High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
| Control (Sham-OP) | |
|---|---|
| Co (n = 10) | Co-K2 (n = 10) |
| Standard diet: | MK-7-supplemented standard diet: |
| 0.36% Phosphate | 0.36% Phosphate |
| 0.6% Calcium | 0.6% Calcium |
| 0.5 µg/g VK1 | 0.5 µg/g VK1 |
| 0 MK-7 | 100 µg/g MK-7 |
| Intervention (5/6-Nephrectomy + high phosphate diet) | |
| CKD (n = 11) | CKD-K2 (n = 11) |
| High phosphate diet: | Phosphate- and MK-7-rich diet: |
| 1.2% Phosphate | 1.2% Phosphate |
| 0.6% Calcium | 0.6% Calcium |
| 0.5 µg/g VK1 | 0.5 µg/g VK1 |
| 0 MK-7 | 100 µg/g MK-7 |
2.2. Study Design
2.3. Echocardiography
2.4. Blood/Urine Analyses
2.5. Histology
2.6. qRT-PCR
2.7. Statistics
3. Results
3.1. Experimental CKD
| Parameters | Co | Co-K2 | CKD | CKD-K2 |
|---|---|---|---|---|
| Serum creatinine (mg/dl) | ||||
| Week 12 | 0.3 ± 0 | 0.3 ± 0 | 0.5 ± 0 | 0.5 ± 0 |
| BUN (mg/dl) | ||||
| Week 12 | 40 ± 2.2 | 42 ± 1.3 | 51 ± 2.9 | 50 ± 1.9 |
| Serum phosphate (mmol/l) | ||||
| Week 12 | 1.8 ± 0.1 | 1.6 ± 0.1 | 2.2 ± 0.2 | 1.8 ± 0.1 |
| Hb (g/dl) | ||||
| Week 12 | 15.0 ± 0.5 | 15.5 ± 0.2 | 15.5 ± 0.2 | 15.5 ± 0.1 |
| Hkt (%) | ||||
| Week 12 | 45.8 ± 1.3 | 47.9 ± 0.9 | 44.0 ± 1.5 | 45.0 ± 0.3 |
| Creatinine clearance (ml/min) | ||||
| Week 12 | 3.2 ± 0.6 | 2.5 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.1 |
| Urine phosphate excretion | ||||
| (mg/24 h) | 33.1 ± 1 | 23.6 ± 3.6 | 82.8 ± 29.9 | 59.8 ± 7.5 |
3.2. Cardiovascular Calcification
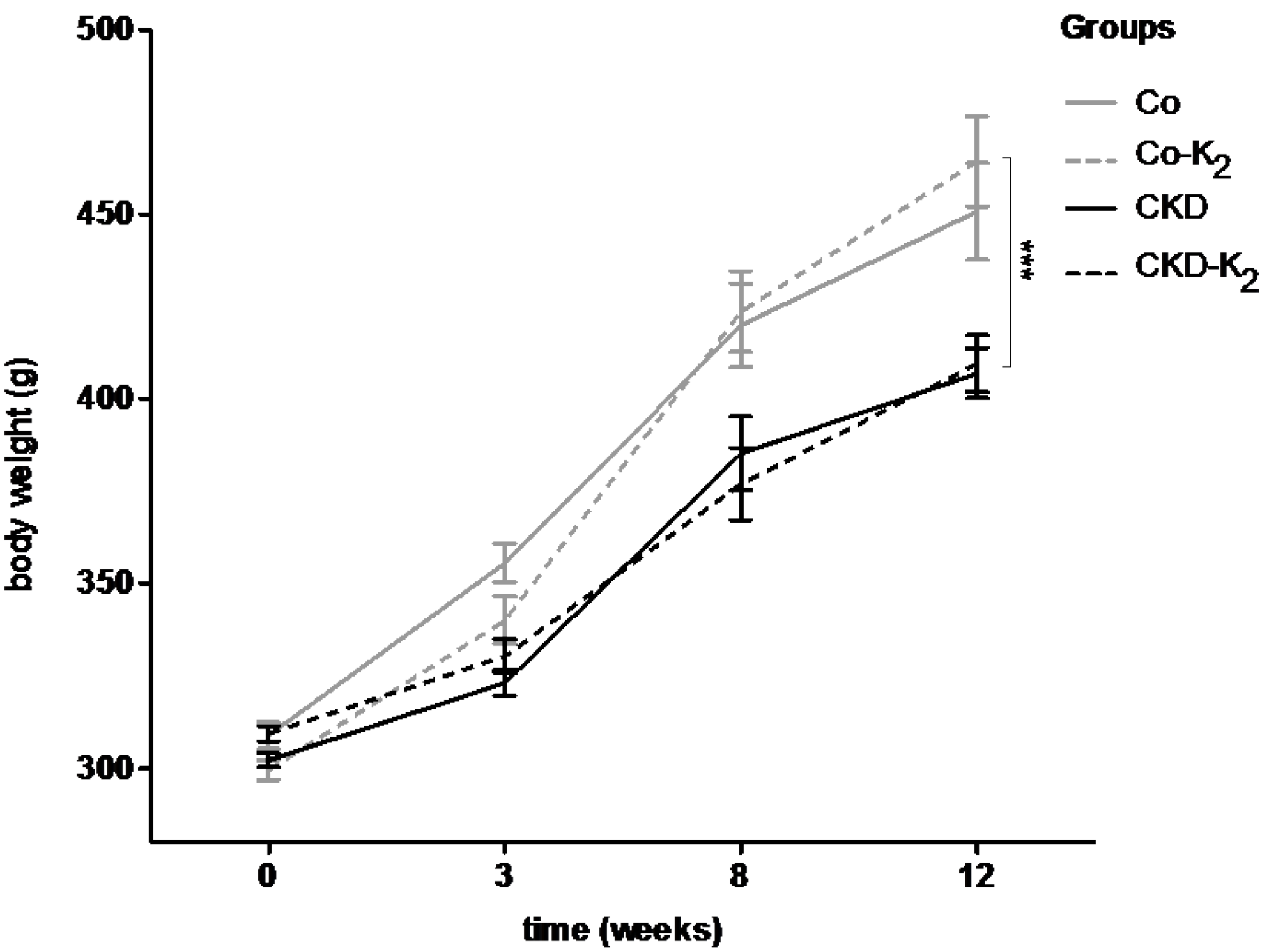
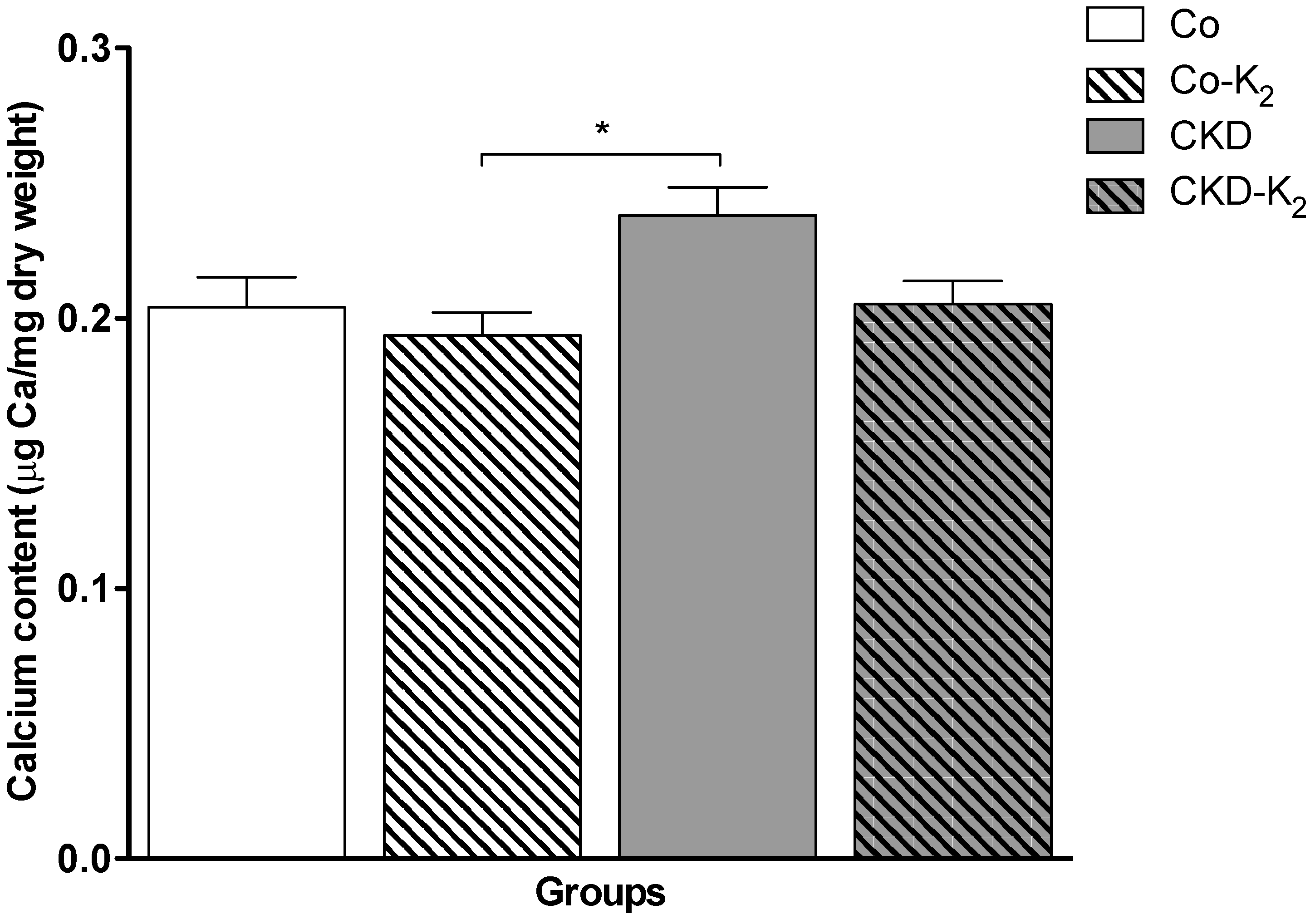
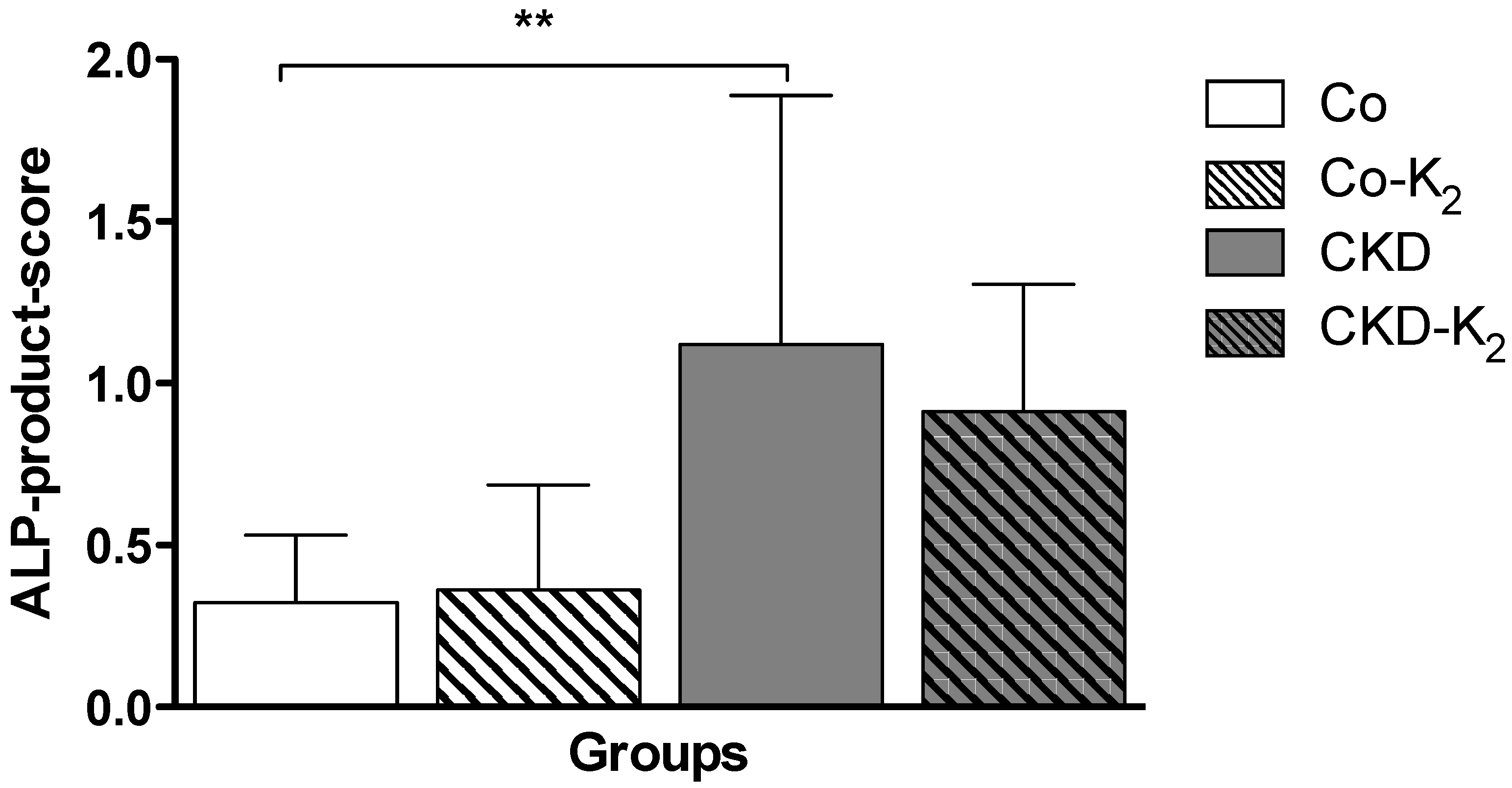
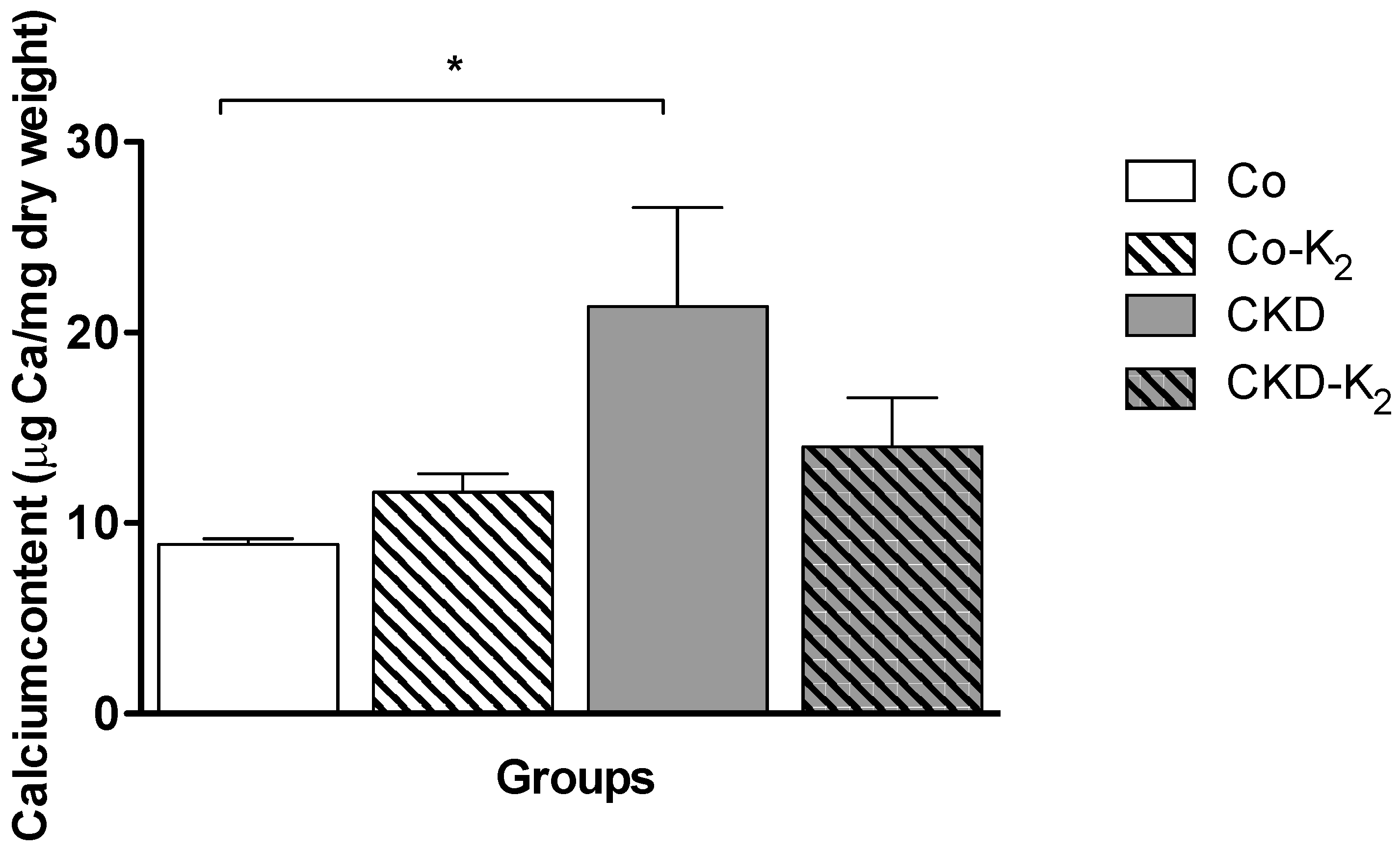
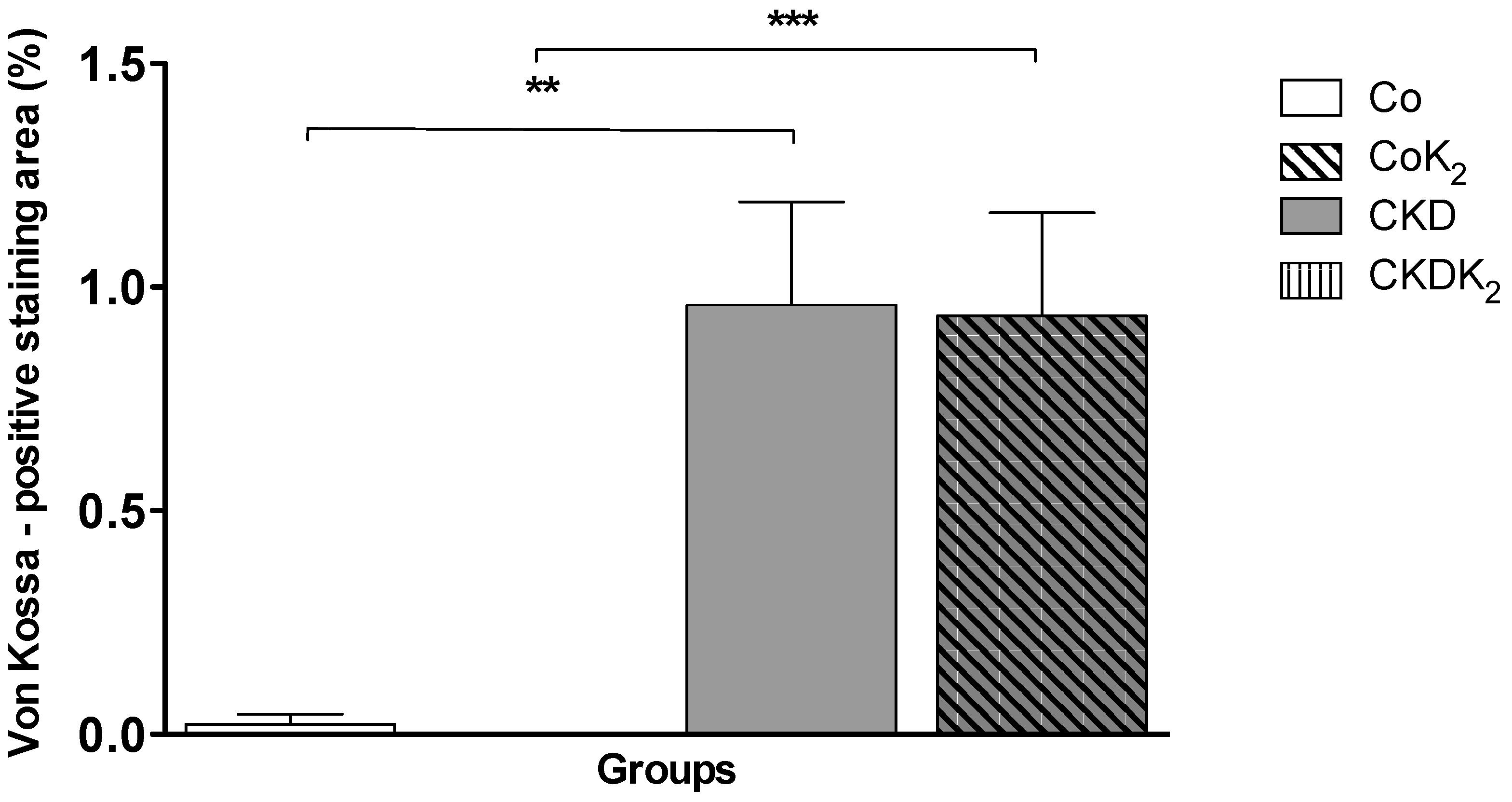

3.3. Structural Alterations in Experimental CKD
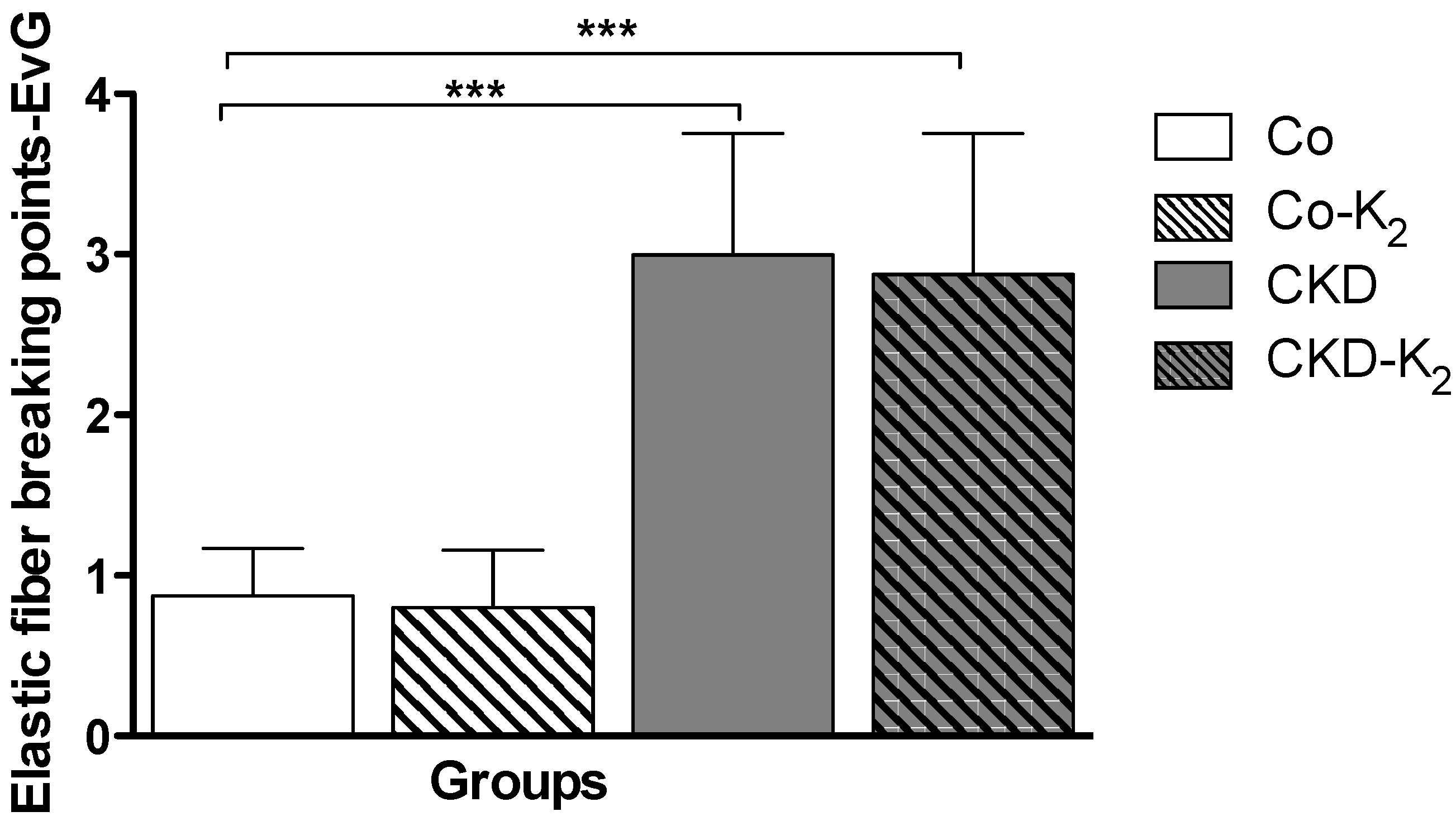

3.4. Cardiovascular Function
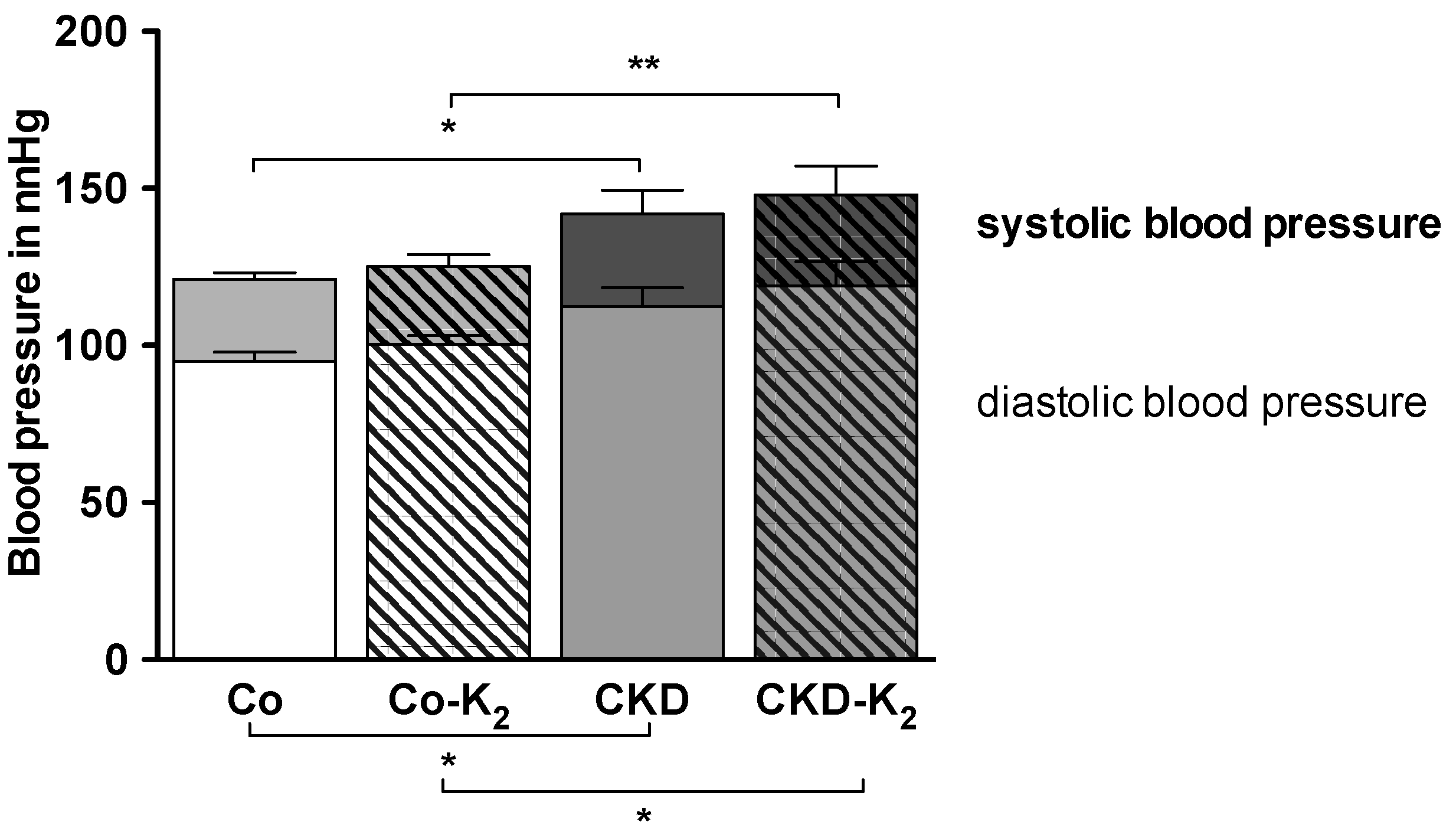
