Protopanaxadiol, an Active Ginseng Metabolite, Significantly Enhances the Effects of Fluorouracil on Colon Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Biotransformation
2.2. Preparation of Protopanaxadiol (PPD) and Chemicals Used in this Study
2.3. Cell Culture
2.4. Cell Proliferation Assay
2.5. Cell Cycle Analysis
2.6. Apoptosis Analysis
2.7. In Vivo Antitumor Evaluation
2.8. Statistical Analysis
3. Results
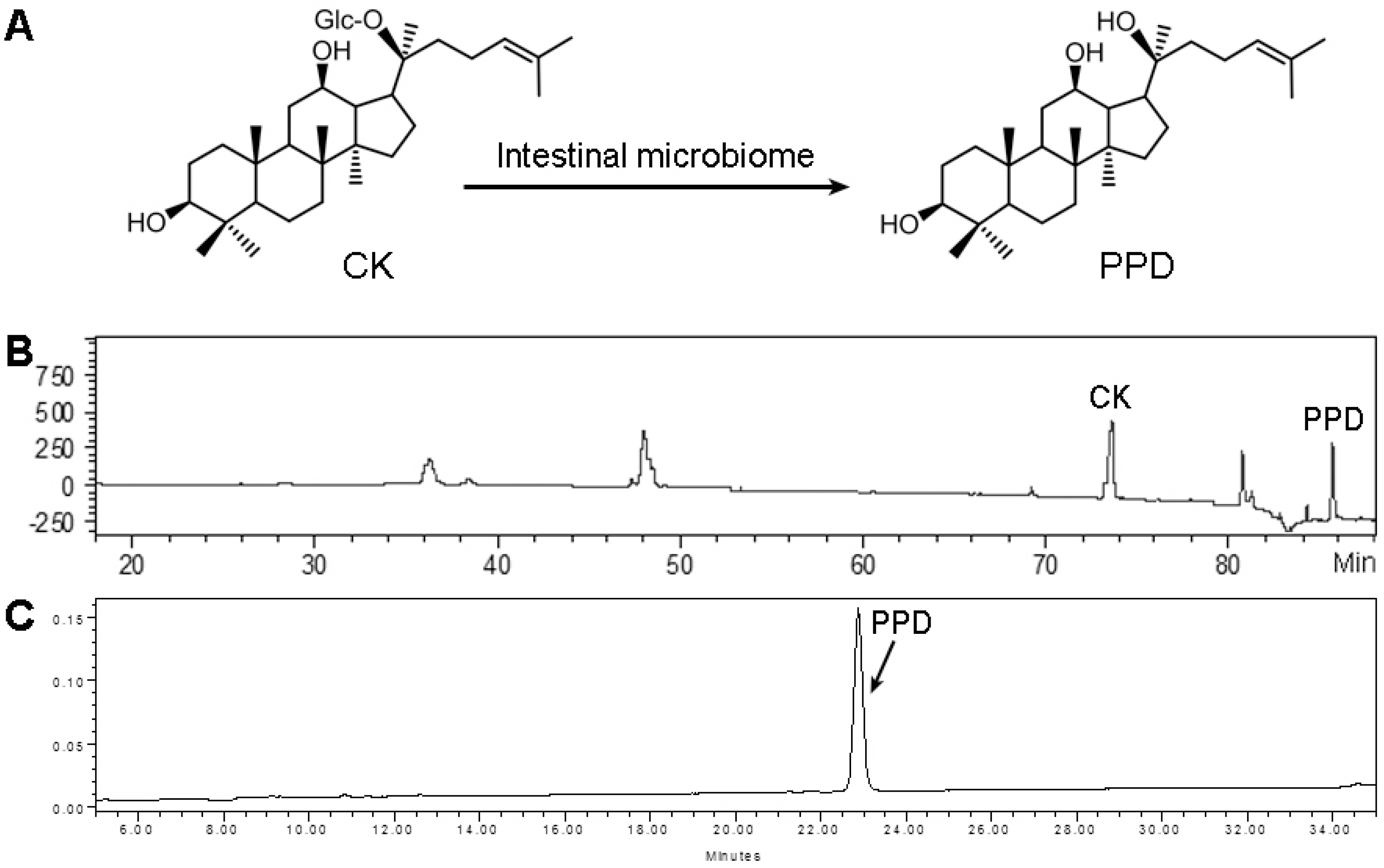
3.1. Compound K Biotransformation and PPD Purity Analysis
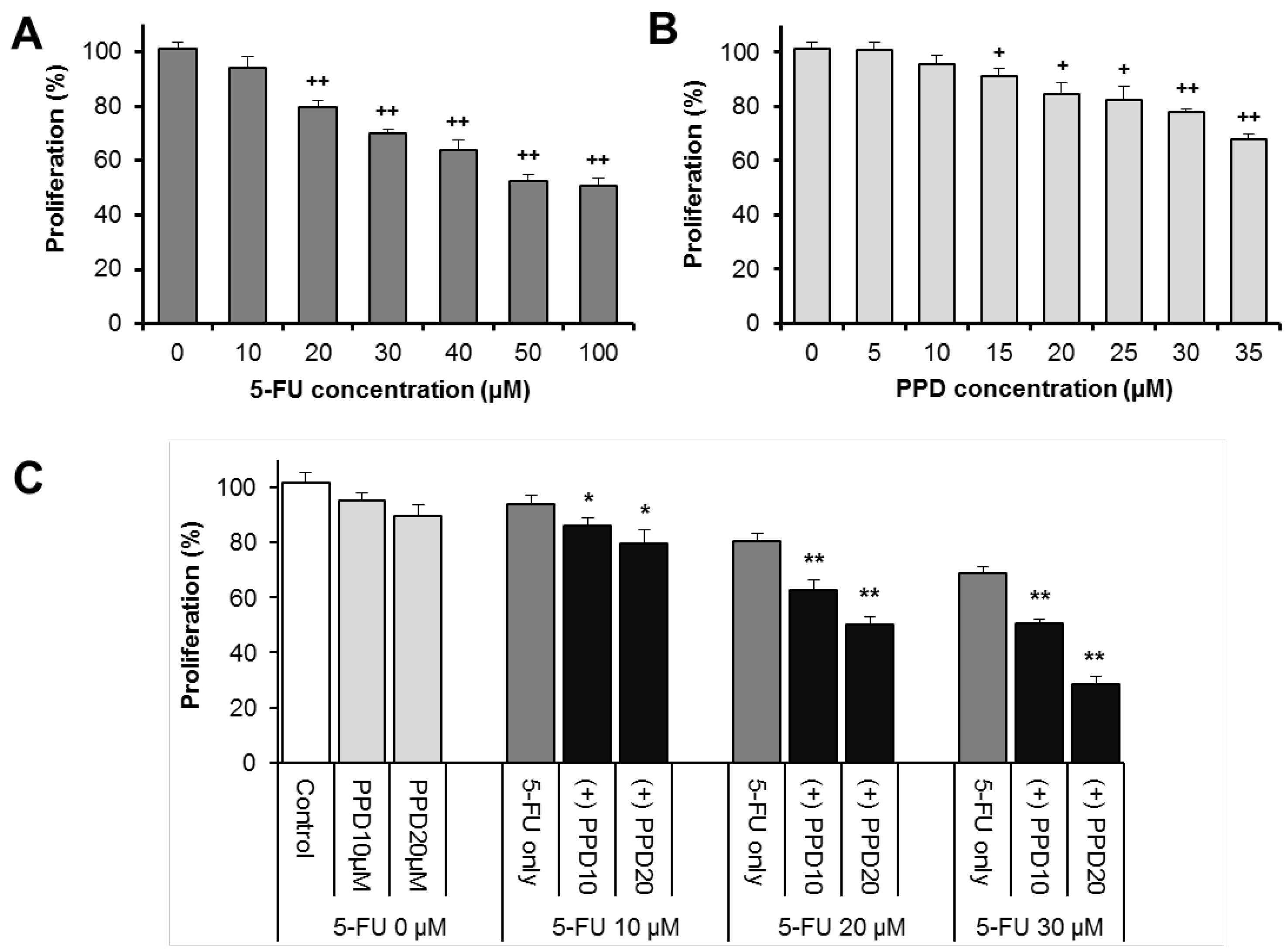
3.2. Effects of PPD and 5-FU on HCT-116 Colon Cell Proliferation
3.3. Effect of PPD and 5-FU on Cell Cycle

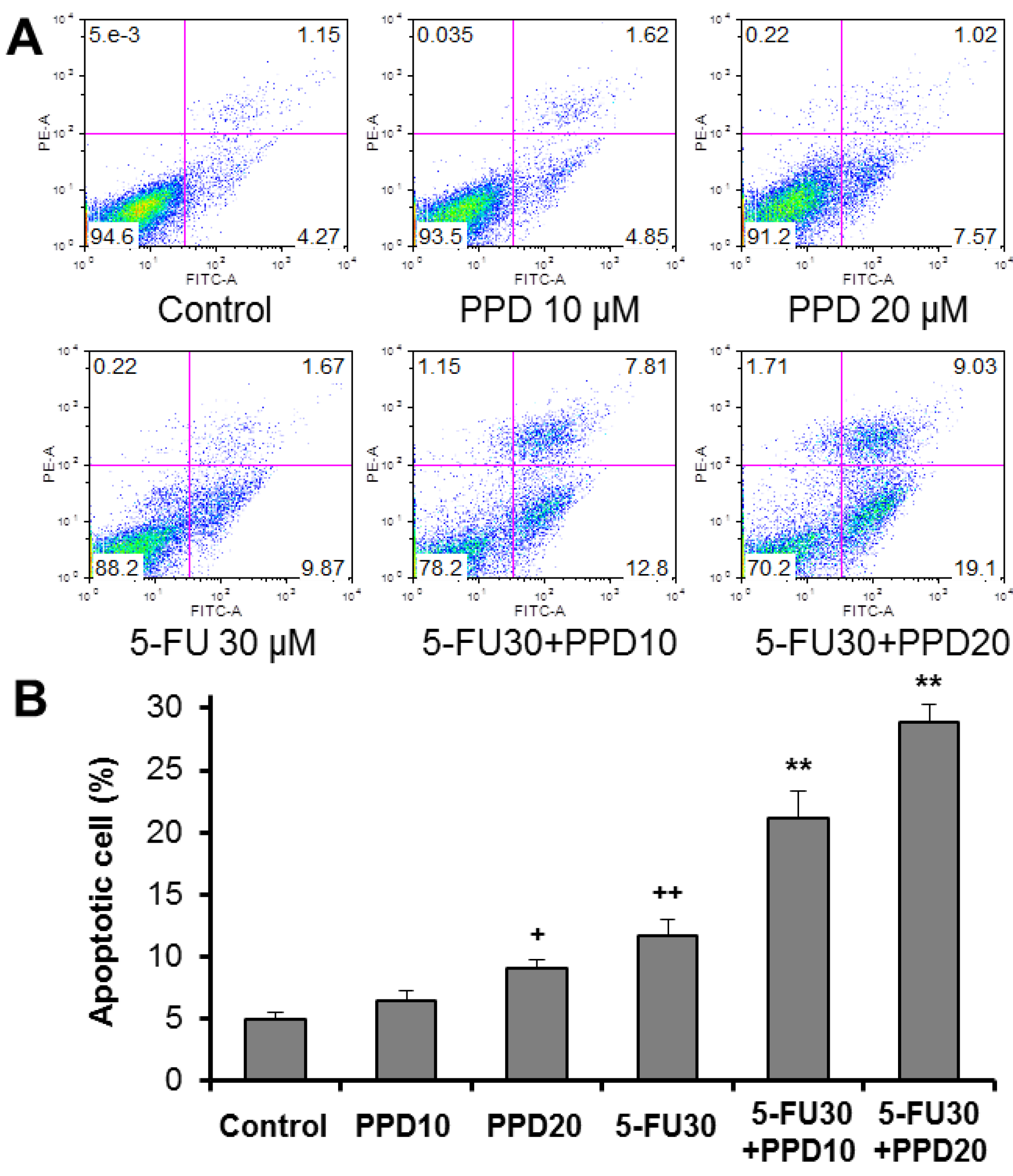
3.4. Apoptotic Effects of PPD and 5-FU on HCT-116 Cells
3.5. Effects of PPD and 5-FU on Colon Tumor Growth Inhibition using Xenogen Bioluminescence Imaging
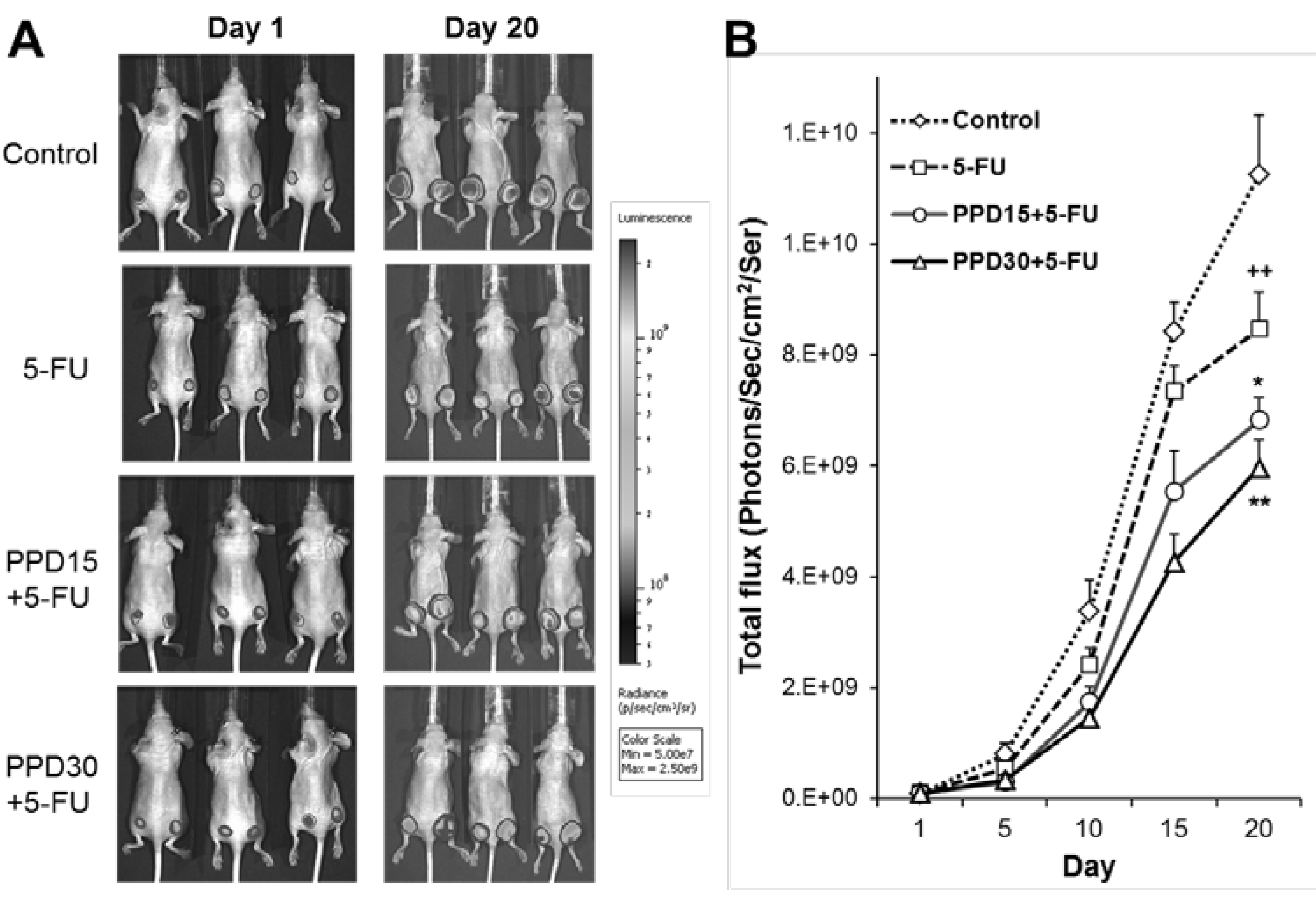
3.6. Effects of PPD and 5-FU on Colon Tumor Size and Tumor Weight Changes
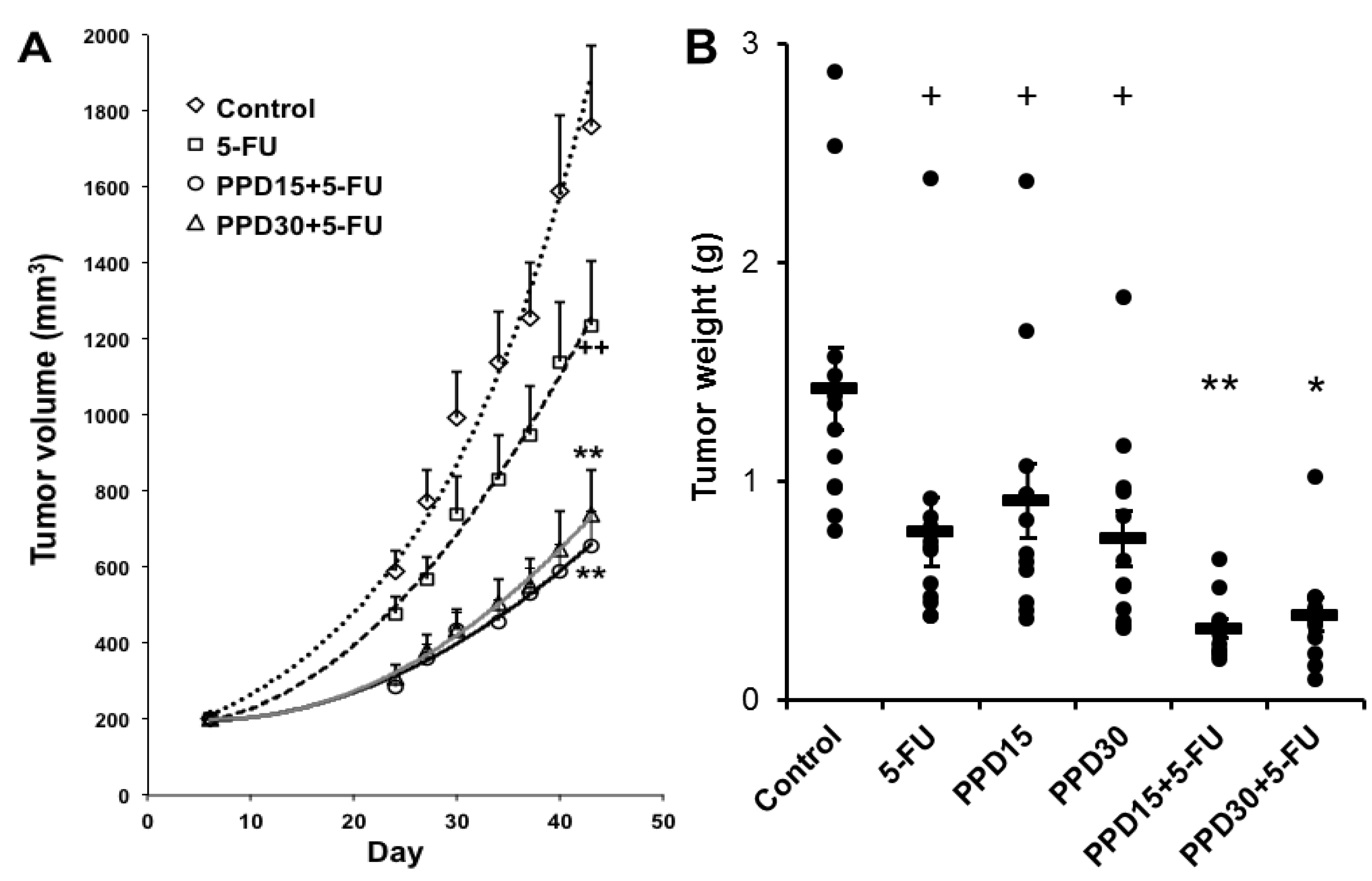
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, M.H.; Kim, E.H.; Lee, E.K.; Park, I.S.; Yang, D.C.; Jun, H.S. Efficacy comparison of Korean ginseng and American ginseng on body temperature and metabolic parameters. Am. J. Chin. Med. 2014, 42, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, H.Y.; Zhang, H.; Wang, C.Z.; Li, P.; Yuan, C.S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1230, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.K.; Choi, S.Y. Preventive