Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution
Abstract
:1. Introduction
2. Diet and the Shaping of the Gut Microbiota
3. The Immunity-Diet-Microbiome Consortium: Towards T1D in Early Life
4. The Diabetogenic Microbiome
| Country/Ethnicity | Diagnostic (n) | Age in Years | Microbiota Diversity in Autoimmunity/T1D | Microbiota Relative Abundance in Autoimmunity/T1D | Other Findings |
|---|---|---|---|---|---|
| Finland (DIPP Study)/Caucasians [43,50] | β-cell AI (4) HC (4) | 0–2 0–3 | Reduced | F/B ratio ↓ Increase in: Bacteroides genus, mainly Bacteroides ovatus. Decrease in: Prevotella and Faecalibacterium | Non-butyrate producers avoid optimal mucine synthesis in T1D-associated autoimmunity. |
| Spain/Caucasians [51] | T1D at onset (16) HC (16) | 7.16 ± 0.72 7.48 ± 0.87 | Similar to the control group (p > 0.05) | F/B ratio ↓ Increase in: Clostridium, Bacteroides and Veionella. Decrease in: Lactobacillus, Bifidobacterium and Prevotella. | Microbiota differences were associated with glycemic level. |
| Finland (FINDIA and TRIGR studies)/Caucasians [44] | β-cell AI (18) HC (18) | FINDIA/TRIGR: 5.1 ± 1/13.3 ± 1 FINDIA/TRIGR: 5.0 ± 2/12.8 ± 1 | Reduced | F/B ratio ↓ Increase in: Bacteroides genus, Clostridium perfringens. Decrease in: Bifidobacterium adolescentis and Bifidobacterium pseudocatenulatum. | The abundance of lactate- and butyrate-producing bacteria was inversely related to the number of β cell autoantibodies. |
| Mexico/Mestizos [18] | T1D at onset (8) T1D ≥ 2 years evolution (13) HC (8) | 12.3 ± 0.64 11 ± 1.04 | Similar to the control group (p > 0.05) | Unaltered F/B ratio. Increase in: Bacteroides genus. Decrease in: Prevotella, Acidaminococcus and Megamonas. | The glycemic control in the T1D ≥ 2 years treated group partially normalizes the microbiotal profile towards Prevotella-dominant profile. |
| Finland (DIPP Study)/Caucasians [47] | High risk cohort (76): β-cell AI (29) T1D at onset (22) HC (47) | 0–2 | Reduced | F/B ratio ↓ Increase: in Bacteroides genus due to Bacteroides dorei and Bacteroides vulgatus. | B.dorei abundance peaked over 8 months prior to the appearance of the first islet auto antibody. It coincided with the introduction of solid foods. |
| Finland, Estonia (DIABIMMUNE Study)/Caucasians [45] | High risk cohort (33): β-cell AI (7) T1D at onset (4) HC (22) | 0–3 | Reduced | Increase in: Blautia, Rikenellaceae, Ruminococcus gnavus and Streptococcus infantarius in T1D cases at the time of alpha-diversity divergence. | Decreased community diversity occurs after seroconversion but before onset of T1D. T1D onset is preceded by increased inflammation-assoc. organisms and pathways. |
| USA/White Americans (TRIALNET Study) [46] | β-cell AI (21) T1D at onset (35) Seroneg. FDR (32) HC (23) | 4–49 2–20 3–45 4–24 | Similar to the control group (p > 0.05) | Increase in: Bacteroides. Decrease in: Prevotella * In seropositive subjects with multiple versus one autoantibody. | The microbiomes of β-cell AI and seroneg. FDRs clustered together but separate from those of T1D at onset and HC. |
5. Microbiota: Molecular Mechanisms in T1D
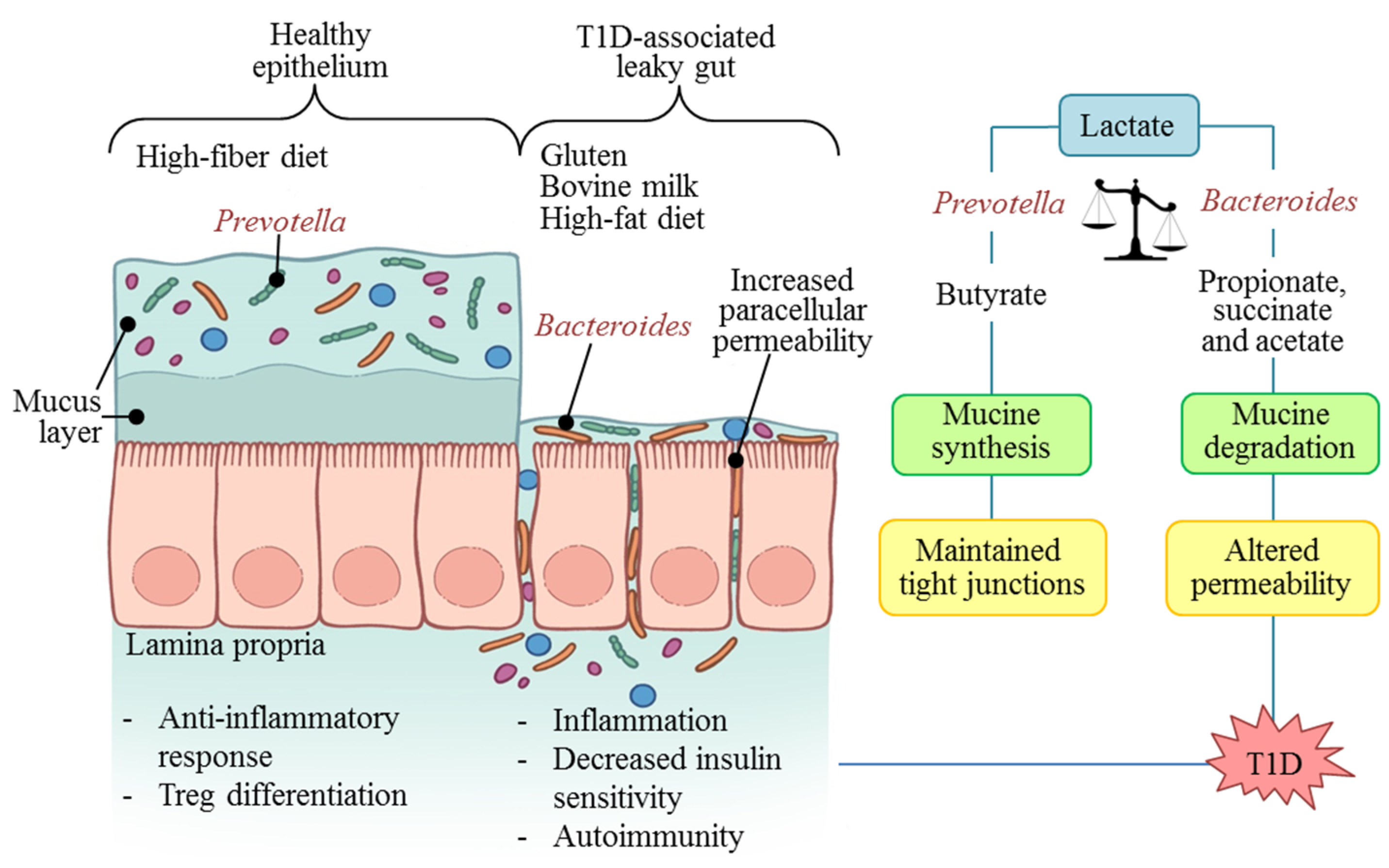
6. T1D Prevention and Control Possibilities
6.1. Primary Prevention of T1D
6.2. Secondary and Tertiary Prevention of T1D
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Standards of medical care in diabetes—2015: Summary of revisions. Diabetes Care 2015, 38, S4. [Google Scholar] [PubMed]
