Dietary Sources of Vitamin B-12 and Their Association with Vitamin B-12 Status Markers in Healthy Older Adults in the B-PROOF Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Dietary Assessment
2.3. Biochemical Analyses
2.4. Covariates
2.5. Data Analyses
3. Results
| Men | Women | Serum Vitamin B-12 < 200 pmol/L | Serum Vitamin B-12 ≥ 200 pmol/L | Impaired Serum Vitamin B-12 and MMA ** | Normal Serum Vitamin B-12 and MMA | |
|---|---|---|---|---|---|---|
| N | 348 | 252 | 134 | 466 | 68 | 531 |
| Men n (%) | 348 (100) | 0 | 87 (65) | 261 (56) | 46 (68) | 302 (57) |
| Age, years | 72.1 ± 5.1 | 72.4 ± 5.7 | 73.0 ± 5.8 | 72.0 ± 5.2 # | 73.8 ± 5.7 | 72 ± 5 # |
| Body Mass Index, kg/m2 | 26.2 ± 3.0 | 27.3 ± 4.3 # | 27.0 ± 4.0 | 26.9 ± 3.5 | 26.4 ± 3.9 | 27.0 ± 3.5 |
| Smoking, n (%) | ||||||
| Non-smoker | 70 (20) | 115 (46) # | 35 (26) | 150 (32) | 13 (19) | 172 (32) |
| Smoker | 41 (12) | 21 (8) | 17 (13) | 45 (10) | 10 (15) | 52 (10) |
| Former smoker | 237 (68) | 116 (46) | 82 (61) | 271 (58) | 45 (66) | 307 (58) |
| Physical activity, kcal/day | 139 ± 91 | 161 ± 109 # | 158 ± 102 | 145 ± 98 | 139 ± 69 | 149 ± 102 |
| Education, n (%) | ||||||
| Primary | 112 (32) | 142 (56) # | 57 (42) | 197 (42) | 36 (53) | 218 (41) |
| Secondary | 97 (28) | 48 (19) | 36 (27) | 109 (24) | 14 (21) | 131 (25) |
| Higher | 139 (40) | 62 (25) | 41 (31) | 160 (34) | 18 (26) | 182 (34) |
| Alcohol intake, n (%) | ||||||
| Light | 203 (58) | 181 (72) # | 87 (65) | 297 (64) | 47 (69) | 336 (63) # |
| Moderate | 133 (38) | 65 (26) | 43 (32) | 155 (33) | 17 (25) | 181 (34) |
| Excessive | 12 (4) | 6 (2) | 4 (3) | 14 (3) | 4 (6) | 14 (3) |
| Serum vitamin B-12, pmol/L | 275 ± 104 | 290 ± 113 | 163 ± 29 | 315 ± 98 # | 157 ± 30 | 297 ± 104 # |
| Serum methylmalonic acid, µmol/L (n = 1 missing) | 0.23 (0.19–0.30) | 0.24 (0.19–0.41) | 0.28 (0.22–0.39) | 0.22 (0.18–0.28) # | 0.39 (0.31–0.59) | 0.22 (0.18–0.27) # |
| Serum HoloTC, pmol/L (n = 1 missing) | 57 (43–76) | 63 (48–87) # | 39 (29–52) | 67 (52–86) # | 35 (26–44) | 64 (49–82) # |
| Plasma homocysteine, μmol/L | 15.2 ± 3.3 | 14.9 ± 3.2 | 16.4 ± 4.2 | 14.7 ± 2.8 # | 17.4 ± 4.8 | 14.8 ± 2.9 # |