effect of ginseng intake against various human cancers: A case-control study on 1987 pairs. Cancer Epidemiol. Biomark. Prev. 1995, 4, 401–408. [Google Scholar]
- Yun, T.K. Panax ginseng—A non-organ-specific cancer preventive? Lancet Oncol. 2001, 2, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhang, B.; Song, W.X.; Wang, A.; Ni, M.; Luo, X.; Aung, H.H.; Xie, J.T.; Tong, R.; He, T.C.; et al. Steamed American ginseng berry: Ginsenoside analyses and anticancer activities. J. Agric. Food Chem. 2006, 54, 9936–9942. [Google Scholar]
- Wang, C.Z.; Aung, H.H.; Ni, M.; Wu, J.A.; Tong, R.; Wicks, S.; He, T.C.; Yuan, C.S. Red American ginseng: Ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007, 73, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Du, G.J.; Zhang, Z.; Wen, X.D.; Calway, T.; Zhen, Z.; Musch, M.W.; Bissonnette, M.; Chang, E.B.; Yuan, C.S. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. Int. J. Oncol. 2012, 40, 1970–1976. [Google Scholar] [PubMed]
- Gao, J.L.; Lv, G.Y.; He, B.C.; Zhang, B.Q.; Zhang, H.; Wang, N.; Wang, C.Z.; Du, W.; Yuan, C.S.; He, T.C. Ginseng saponin metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncol. Rep. 2013, 30, 292–298. [Google Scholar] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Kinross, J.; Gibson, G.R.; Burcelin, R.; Jia, W.; Pettersson, S.; Nicholson, J.K. Therapeutic modulation of microbiota-host metabolic interactions. Sci. Transl. Med. 2012, 4, 137rv6. [Google Scholar] [CrossRef]