- Pettitt, D.J.; Talton, J.; Dabelea, D.; Divers, J.; Imperatore, G.; Lawrence, J.M.; Liese, A.D.; Linder, B.; Mayer-Davis, E.J.; Pihoker, C.; et al. Prevalence of diabetes in U.S. Youth in 2009: The search for diabetes in youth study. Diabetes Care 2014, 37, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Kantarova, D.; Buc, M. Genetic susceptibility to type 1 diabetes mellitus in humans. Physiol. Res. 2007, 56, 255–266. [Google Scholar] [PubMed]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.H.; Vehik, K.; Gesualdo, P.; Akolkar, B.; Hagopian, W.; Krischer, J.; Lernmark, Å.; Rewers, M.; Simell, O.; She, J.-X.; et al. Children followed in the teddy study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr. Diabetes 2014, 15, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet 2001, 358, 221–229. [Google Scholar] [CrossRef]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O. Is the origin of type 1 diabetes in the gut? Immunol. Cell Biol. 2012, 90, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Salvatori, G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell. Infect. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Blaser, M.J.; Ley, R.E.; Knight, R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 2011, 140, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R.; Poulsen, S.K.; Larsen, T.M.; Bahl, M.I. Microbial enterotypes, inferred by the prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new nordic diet. Appl. Environ. Microbiol. 2014, 80, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Leon, M.E.; Petrosino, J.F.; Ajami, N.J.; Dominguez-Bello, M.G.; Calderon de la Barca, A.M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.-H.; Haryono, P.; La-ongkham, O.; Sarwoko, M.-A.; Sujaya, I.N.; Zhao, L.; et al. Diversity in gut bacterial community of school-age children in Asia. Sci. Rep. 2015, 5, 8397. [Google Scholar] [CrossRef] [PubMed]
- La-ongkham, O.; Nakphaichit, M.; Leelavatcharamas, V.; Keawsompong, S.; Nitisinprasert, S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch. Microbiol. 2015, 197, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Knights, D.; Ward, T.L.; McKinlay, C.E.; Miller, H.; Gonzalez, A.; McDonald, D.; Knight, R. Rethinking “enterotypes”. Cell Host Microb. 2014, 16, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Disease 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Perdijk, O.; van Neerven, R.J.J.; Savelkoul, H.J. Mucosal immune development in early life: Setting the stage. Arch. Immunol. Ther. Exp. 2015, 63, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Calderón de la Barca, A.M.; Mejia-León, M.E. Are dietary caseins related to the onset and evolution of type 1 diabetes and celiac disease? In Casein: Production, Uses and Health Effects, 1st ed.; Ventimiglia, A.M., Birkenhäger, J.M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; Volume 1, pp. 195–208. [Google Scholar]
- Longman, R.S.; Yang, Y.; Diehl, G.E.; Kim, S.V.; Littman, D.R. Microbiota: Host interactions in mucosal homeostasis and systemic autoimmunity. Cold Spring Harbor Symp. Quant. Biol. 2013, 78, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. The gut as communicator between environment and host: Immunological consequences. Eur. J. Pharmacol. 2011, 668 (Suppl. 1), S16–S32. [Google Scholar] [CrossRef] [PubMed]
- Cerf-Bensussan, N.; Gaboriau-Routhiau, V. The immune system and the gut microbiota: Friends or foes? Nat. Rev. Immunol. 2010, 10, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Young, C.; Neu, J. Molecular modulation of intestinal epithelial barrier: Contribution of microbiota. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.C.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3074–3079. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.M.; Nevalainen, J.; Kronberg-Kippilä, C.; Ahonen, S.; Tapanainen, H.; Uusitalo, L.; Takkinen, H.-M.; Niinistö, S.; Ovaskainen, M.