| Serum folate, μg/day (n = 9 missing) | 19.0 ± 6.9 | 19.4 ± 6.8 | 17.6 ± 6.2 | 19.5 ± 7.0 # | 17.8 ± 6.2 | 19.2 ± 6.9 |
| Serum creatinine, μmol/L (n = 1 missing) | 91 ± 17 | 75 ± 14 # | 82 ± 18 | 85 ± 18 # | 83 ± 19 | 84 ± 18 |
| Men | Women | Serum Vitamin B-12 <200 pmol/L | Serum Vitamin B-12 ≥200 pmol/L | Impaired Serum Vitamin B-12 and MMA ** | Normal Serum Vitamin B-12 and MMA | |
|---|---|---|---|---|---|---|
| N | 348 | 252 | 134 | 466 | 68 | 531 |
| Energy intake, kcal/day | 2170 ± 484 | 1778 ± 350 # | 2058 ± 554 | 1990 ± 448 | 2075 ± 590 | 1998 ± 457 |
| Fat, En% | 36 ± 6 | 36 ± 6 | 36 ± 6 | 36 ± 6 | 37 ± 7 | 36 ± 6 |
| Protein, En% | 15 ± 2 | 16 ± 2 # | 14 ± 2 | 15 ± 2 # | 14 ± 3 | 15 ± 2 # |
| Carbohydrates, En% | 44 ± 6 | 44 ± 7 | 44 ± 7 | 44 ± 6 | 44 ± 8 | 44 ± 6 |
| Fibre, gram/day | 25 ± 7 | 23 ± 6 # | 24 ± 7 | 24 ± 6 | 24 ± 8 | 24 ± 6 |
| Folate, μg/day | 199 ± 56 | 181 ± 48 # | 184 ± 53 | 194 ± 54 | 182 ± 52 | 193 ± 54 |
| Total vitamin B-12, μg/day | 4.18 (3.29–5.38) | 3.47 (2.64–4.40) # | 3.56 (2.54–4.61) | 3.92 (3.10–5.17) # | 3.47 (2.59–4.29) | 3.92 (3.00–5.17) # |
| Vitamin B-12 from foods, μg/day | 4.50 (3.18–5.17) | 3.41 (2.62–4.31) # | 3.52 (2.43–4.61) | 3.80 (3.00–5.01) # | 3.38 (2.59–4.29) | 3.80 (2.94–5.00) # |
| Vitamin B-12 from supplements, μg/day | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Vitamin B-12 from meat, μg/day | 1.31 (0.91–1.89) | 1.01 (0.69–1.58) # | 1.08 (0.62–1.74) | 1.20 (0.85–1.77) # | 1.11 (0.62–1.90) | 1.19 (0.84–1.74) |
| Vitamin B-12 from fish and shellfish, μg/day | 0.61 (0.26–1.20) | 0.44 (0.19–0.82) # | 0.34 (0.13–0.93) | 0.54 (0.26–1.08) # | 0.30 (0.09–0.65) | 0.53 (0.25–1.12) # |
| Vitamin B-12 from eggs, μg/day | 0.16 (0.08–0.24) | 0.16 (0.08–0.16) | 0.16 (0.08–0.24) | 0.16 (0.08–0.16) | 0.16 (0.06–0.24) | 0.16 (0.08–0.16) |
| Vitamin B-12 from dairy, μg/day | 1.32 (0.89–1.71) | 1.17 (0.83–1.56) # | 1.07 (0.77–1.58) | 1.30 (0.90–1.67) # | 1.03 (0.73–1.58) | 1.28 (0.88–1.67) # |
| Vitamin B-12 from milk, μg/day | 0.57 (0.22–1.00) | 0.35 (0.10–0.69) # | 0.36 (0.09–0.71) | 0.52 (0.19–0.93) # | 0.34 (0.06–0.65) | 0.50 (0.17–0.90) # |