- Wan, J.Y.; Liu, P.; Wang, H.Y.; Qi, L.W.; Wang, C.Z.; Li, P.; Yuan, C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1286, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, U.; Mustafi, R.; Wang, Y.; Musch, M.W.; Wang, C.Z.; Konda, V.J.; Kulkarni, A.; Hart, J.; Dawson, G.; Kim, K.E.; et al. American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: Role of EGFR. BMC Complement. Altern. Med. 2011, 11, 111. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sung, J.H.; Matsumiya, S.; Uchiyama, M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996, 62, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Park, S.Y.; Kim, D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol. Pharm. Bull. 2000, 23, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.J. Complementary and alternative therapies in cancer symptom management. Cancer Pract. 2002, 10, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Bai, X.Y.; Wang, C.H. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.S.; Wei, G.; Dey, L.; Karrison, T.; Nahlik, L.; Maleckar, S.; Kasza, K.; Ang-Lee, M.; Moss, J. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: A randomized, controlled Trial. Ann. Intern. Med. 2004, 141, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Qiao, J.; Zhu, M.; Du, J.; Shang, W.; Yin, W.; Wang, W.; Han, M.; Lu, W. Preliminary evaluation of the interactions of Panax ginseng and Salvia miltiorrhiza Bunge with 5-fluorouracil on pharmacokinetics in rats and pharmacodynamics in human cells. Am. J. Chin. Med. 2013, 41, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, H.M.; Peters, G.F. Fluorouracil: Biochemistry and pharmacology. J. Clin. Oncol. 1988, 6, 1653–1664. [Google Scholar] [PubMed]
- Smorenburg, C.H.; Peters, G.J.; van Groeningen, C.J.; Noordhuis, P.; Smid, K.; van Riel, A.M.; Dercksen, W.; Pinedo, H.M.; Giaccone, G. Phase II study of tailored chemotherapy for advanced colorectal cancer with either 5-fluouracil and leucovorin or oxaliplatin and irinotecan based on the expression of thymidylate synthase and dihydropyrimidine dehydrogenase. Ann. Oncol. 2006, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Luo, X.; Zhang, B.; Song, W.X.; Ni, M.; Mehendale, S.; Xie, J.T.; Aung, H.H.; He, T.C.; Yuan, C.S. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother. Pharmacol. 2007, 60, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wang, C.Z.; Mehendale, S.R.; Sun, S.; Wang, Q.; Yuan, C.S. Panaxadiol, a purified ginseng component, enhances the anti-cancer effects of 5-fluorouracil in human colorectal cancer cells. Cancer Chemother. Pharmacol. 