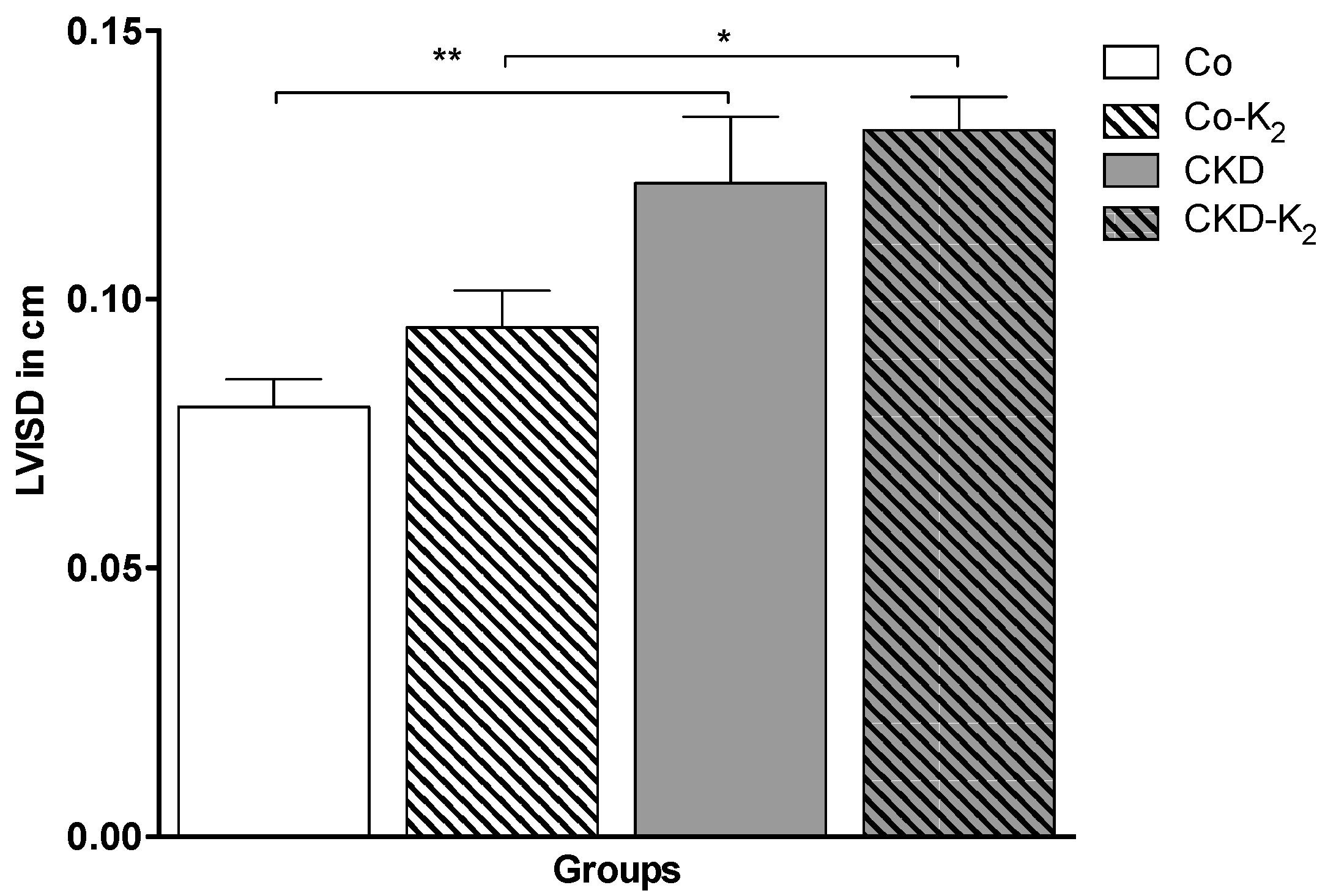
3.5. Aortic mRNA Expression
4. Discussion
4.1. CKD and High Phosphate Diet

4.2. Cardiovascular Calcification
4.3. Structural and Functional Alterations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allison, M.A.; Criqui, M.H.; Wright, C.M. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009, 75, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Schurgin, S.; Rich, S.; Mazzone, T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care 2001, 24, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart, R.; Oudkerk, M.; Hofman, A.; Oei, H.H.; van Dijck, W.; van Rooij, F.J.; Witteman, J.C. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005, 112, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Schlieper, G.; Floege, J. Calcification and cardiovascular health—New insights into an old phenomenon. Hypertension 2006, 47, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; O’Neill, K.D.; Duan, D.; Ahmed, S.; Chen, N.X.; Leapman, S.B.; Fineberg, N.; Kopecky, K. Medial artery calcification in esrd patients is associated with deposition of bone matrix proteins. Kidney Int. 2002, 61, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Chatrou, M.L.; Winckers, K.; Hackeng, T.M.; Reutelingsperger, C.P.; Schurgers, L.J. Vascular calcification: The price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012, 26, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Reaven, P.D.; Sacks, J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia 2005, 48, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Okuno, S.; Ishimura, E.; Kitatani, K.; Fujino, Y.; Kohno, K.; Maeno, Y.; Maekawa, K.; Yamakawa, T.; Imanishi, Y.; Inaba, M.; et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis. Am. J. Kidney Dis. 2007, 49, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Cranenburg, E.C.M.; Vermeer, C. Matrix gla-protein: The calcification inhibitor in need of vitamin k. Thromb. Haemost. 