| Vitamin B-12 from yogurt, μg/day | 0.37 (0.08–0.56) | 0.37 (0.13–0.56) | 0.39 (0.13–0.55) | 0.36 (0.09–0.56) | 0.35 (0.02–0.55) | 0.36 (0.12–0.56) |
| Vitamin B-12 from cheese, μg/day | 0.17 (0.09–0.27) | 0.20 (0.11–0.33) # | 0.17 (0.09–0.28) | 0.18 (0.10–0.29) | 0.16 (0.07–0.28) | 0.18 (0.10–0.29) |
| T1 | T2 | T3 | P for Trend | |
|---|---|---|---|---|
| Total vitamin B-12 intake, μg/day | ≤3.19 | 3.20–4.40 | ≥4.41 | |
| Serum B-12, pmol/L | 250 (214; 286) | 273 (237; 310) | 287 (250; 324) | 0.009 |
| Vitamin B-12 intake from meat, μg/day | ≤0.92 | 0.93–1.51 | ≥1.52 | |
| Serum B-12, pmol/L | 247 (211; 284) | 275 (240; 311) | 290 (253; 327) | 0.001 |
| Vitamin B-12 intake from fish and shellfish, μg/day | ≤0.31 | 0.32–0.76 | ≥0.77 | |
| Serum B-12, pmol/L | 254 (218; 290) | 274 (238; 310) | 281 (244; 317) | 0.04 |
| Vitamin B-12 intake from eggs, μg/day | ≤0.078 | 0.079–0.157 | ≥0.158 | |
| Serum B-12, pmol/L | 265 (229; 301) | 277 (241; 312) | 265 (228; 302) | 0.43 |
| Vitamin B-12 intake from dairy products, μg/day | ≤0.96 | 0.97–1.48 | ≥1.49 | |
| Serum B-12, pmol/L | 249 (214; 284) | 286 (250; 323) | 287 (250; 324) | 0.0006 |
| Probability of Having Serum Vitamin B-12 ≥200 pmol/L | Probability of Having Normal Serum Vitamin B-12 and MMA | |||||
|---|---|---|---|---|---|---|
| Dietary vitamin B-12 intake from food items (μg/day) | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Total vitamin B-12 intake | ||||||
| ≤3.19 | 1 (ref)* | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 3.20–4.40 | 1.16 (1.04–1.30) | 1.16 (1.05–1.30) | 1.19 (1.07–1.33) | 1.05 (0.97–1.13) | 1.05 (0.97–1.13) | 1.07 (0.99–1.15) |
| ≥4.41 | 1.14 (1.02–1.28) | 1.14 (1.02–1.28) | 1.20 (1.06–1.35) | 1.11 (1.04–1.19) | 1.10 (1.03–1.18) | 1.14 (1.04–1.24) |
| P for trend | 0.04 | 0.03 | 0.007 | 0.003 | 0.005 | 0.003 |
| Vitamin B-12 intake from meat | ||||||
| ≤0.92 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 0.93–1.51 | 1.13 (1.01–1.26) | 1.13 (1.01–1.26) | 1.17 (1.04–1.31) | 1.06 (0.99–1.14) | 1.06 (0.99–1.14) | 1.09 (1.01–1.17) |
| ≥1.52 | 1.14 (1.02–1.28) | 1.15 (1.02–1.28) | 1.22 (1.08–1.37) | 1.05 (0.97–1.13) | 1.04 (0.97–1.12) | 1.07 (0.99–1.17) |
