Glucose Homeostasis Variables in Pregnancy versus Maternal and Infant Body Composition
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants and Study Outline
2.2. Body Composition of Women in Gestational Week 32
2.3. Glucose Homeostasis Variables of Women in Gestational Week 32
2.4. Body Size and Composition of Infants
2.5. Statistics
3. Results
3.1. Characteristics of Mothers and Their Infants
3.2. Glucose Homeostasis Variables vs. Body Composition of Women in Gestational Week 32
| Women | All ( n = 209) | Mothers of Girls | Mothers of Boys |
|---|---|---|---|
| ( n = 99) | ( n = 110) | ||
| Stage of Gestation (weeks) | 31.4 ± 0.3 | 31.4 ± 0.3 | 31.4 ± 0.3 |
| Body mass index (kg/m2) | 26.6 ± 3.4 | 26.5 ± 3.7 | 26.7 ± 3.0 |
| Fat-free mass index (kg/m2) | 17.3 ± 1.4 | 17.4 ± 1.5 | 17.3 ± 1.4 |
| Fat mass index (kg/m2) | 9.3 ± 2.6 | 9.2 ± 2.8 | 9.4 ± 2.4 |
| Fat-free mass (kg) | 49.6 ± 5.3 | 49.8 ± 5.5 | 49.4 ± 5.1 |
| Fat mass (kg) | 26.5 ± 7.4 | 26.2 ± 7.8 | 26.7 ± 7.1 |
| Fat mass (%) | 34.3 ± 5.8 | 33.9 ± 6.2 | 34.6 ± 5.5 |
| Glucose in plasma (mmol/L) | 4.8 ± 0.4 | 4.9 ± 0.4 | 4.8 ± 0.3 |
| Insulin in serum (pmol/L) | 66 ± 37 | 65 ± 38 | 67 ± 36 |
| HOMA-IR | 2.1 ± 1.3 | 2.1 ± 1.4 | 2.1 ± 1.2 |
| HbA1c * (mmol/mol) | 31 ± 3 | 31 ± 3 | 32 ± 3 |
| IGFBP-1 in serum † (ng/mL) | 108 ± 71 | 110 ± 81 | 107 ± 62 |
| Infants | All (n = 209) | Girls (n = 99) | Boys (n = 110) |
| Age (weeks) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.2 |
| Length (cm) | 51.5 ± 1.5 | 51.0 ± 1.5 | 52.0 ± 1.5 |
| Weight (g) | 3591 ± 458 | 3523 ± 445 | 3653 ± 462 |
| Fat-free mass (g) | 3151 ± 343 | 3049 ± 318 | 3243 ± 339 |
| Fat mass (g) | 440 ± 183 | 474 ± 171 | 409 ± 188 |
| Fat mass (%) | 12.0 ± 4.0 | 13.2 ± 3.6 | 10.9 ± 4.1 |
3.3. Glucose Homeostasis Variables of Women in Gestational Week 32 vs. Size and Body Composition of Their Infants
| Body Composition † | Glucose (SDS ‡) | HOMA-IR (SDS ‡) | HbA1c (SDS ‡) | IGFBP-1 (SDS ‡) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Independent Variables) | n = 209 | n = 209 | n = 208 | n = 204 | ||||||||
| b | r2 | p | b | r2 | p | b | r2 | p | b | r2 | p | |
| Fat mass (kg) | 0.04 | 0.07 | <0.001 | 0.08 | 0.32 § | <0.001 | 0.02 | 0.03 | 0.012 | −0.05 | 0.14 | <0.001 |
| Fat mass index (kg/m2) | 0.10 | 0.07 | <0.001 | 0.22 | 0.32 § | <0.001 | 0.08 | 0.04 | 0.003 | −0.14 | 0.14 | <0.001 |
| Fat-free mass index (kg/m2) | 0.22 | 0.10 | <0.001 | 0.26 | 0.14 § | <0.001 | 0.16 | 0.05 | 0.001 | −0.26 | 0.13 | <0.001 |