2008. [Google Scholar] [CrossRef]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix gla protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Munroe, P.B.; Olgunturk, R.O.; Fryns, J.P.; van Maldergem, L.; Ziereisen, F.; Yuksel, B.; Gardiner, R.M.; Chung, E. Mutations in the gene encoding the human matrix gla protein cause keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin k-dependent carboxylation of matrix gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M.; Schinke, T.; McKee, M.D.; Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 2004, 165, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.; Knapen, M.H.; Kwaijtaal, M.; van Diest, R.; Appels, A.; Reutelingsperger, C.P.; Cleutjens, J.P.; Vermeer, C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (gla) protein: Undercarboxylated matrix gla protein as marker for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Berkner, K.L.; Runge, K.W. The physiology of vitamin k nutriture and vitamin k-dependent protein function in atherosclerosis. J. Thromb. Haemost. 2004, 2, 2118–2132. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, R.J.M.W.; van Varik, B.J.; Schurgers, L.J.; Hamulyak, K.; ten Cate, H.; Leiner, T.; Vermeer, C.; de Leeuw, P.W.; Kroon, A.A. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood 2010, 115, 5121–5123. [Google Scholar] [CrossRef] [PubMed]
- Pilkey, R.M.; Morton, A.R.; Boffa, M.B.; Noordhof, C.; Day, A.G.; Su, Y.H.; Miller, L.M.; Koschinsky, M.L.; Booth, S.L. Subclinical vitamin k deficiency in hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; O’Donnell, C.J.; Vermeer, C.; Magdeleyns, E.J.P.; Crosier, M.D.; Gundberg, C.M.; Ordovas, J.M.; Kritchevsky, S.B.; Booth, S.L. Circulating uncarboxylated matrix gla protein is associated