| P for trend | 0.03 | 0.02 | 0.02 | 0.30 | 0.37 | 0.13 |
| Vitamin B-12 intake from fish and shellfish | ||||||
| ≤0.31 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 0.32-0.76 | 1.18 (1.06–1.31) | 1.17 (1.05–1.31) | 1.17 (1.05–1.30) | 1.12 (1.03–1.20) | 1.11 (1.03–1.19) | 1.10 (1.02–1.18) |
| ≥0.77 | 1.15 (1.03–1.29) | 1.15 (1.03–1.29) | 1.16 (1.04–1.30) | 1.14 (1.06–1.23) | 1.13 (1.04–1.21) | 1.13 (1.05–1.23) |
| P for trend | 0.03 | 0.04 | 0.02 | 0.01 | 0.004 | 0.003 |
| Vitamin B-12 intake from eggs | ||||||
| ≤0.078 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 0.079–0.157 | 1.10 (1.00–1.21) | 1.11 (1.00–1.22) | 1.10 (0.99–1.21) | 1.04 (0.97-1.10) | 1.04 (0.98–1.11) | 1.04 (0.97–1.11) |
| ≥0.158 | 1.04 (0.92–1.17) | 1.06 (0.94–1.19) | 1.05 (0.93–1.18) | 1.00 (0.93-1.08) | 1.01 (0.94–1.09) | 1.01 (0.93–1.09) |
| P for trend | 0.51 | 0.35 | 0.43 | 0.94 | 0.76 | 0.87 |
| Vitamin B-12 intake from dairy products | ||||||
| ≤0.96 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 0.97–1.48 | 1.19 (1.06–1.33) | 1.17 (1.05–1.31) | 1.20 (1.08–1.34) | 1.10 (1.03–1.19) | 1.09 (1.01–1.17) | 1.10 (1.02–1.19) |
| ≥1.49 | 1.18 (1.06–1.33) | 1.18 (1.06–1.33) | 1.24 (1.10–1.39) | 1.10 (1.02–1.18) | 1.09 (1.01–1.18) | 1.11 (1.02–1.21) |
| P for trend | 0.006 | 0.005 | 0.0007 | 0.03 | 0.04 | 0.02 |
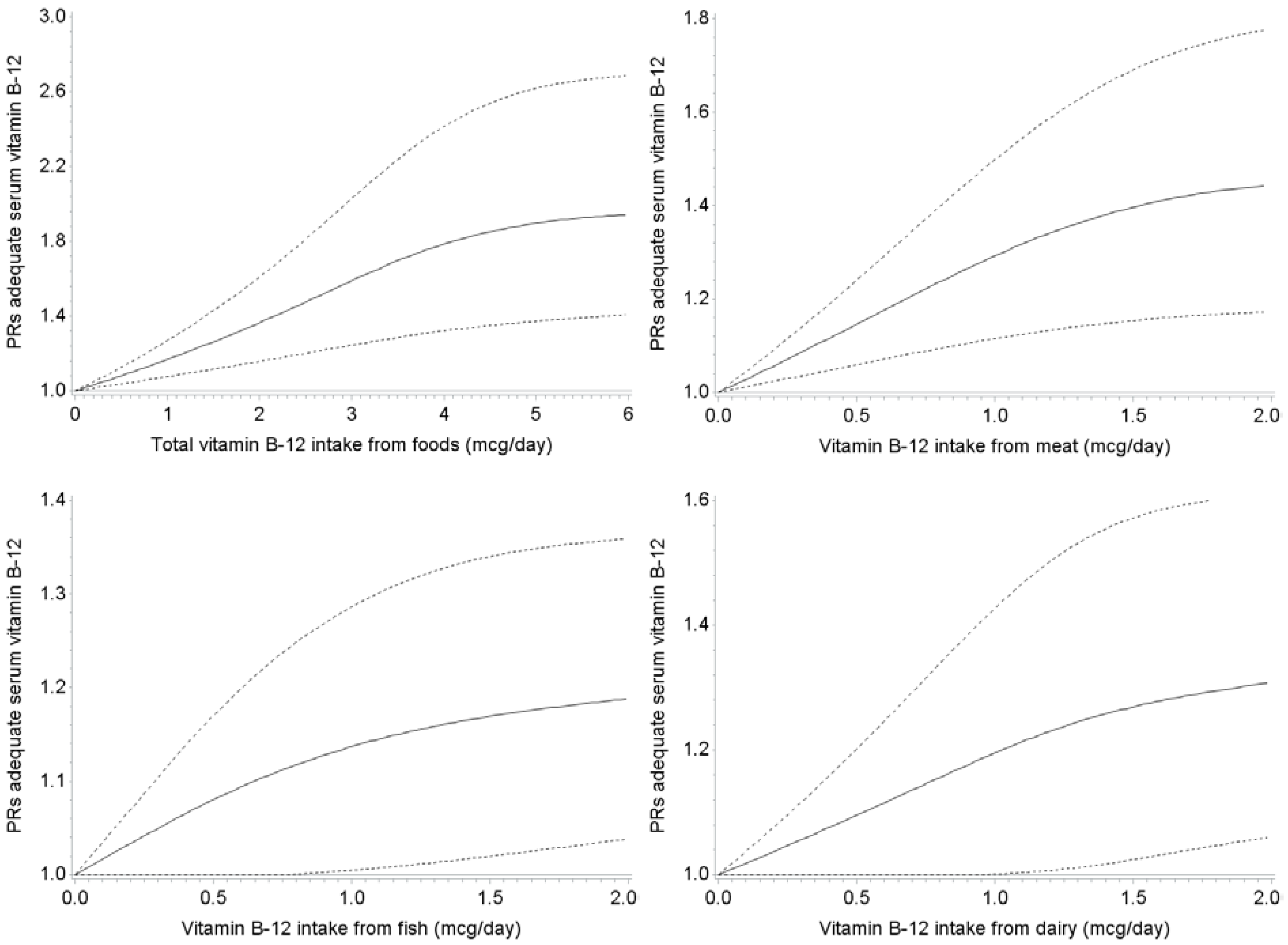
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Troen, A.; Rosenberg, I.H. B vitamins and the aging brain. Nutr. Rev. 2010, 68, S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Clinical practice, Vitamin B-12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Dullemeijer, C.; Souverein, O.W.; Doets, E.L.; van der Voet, H.; van Wijngaarden, J.P.; de Boer, W.J.; Plada, M.; Dhonukshe-Rutten, R.A.; Inʼt Veld, P.H.; Cavelaars, A.E.; et al. Systematic review with dose-response meta-analyses between vitamin B-12 intake and European Micronutrient Recommendations Alignedʼs prioritized biomarkers of vitamin B-12 including randomized controlled trials and observational studies in adults and elderly persons. Am. J. Clin. Nutr. 2013, 97, 390–402. [Google Scholar] [PubMed]