-L.; Kenward, M.G.; et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: A nested case-control design. Am. J. Clin. Nutr. 2012, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.M.; Miller, M.; Seifert, J.A.; Frederiksen, B.; Kroehl, M.; Rewers, M.; Norris, J.M. The effect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: The diabetes autoimmunity study in the young. Pediatr. Diabetes 2015, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, B.E.; Hill, J.P.; Thorsson, A.V.; Thorsdottir, I. Lower consumption of cow milk protein A1 β-casein at 2 years of age, rather than consumption among 11- to 14-year-old adolescents, may explain the lower incidence of type 1 diabetes in Iceland than in Scandinavia. Ann. Nutr. Metab. 2006, 50, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Davis-Richardson, A.; Triplett, E. A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia 2015, 58, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Weile, C.; Antvorskov, J.C.; Engkilde, K.; Nielsen, S.M.B.; Josefsen, K.; Buschard, K. Effect of dietary gluten on dendritic cells and innate immune subsets in BALB/c and nod mice. PLoS ONE 2015, 10, e0118618. [Google Scholar] [CrossRef] [PubMed]
- Mejía-León, M.E.; Calderón de la Barca, A.M. Serum IgG subclasses against dietary antigens in children with type 1 diabetes. Pediatr. Diabetes 2015. submitted for publication. [Google Scholar]
- Hamari, S.; Kirveskoski, T.; Glumoff, V.; Kulmala, P.; Simell, O.; Knip, M.; Ilonen, J.; Veijola, R. CD4+ T-cell proliferation responses to wheat polypeptide stimulation in children at different stages of type 1 diabetes autoimmunity. Pediatr. Diabetes 2015, 16, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Klatter, F.A.; Visser, J.T.J.; Wildeboer-Veloo, A.C.M.; Harmsen, H.J.M.; Rozing, J.; Bos, N.A. Antibiotic treatment partially protects against type 1 diabetes in the bio-breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 2006, 49, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Candon, S.; Perez-Arroyo, A.; Marquet, C.; Valette, F.; Foray, A.-P.; Pelletier, B.; Milani, C.; Ventura, M.; Bach, J.-F.; Chatenoud, L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 2015, 10, e0125448. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Godovannyi, A.; Ma, C.; Zhang, Y.; Ahmadi-Vand, Z.; Dai, C.; Gorzelak, M.A.; Chan, Y.; Chan, J.M.; Lochner, A.; et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in nod mice. ISME J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyoty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- De Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microb. 2015, 17, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Alkanani, A.K.; Hara, N.; Gottlieb, P.A.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 2015, 64, 3510–3520. [Google Scholar] [CrossRef] [PubMed]
- Davis-Richardson, A.G.; Ardissone, A.N.; Dias, R.; Simell, V.; Leonard, M.T.; Kemppainen, K.M.; Drew, J.C.; Schatz, D.; Atkinson, M.A.; Kolaczkowski, B.; et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 2014. [Google Scholar] [CrossRef]
- Virtanen, S.M.; Ylonen, K.; Rasanen, L.; Ala-Venna, E.; Maenpaa, J.; Akerblom, H.K. Two year prospective dietary survey of newly diagnosed children with diabetes aged less than 6 years. Arch. Dis. Childh. 2000, 82, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the american heart association nutrition committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R. The microbiome in early life: Self-completion and microbiota protection as health priorities. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2014, 101, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.; Cardona, F.; Soriguer, F.; Queipo-Ortuno, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourlanos, S.; Harrison, L.C.; Colman, P.G. The accelerator hypothesis and increasing incidence of type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Fourlanos, S.; Narendran, P.; Byrnes, G.B.; Colman, P.G.; Harrison, L.C. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 2004, 47, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; Simell, S.; Sysi-Aho, M.; Näntö-Salonen, K.; Seppänen-Laakso, T.; Parikka, V.; Katajamaa, M.; Hekkala, A.; Mattila, I.; Keskinen, P.; et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J. Exp. Med. 2008, 205, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Mejía-León, M.E.; Ruiz-Dyck, K.M.; Calderón de la Barca, A.M. HLA-DQ genetic risk gradient for type 1 diabetes and celiac disease in North-Western Mexico. Rev. Gastroenterol. de Méx. 2015, 80, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Teddy Study Group. The environmental determinants of diabetes in the young (TEDDY) study. Ann. N. Y. Acad. Sci. 2008, 1150, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S. Toward primary prevention of type 1 diabetes. JAMA 2015, 313, 1520–1521. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.; Michels, A.W. Lessons from type 1 diabetes for understanding natural history and prevention of autoimmune disease. Rheumatic Dis. Clin. N. Am. 2014, 40, 797–811. [Google Scholar] [CrossRef] [PubMed]
- De Jesus-Laboy, K.M.; Cox, L.M.; Rodriguez-Rivera, S.M.; Rivera-Vinas, J.; Mendez, K.; Clemente, J.C.; Knight, R.; Dominguez-Bello, M.G. Restoring the normal microbiota of cesarean-section born infants. In Proceedings of American society for microbiology 114th general meeting, Boston, MA, USA, 18 May 2014; pp. I–741.
- Niinistö, S.; Takkinen, H.M.; Uusitalo, L.; Rautanen, J.; Vainio, N.; Ahonen, S.; Nevalainen, J.; Kenward, M.G.; Lumia, M.; Simell, O.; et al. Maternal intake of fatty acids and their food sources during lactation and the risk of preclinical and clinical type 1 diabetes in the offspring. Acta Diabetol. 2015, 52, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Virtanen, S.M.; Becker, D.; Dupré, J.; Krischer, J.P.; Åkerblom, H.K. Early feeding and risk of type 1 diabetes: Experiences from the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk (TRIGR). Am. J. Clin. Nutr. 2011, 94, 1814S–1820S. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.; Frederiksen, B.; Seifert, J.; Kroehl, M.; Rewers, M.; Norris, J. Sugar intake is associated with progression from islet autoimmunity to type 1 diabetes: The diabetes autoimmunity study in the young. Diabetologia 2015, 58, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shan, Y.; Song, W. Targeting gut microbiota as a possible therapy for diabetes. Nutr. Res. 2015, 35, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.; Bird, A. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Bruno, G.; Petito, V.; Franceschi, F.; Gasbarrini, A. The therapeutic management of gut barrier leaking: The emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1068–1076. [Google Scholar] [PubMed]
- Vardanyan, M.; Parkin, E.; Gruessner, C.; Rodriguez Rilo, H.L. Pancreas vs. Islet transplantation: A call on the future. Curr. Opin. Organ Transpl. 2010, 15, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; Brayman, K.L. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Transl. Med. 2013, 2, 328–336. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-León, M.E.; Barca, A.M.C.d.l. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients 2015, 7, 9171-9184. https://doi.org/10.3390/nu7115461
Mejía-León ME, Barca AMCdl. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients. 2015; 7(11):9171-9184. https://doi.org/10.3390/nu7115461
Chicago/Turabian StyleMejía-León, María E., and Ana M. Calderón de la Barca. 2015. "Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution" Nutrients 7, no. 11: 9171-9184. https://doi.org/10.3390/nu7115461