3.4. HOMA-IR of Women in Gestational Week 32 vs. Fat Mass of Their Infant Sons and Daughters
3.5. Fat Mass of Infant Girls vs. Fat Mass and HOMA-IR of Their Mothers in Gestational Week 32
| Model | Maternal (Independent) Variables | b | r2 | p | Model Adjusted r2 |
|---|---|---|---|---|---|
| A * | Fat mass (kg) | 5.8 | 0.09 | 0.004 | 0.27 |
| B * | HOMA-IR (SDS †) | 52.7 | 0.13 | <0.001 | 0.30 |
| C * | Fat mass (kg) | 2.7 | 0.01 | 0.24 | 0.31 |
| HOMA-IR (SDS †) | 41.4 | 0.06 | 0.017 |

- (A)
- Infant weightglucose: b = 61.0, r2 = 0.03, n = 209, p = 0.022HOMA-IR: b = 55.6, r2 = 0.02, n = 209, p = 0.035IGFBP-1: b = −68.2, r2 = 0.03, n = 204, p = 0.011
- (B)
- Infant fat-free massglucose: n = 209, p = 0.13HOMA-IR: n = 209, p = 0.21IGFBP-1: n = 204, p = 0.13
- (C)
- Infant fat massglucose: b = 31.0, r2 = 0.04, n = 209, p = 0.006HOMA-IR: b = 31.3, r2 = 0.04, n = 209, p = 0.005IGFBP-1: b = −38.5, r2 = 0.06, n = 204, p = 0.001
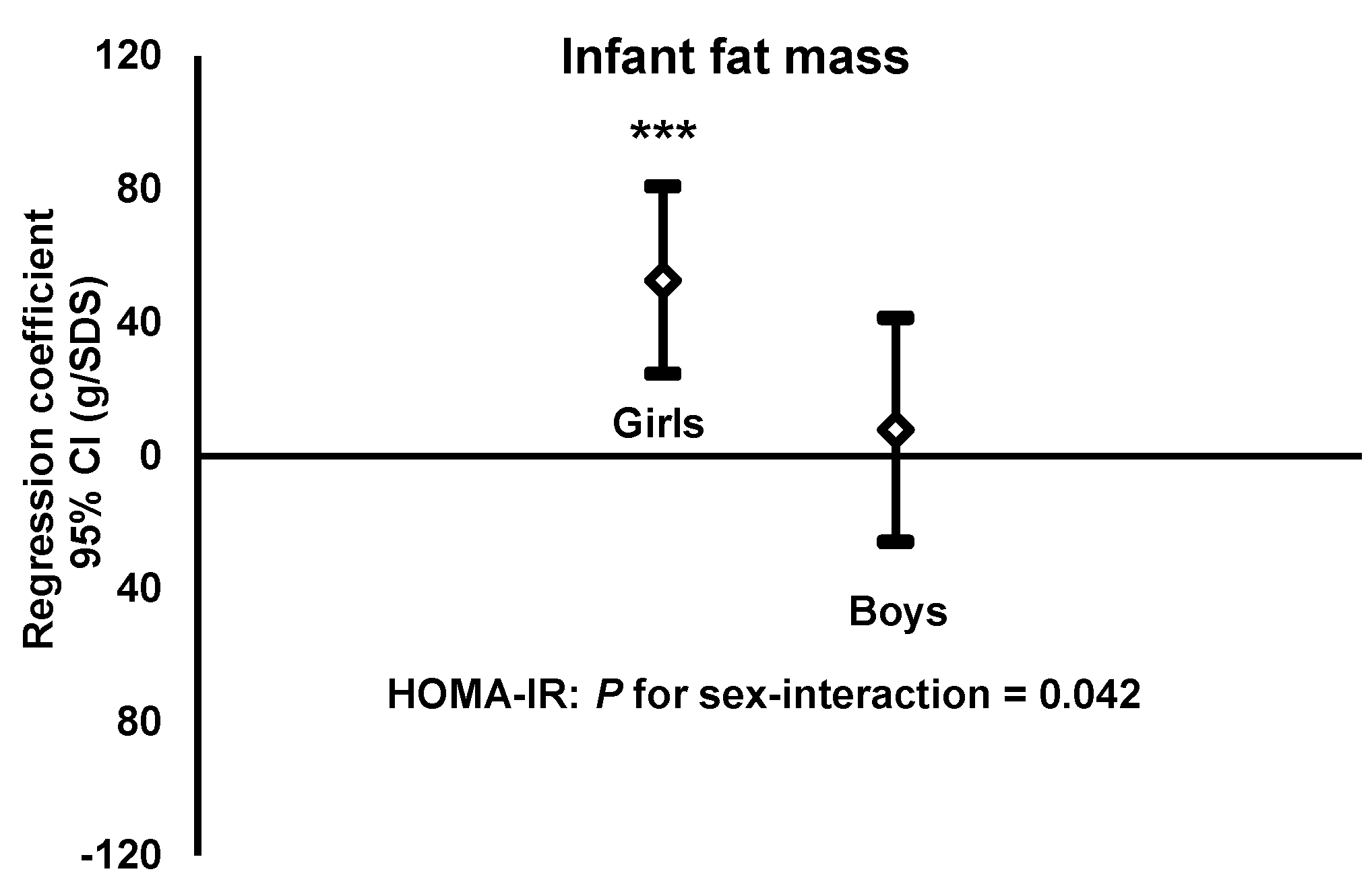
- HOMA-IRGirls: b = 52.7, r2 = 0.13, n = 99, p < 0.001Boys: b = 7.8, r2 = 0.00, n = 110, p = 0.65.
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Childhood Overweight and Obesity. Available online: http://www.who.int/dietphysicalactivity/childhood/en/ (accessed on 12 May 2015).
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes 2009, 58, 453–459. [Google Scholar]
- Henriksson, P.; Löf, M.; Forsum, E. Parental fat-free mass is related to fat-free mass of infants and maternal fat mass is related to fat mass of infant girls. Acta Paediatr. 2015, 104, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, B.E.; Henry, A.M.; d’Emden, M.C.; Fullerton, A.M.; Mortimer, R.H.; Colditz, P.B.; Lê Cao, K.A.; Callaway, L.K. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care 2011, 34, 2581–2585. [Google Scholar] [CrossRef] [PubMed]
- Friis, C.M.; Qvigstad, E.; Paasche Roland, M.C.; Godang, K.; Voldner, N.; Bollerslev, J.; Henriksen, T. Newborn body fat: Associations with maternal metabolic state and placental size. PLoS ONE 2013, 8, e57467. [Google Scholar] [CrossRef] [PubMed]