- Doets, E.L.; Cavelaars, A.E.; Dhonukshe-Rutten, R.A.; vanʼt Veer, P.; de Groot, L.C. Explaining the variability in recommended intakes of folate, vitamin B-12, iron and zinc for adults and elderly people. Public Health Nutr. 2012, 15, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Toh, B.H.; Chan, J.; Kyaw, T.; Alderuccio, F. Cutting edge issues in autoimmune gastritis. Clin. Rev. Allergy Immunol. 2012, 42, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Weck, M.N.; Brenner, H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Venn, B.J.; Skeaff, C.M.; Williams, S.M. Serum vitamin B-12 concentrations and atrophic gastritis in older New Zealanders. Eur. J. Clin. Nutr. 2005, 59, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lewerin, C.; Jacobsson, S.; Lindstedt, G.; Nilsson-Ehle, H. Serum biomarkers for atrophic gastritis and antibodies against Helicobacter pylori in the elderly: Implications for vitamin B-12, folic acid and iron status and response to oral vitamin therapy. Scand. J. Gastroenterol. 2008, 43, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [PubMed]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, J.P.; Dhonukshe-Rutten, R.A.; van Schoor, N.M.; van der Velde, N.; Swart, K.M.; Enneman, A.W.; van Dijk, S.C.; Brouwer-Brolsma, E.M.; Zillikens, M.C.; van Meurs, J.B.; et al. Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B-12 and folic acid on fracture incidence. BMC Geriatr. 2011, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Verkleij-Hagoort, A.C.; de Vries, J.H.; Stegers, M.P.; Lindemans, J.; Ursem, N.T. Steegers-Theunissen, R.P. Validation of the assessment of folate and vitamin B-12 intake in women of reproductive age: The method of triads. Eur. J. Clin. Nutr. 2007, 61, 610–615. [Google Scholar] [PubMed]
- Feunekes, G.I.; van Staveren, W.A.; de Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [PubMed]
- Dutch Nutrition Center. Zo eet Nederland: Resultaten van de Voedselconsumptiepeiling 1997–1998; Dutch Nutrition Center: The Hague, The Netherlands, 1998. [Google Scholar]
- NEVO-online. Available online: http://nevo-online.rivm.nl/ProductenZoeken.aspx (accessed on 25 June 2015).