2009, 64, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Hong, S.W.; Kim, Y.; Jang, S.E.; Kim, N.J.; Han, M.J.; Kim, D.H. Metabolic activities of ginseng and its constituents, ginsenoside rb1 and rg1, by human intestinal microflora. J. Ginseng Res. 2011, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Wang, M.; Venema, K.; Maathuis, A.; van der Heijden, R.; van der Greef, J.; Xu, G.; Hankemeier, T. Bioconversion of red ginseng saponins in the gastro-intestinal tract in vitro model studied by high-performance liquid chromatography-high resolution Fourier transform ion cyclotron resonance mass spectrometry. J. Chromatogr. A 2009, 1216, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- He, B.C.; Gao, J.L.; Zhang, B.Q.; Luo, Q.; Shi, Q.; Kim, S.H.; Huang, E.; Gao, Y.; Yang, K.; Wagner, E.R.; et al. Tetrandrine inhibits Wnt/beta-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol. Pharmacol. 2011, 79, 211–219. [Google Scholar]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Macarulla, T.; Sauri, T.; Tabernero, J. Evaluation of aflibercept in the treatment of metastatic colorectal cancer. Expert Opin. Biol. Ther. 2014, 14, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qu, X.; Wan, P.; Li, Q.W.; Wang, Z.; Guo, F.; Bai, L.; Hu, Z.; Tan, W.; Li, J. Norcantharidin inhibits pre-replicative complexes assembly of HepG2 cells. Am. J. Chin. Med. 2013, 41, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.J.; Liu, Y.; Cui, Y.F. PI3K/AKT/mTOR signaling is involved in (−)-epigallocatechin-3-gallate-induced apoptosis of human pancreatic carcinoma cells. Am. J. Chin. Med. 2013, 41, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.D.; Wang, C.Z.; Yu, C.; Zhang, Z.; Calway, T.; Wang, Y.; Li, P.; Yuan, C.S. Salvia miltiorrhiza (dan shen) significantly ameliorates colon inflammation in dextran sulfate sodium induced colitis. Am. J. Chin. Med. 2013, 41, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Pae, H.O.; Choi, B.M.; Seo, E.A.; Kim, D.H.; Shin, M.K.; Kim, J.D.; Kim, J.B.; Chung, H.T. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004, 205, 23–29. [Google Scholar]
- Wang, C.Z.; Li, B.; Wen, X.D.; Zhang, Z.; Yu, C.; Calway, T.D.; He, T.C.; Du, W.; Yuan, C.S. Paraptosis and NF-kappaB activation are associated with protopanaxadiol-induced cancer chemoprevention. BMC Complement. Altern. Med. 2013, 13, 2. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Mayer, R.J. Systemic treatment of colorectal cancer. Gastroenterology 2008, 134, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, M.; Martignoni, G.; Frontini, L.; Zonato, S.; Pavia, G.; Beretta, G. Increasing doses of 5-fluorouracil and high-dose folinic acid in the treatment of metastatic colorectal cancer. Tumori 1998, 84, 662–665. [Google Scholar] [PubMed]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.Z.; Huang, W.H.; Li, S.P.; He, T.C.; Yuan, C.S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef]