with vitamin k nutritional status, but not coronary artery calcium, in older adults. J. Nutr. 2011, 141, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Gullestad, L.; Dahl, C.P.; Aukrust, P.; Aakhus, S.; Solberg, O.G.; Vermeer, C.; Schurgers, L.J. Undercarboxylated matrix gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J. Intern. Med. 2010, 268, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Dahl, C.P.; Gullestad, L.; Aakhus, S.; Broch, K.; Skardal, R.; Vermeer, C.; Aukrust, P.; Schurgers, L.J. Circulating levels of non-phosphorylated undercarboxylated matrix gla protein are associated with disease severity in patients with chronic heart failure. Clin. Sci. 2011, 121, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewe, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix gla protein (mgp) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [PubMed]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin k2 supplementation on functional vitamin k deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control. Release 2010, 146, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, B.L.; Jie, K.S.; Vermeer, C. Effect of food composition on vitamin k absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin k concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [PubMed]
- Beulens, J.W.J.; Bots, M.L.; Atsma, F.; Bartelink, M.L.E.L.; Prokop, M.; Geleijnse, J.M.; Witteman, J.C.M.; Grobbee, D.E.; van der Schouw, Y.T. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.; van der Meer, I.M.; Hofman, A.; Witteman, J.C. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The rotterdam study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [PubMed]