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.; Moller, J. Total homocysteine measurement in clinical practice. Ann. Clin. Biochem. 2000, 37, 627–648. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, J.B.; Hayward Vermaak, W.J.; Bissbort, S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J. Chromatogr. 1991, 565, 441–446. [Google Scholar] [CrossRef]
- Van Driel, L.M.; Eijkemans, M.J.; de Jonge, R.; de Vries, J.H.; van Meurs, J.B.; Steegers, E.A.; Steegers-Theunissen, R.P. Body mass index is an important determinant of methylation biomarkers in women of reproductive ages. J. Nutr. 2009, 39, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Heil, S.G.; de Jonge, R.; de Rotte, M.C.; van Wijnen, M.; Heiner-Fokkema, R.M.; Kobold, A.C.; Pekelharing, J.M.; Adriaansen, H.J.; Sanders, E.; Trienekens, P.H.; et al. Screening for metabolic vitamin B-12 deficiency by holotranscobalamin in patients suspected of vitamin B-12 deficiency: A multicentre study. Ann. Clin. Biochem. 2012, 49, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Stel, V.S.; Smit, J.H.; Pluijm, S.M.; Visser, M.; Deeg, D.J.; Lips, P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J. Clin. Epidemiol. 2004, 57, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.J.; Hirakata, V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Council of The Netherlands. Dietary Reference Intakes: Vitamin B6, Folic Acid, and Vitamin B12; Health Council of The Netherlands: The Hague, The Netherlands, 2003. [Google Scholar]
- Bor, M.V.; Lydeking-Olsen, E.; Moller, J.; Nexo, E. A daily intake of approximately 6 microg vitamin B-12 appears to saturate all the vitamin B-12-related variables in Danish postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 52–58. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouwer-Brolsma, E.M.; Dhonukshe-Rutten, R.A.M.; Van Wijngaarden, J.P.; Zwaluw, N.L.v.d.; Velde, N.V.d.; De Groot, L.C.P.G.M. Dietary Sources of Vitamin B-12 and Their Association with Vitamin B-12 Status Markers in Healthy Older Adults in the B-PROOF Study. Nutrients 2015, 7, 7781-7797. https://doi.org/10.3390/nu7095364
Brouwer-Brolsma EM, Dhonukshe-Rutten RAM, Van Wijngaarden JP, Zwaluw NLvd, Velde NVd, De Groot LCPGM. Dietary Sources of Vitamin B-12 and Their Association with Vitamin B-12 Status Markers in Healthy Older Adults in the B-PROOF Study. Nutrients. 2015; 7(9):7781-7797. https://doi.org/10.3390/nu7095364
Chicago/Turabian StyleBrouwer-Brolsma, Elske M., Rosalie A. M. Dhonukshe-Rutten, Janneke P. Van Wijngaarden, Nikita L. van der Zwaluw, Nathalie Van der Velde, and Lisette C. P. G. M. De Groot. 2015. "Dietary Sources of Vitamin B-12 and Their Association with Vitamin B-12 Status Markers in Healthy Older Adults in the B-PROOF Study" Nutrients 7, no. 9: 7781-7797. https://doi.org/10.3390/nu7095364





