron, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. The role of vitamin A on the inhibitors of nonheme iron absorption: Preliminary results. J. Nutr. Biochem. 1997, 8, 61–67. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perignon, M.; Fiorentino, M.; Kuong, K.; Dijkhuizen, M.A.; Burja, K.; Parker, M.; Chamnan, C.; Berger, J.; Wieringa, F.T. Impact of Multi-Micronutrient Fortified Rice on Hemoglobin, Iron and Vitamin A Status of Cambodian Schoolchildren: a Double-Blind Cluster-Randomized Controlled Trial. Nutrients 2016, 8, 29. https://doi.org/10.3390/nu8010029
Perignon M, Fiorentino M, Kuong K, Dijkhuizen MA, Burja K, Parker M, Chamnan C, Berger J, Wieringa FT. Impact of Multi-Micronutrient Fortified Rice on Hemoglobin, Iron and Vitamin A Status of Cambodian Schoolchildren: a Double-Blind Cluster-Randomized Controlled Trial. Nutrients. 2016; 8(1):29. https://doi.org/10.3390/nu8010029
Chicago/Turabian StylePerignon, Marlène, Marion Fiorentino, Khov Kuong, Marjoleine A. Dijkhuizen, Kurt Burja, Megan Parker, Chhoun Chamnan, Jacques Berger, and Frank T. Wieringa. 2016. "Impact of Multi-Micronutrient Fortified Rice on Hemoglobin, Iron and Vitamin A Status of Cambodian Schoolchildren: a Double-Blind Cluster-Randomized Controlled Trial" Nutrients 8, no. 1: 29. https://doi.org/10.3390/nu8010029
APA StylePerignon, M., Fiorentino, M., Kuong, K., Dijkhuizen, M. A., Burja, K., Parker, M., Chamnan, C., Berger, J., & Wieringa, F. T. (2016). Impact of Multi-Micronutrient Fortified Rice on Hemoglobin, Iron and Vitamin A Status of Cambodian Schoolchildren: a Double-Blind Cluster-Randomized Controlled Trial. Nutrients, 8(1), 29. https://doi.org/10.3390/nu8010029