- Jin, H.R.; Liao, Y.; Li, X.; Zhang, Z.; Zhao, J.; Wang, C.Z.; Huang, W.H.; Li, S.P.; Yuan, C.S.; Du, W. Anticancer compound oplopantriol A kills cancer cells through inducing ER stress and BH3 proteins Bim and Noxa. Cell Death Dis. 2014, 5, e1190. [Google Scholar] [CrossRef]
- Tiffen, J.C.; Bailey, C.G.; Ng, C.; Rasko, J.E.; Holst, J. Luciferase expression and bioluminescence does not affect tumor cell growth in vitro or in vivo. Mol. Cancer 2010, 9, 299. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Z.; Zhang, Z.; Wan, J.-Y.; Zhang, C.-F.; Anderson, S.; He, X.; Yu, C.; He, T.-C.; Qi, L.-W.; Yuan, C.-S. Protopanaxadiol, an Active Ginseng Metabolite, Significantly Enhances the Effects of Fluorouracil on Colon Cancer. Nutrients 2015, 7, 799-814. https://doi.org/10.3390/nu7020799
Wang C-Z, Zhang Z, Wan J-Y, Zhang C-F, Anderson S, He X, Yu C, He T-C, Qi L-W, Yuan C-S. Protopanaxadiol, an Active Ginseng Metabolite, Significantly Enhances the Effects of Fluorouracil on Colon Cancer. Nutrients. 2015; 7(2):799-814. https://doi.org/10.3390/nu7020799
Chicago/Turabian StyleWang, Chong-Zhi, Zhiyu Zhang, Jin-Yi Wan, Chun-Feng Zhang, Samantha Anderson, Xin He, Chunhao Yu, Tong-Chuan He, Lian-Wen Qi, and Chun-Su Yuan. 2015. "Protopanaxadiol, an Active Ginseng Metabolite, Significantly Enhances the Effects of Fluorouracil on Colon Cancer" Nutrients 7, no. 2: 799-814. https://doi.org/10.3390/nu7020799