- Kruger, T.; Oelenberg, S.; Kaesler, N.; Schurgers, L.J.; van de Sandt, A.M.; Boor, P.; Schlieper, G.; Brandenburg, V.M.; Fekete, B.C.; Veulemans, V.; et al. Warfarin induces cardiovascular damage in mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Spronk, H.M.; Soute, B.A.; Schiffers, P.M.; DeMey, J.G.; Vermeer, C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin k in rats. Blood 2007, 109, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.; Soute, B.A.; Schurgers, L.J.; Thijssen, H.H.; de Mey, J.G.; Vermeer, C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J. Vasc. Res. 2003, 40, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, L.; Ros-Lopez, S.; Cardus, A.; Fernandez, E.; Valdivielso, J.M. A forgotten method to induce experimental chronic renal failure in the rat by ligation of the renal parenchyma. Nephron. Exp. Nephrol. 2006, 103, e126–e130. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Hildreth, C.M.; Phillips, J.K.; Avolio, A.P. Aortic stiffness is associated with vascular calcification and remodeling in a chronic kidney disease rat model. Am. J. Physiol. Renal Physiol. 2011, 300, F1431–F1436. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, N.; Adams, M.A.; Holden, R.M. Vascular calcification in animal models of ckd: A review. Am. J. Nephrol. 2010, 31, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.; Appenroth, D.; Jonas, P.; Koch, M.; Kundt, G.; Nizze, H.; Stein, G. Suitability of 5/6 nephrectomy (5/6nx) for the induction of interstitial renal fibrosis in rats—Influence of sex, strain, and surgical procedure. Exp. Toxicol. Pathol. 2006, 57, 195–205. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Ohmit, S.E.; McCullough, P.A.; Crook, E.D.; Brown, W.W.; Grimm, R.; Bakris, G.L.; Keane, W.F.; Flack, J.M. Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The kidney early evaluation program. Kidney Int. 2005, 67, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin m derived from macrophages. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Chonchol, M. The role of phosphorus in the development and progression of vascular calcification. Am. J. Kidney Dis. 2011, 58, 826–834. [Google Scholar] [CrossRef] [PubMed]