- Olausson, H.; Löf, M.; Brismar, K.; Forsum, E.; Sohlström, A. Maternal serum concentrations of insulin-like growth factor (IGF)-I and IGF binding protein-1 before and during pregnancy in relation to maternal body weight and composition and infant birth weight. Br. J. Nutr. 2010, 104, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Olausson, H.; Löf, M.; Brismar, K.; Lewitt, M.; Forsum, E.; Sohlström, A. Longitudinal study of the maternal insulin-like growth factor system before, during and after pregnancy in relation to fetal and infant weight. Horm. Res. 2008, 69, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Åsvold, B.O.; Eskild, A.; Jenum, P.A.; Vatten, L.J. Maternal concentrations of insulin-like growth factor I and insulin-like growth factor binding protein 1 during pregnancy and birth weight of offspring. Am. J. Epidemiol. 2011, 174, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.J. The role of the insulin-like growth factors and their binding proteins in glucose homeostasis. Exp. Diabetes Res. 2003, 4, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.A.; Huston, L.; Amini, S.B.; Catalano, P.M. Longitudinal changes in the relationship between body mass index and percent body fat in pregnancy. Obstet. Gynecol. 1997, 89, 377–382. [Google Scholar] [CrossRef]
- Knight, B.; Shields, B.M.; Hill, A.; Powell, R.J.; Wright, D.; Hattersley, A.T. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care 2007, 30, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Jörgensen, C. Fetometri och graviditetsbestämning. In Obstetriska Ultraljud; Sjöberg, S.O., Ed.; Västra Aros tryckeri AB: Västerås, Sweden, 1997. [Google Scholar]
- Henriksson, P.; Löf, M.; Forsum, E. Assessment and prediction of thoracic gas volume in pregnant women: An evaluation in relation to body composition assessment using air displacement plethysmography. Br. J. Nutr. 2013, 109, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Van Raaij, J.M.; Peek, M.E.; Vermaat-Miedema, S.H.; Schonk, C.M.; Hautvast, J.G. New equations for estimating body fat mass in pregnancy from body density or total body water. Am. J. Clin. Nutr. 1988, 48, 24–29. [Google Scholar] [PubMed]
- Forsum, E.; Henriksson, P.; Löf, M. The two-component model for calculating total body fat from body density: An evaluation in healthy women before, during and after pregnancy. Nutrients 2014, 6, 5888–5899. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, J.O.; Kobold, U.; Barr, J.; Finke, A.; Hoelzel, W.; Hoshino, T.; Miedema, K.; Mosca, A.; Mauri, P.; Paroni, R.; et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin. Chem. Lab. Med. 2002, 40, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J.; Yao, M.; Shypailo, R.J.; Urlando, A.; Wong, W.W.; Heird, W.C. Body-composition assessment in infancy: Air-displacement plethysmography compared with a reference 4-compartment model. Am. J. Clin. Nutr. 2007, 85, 90–95. [Google Scholar] [PubMed]