- El-Abbadi, M.M.; Pai, A.S.; Leaf, E.M.; Yang, H.Y.; Bartley, B.A.; Quan, K.K.; Ingalls, C.M.; Liao, H.W.; Giachelli, C.M. Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009, 75, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A.; et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and mmp production in humans and rodents. J. Clin. Invest. 2010, 120, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Hixson, J.E.; Shimmin, L.C.; Montasser, M.E.; Kim, D.K.; Zhong, Y.; Ibarguen, H.; Follis, J.; Malcom, G.; Strong, J.; Howard, T.; et al. Common variants in the periostin gene influence development of atherosclerosis in young persons. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Lindenmeyer, M.T.; Gaspert, A.; Eichinger, F.; Neusser, M.A.; Kretzler, M.; Segerer, S.; Cohen, C.D. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am. J. Pathol. 2011, 179, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, K.; Watson, K.E.; Horn, S.; Wortham, C.; Herman, I.M.; Demer, L.L. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Invest. 1993, 91, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Elli, F.; Brenna, I.; Volpi, E.; Romagnoli, S.; Tosi, D.; Braidotti, P.; Brancaccio, D.; Cozzolino, M. Lanthanum prevents high phosphate-induced vascular calcification by preserving vascular smooth muscle lineage markers. Calcif. Tissue Int. 2013, 92, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, N.; Magdeleyns, E.; Herfs, M.; Schettgen, T.; Brandenburg, V.; Fliser, D.; Vermeer, C.; Floege, J.; Schlieper, G.; Kruger, T. Impaired vitamin k recycling in uremia is rescued by vitamin k supplementation. Kidney Int. 2014, 86, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grun, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin k2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor sxr. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.S.; Rafael, M.S.; Enriquez, J.L.; Teixeira, A.; Vitorino, R.; Luis, I.M.; Costa, R.M.; Santos, S.; Cavaco, S.; Neves, J.; et al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Matsushima, S.; Takasu, N.; Kyokawa, Y.; Torii, M. Glomerular calcification induced by bolus injection with dibasic sodium phosphate solution in sprague-dawley rats. Toxicol. Pathol. 2004, 32, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Levin, N.W. K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S14–S266. [Google Scholar]
- McCarty, M.F.; DiNicolantonio, J.J. Bioavailable dietary phosphate, a mediator of cardiovascular disease, may be decreased with plant-based diets, phosphate binders, niacin, and avoidance of phosphate additives. Nutrition 2014, 30, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Pucaj, K.; Rasmussen, H.; Moller, M.; Preston, T. Safety and toxicological evaluation of a synthetic vitamin k2, menaquinone-7. Toxicol. Mech. Methods 2011, 21, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins k and d status in stages 3–5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Noale, M.; Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; et al. Vitamin k, vertebral fractures, vascular calcifications, and mortality: Vitamin k italian (viki) dialysis study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Schurgers, L.J.; Kaesler, N.; Pusche, K.; van Gorp, R.H.; Leftheriotis, G.; Reinartz, S.; Koos, R.; Kruger, T. Prevention of vasculopathy by vitamin k supplementation: Can we turn fiction into fact? Atherosclerosis 2015, 240, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Nami, R.; Bruno, R.M.; Quatrini, I.; Nuti, R. Hypertension, left