- Kleinbaum, D.G.; Kupper, L.L.; Nizam, A.; Muller, K.E. Applied Regression Analysis and Other Multivariable Methods; Duxbury Press: Belmont, CA, USA, 2007. [Google Scholar]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar]
- Eriksson, B.; Löf, M.; Olausson, H.; Forsum, E. Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br. J. Nutr. 2010, 103, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Watve, M.G.; Yajnik, C.S. Evolutionary origins of insulin resistance: A behavioural switch hypothesis. BMC Evol. Biol. 2007, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.C.; Friis, C.M.; Godang, K.; Bollerslev, J.; Haugen, G.; Henriksen, T. Maternal factors associated with fetal growth and birthweight are independent determinants of placental weight and exhibit differential effects by fetal sex. PLoS ONE 2014, 9, e87303. [Google Scholar] [CrossRef] [PubMed]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal-fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [PubMed]
- Simon-Muela, I.; Naf, S.; Ballesteros, M.; Vendrell, J.; Ceperuelo-Mallafre, V.; de la Flor, M.; Megia, A. Gender determines the actions of adiponectin multimers on fetal growth and adiposity. Am. J. Obstet. Gynecol. 2013, 208, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Hill, J.C.; Leary, S.D.; Veena, S.R.; Saperia, J.; Saroja, A.; Karat, S.C.; Fall, C.H. Anthropometry, glucose tolerance, and insulin concentrations in Indian children: Relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care 2005, 28, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Veena, S.R.; Hill, J.C.; Kehoe, S.; Karat, S.C.; Fall, C.H. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010, 33, 402–404. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksson, P.; Löf, M.; Forsum, E. Glucose Homeostasis Variables in Pregnancy versus Maternal and Infant Body Composition. Nutrients 2015, 7, 5615-5627. https://doi.org/10.3390/nu7075243
Henriksson P, Löf M, Forsum E. Glucose Homeostasis Variables in Pregnancy versus Maternal and Infant Body Composition. Nutrients. 2015; 7(7):5615-5627. https://doi.org/10.3390/nu7075243
Chicago/Turabian StyleHenriksson, Pontus, Marie Löf, and Elisabet Forsum. 2015. "Glucose Homeostasis Variables in Pregnancy versus Maternal and Infant Body Composition" Nutrients 7, no. 7: 5615-5627. https://doi.org/10.3390/nu7075243