ventricular hypertrophy and chronic kidney disease. Heart Fail. Rev. 2011, 16, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Feihl, F.; Liaudet, L.; Levy, B.I.; Waeber, B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008, 78, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Li, Y.; Kost, C.K., Jr.; Martin, D.S. Bortezomib, a proteasome inhibitor, attenuates angiotensin ii-induced hypertension and aortic remodeling in rats. PLoS One 2013, 8, e78564. [Google Scholar] [CrossRef] [PubMed]
- Ro, A.; Kageyama, N. Pathomorphometry of ruptured intracranial vertebral arterial dissection: Adventitial rupture, dilated lesion, intimal tear, and medial defect. J. Neurosurg. 2013, 119, 221–227. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiber, D.; Veulemans, V.; Horn, P.; Chatrou, M.L.; Potthoff, S.A.; Kelm, M.; Schurgers, L.J.; Westenfeld, R. High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification. Nutrients 2015, 7, 6991-7011. https://doi.org/10.3390/nu7085318
Scheiber D, Veulemans V, Horn P, Chatrou ML, Potthoff SA, Kelm M, Schurgers LJ, Westenfeld R. High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification. Nutrients. 2015; 7(8):6991-7011. https://doi.org/10.3390/nu7085318
Chicago/Turabian StyleScheiber, Daniel, Verena Veulemans, Patrick Horn, Martijn L. Chatrou, Sebastian A. Potthoff, Malte Kelm, Leon J. Schurgers, and Ralf Westenfeld. 2015. "High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification" Nutrients 7, no. 8: 6991-7011. https://doi.org/10.3390/nu7085318
APA StyleScheiber, D., Veulemans, V., Horn, P., Chatrou, M. L., Potthoff, S. A., Kelm, M., Schurgers, L. J., & Westenfeld, R. (2015). High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification. Nutrients, 7(8), 6991-7011. https://doi.org/10.3390/nu7085318






