Time and Dose-Dependent Effects of Labisia pumila on Bone Oxidative Status of Postmenopausal Osteoporosis Rat Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Treatment
2.2. Labisia Pumila var. Alata (LP) and Estrogen (ERT)
2.3. Bone Sampling
2.4. Measurement of Superoxide Dismutase (SOD) Enzyme
2.5. Measurement of Glutathione Peroxidase (GPx)
2.6. Measurement of Malondialdehyde (MDA)
2.7. Statistical Analysis
3. Results
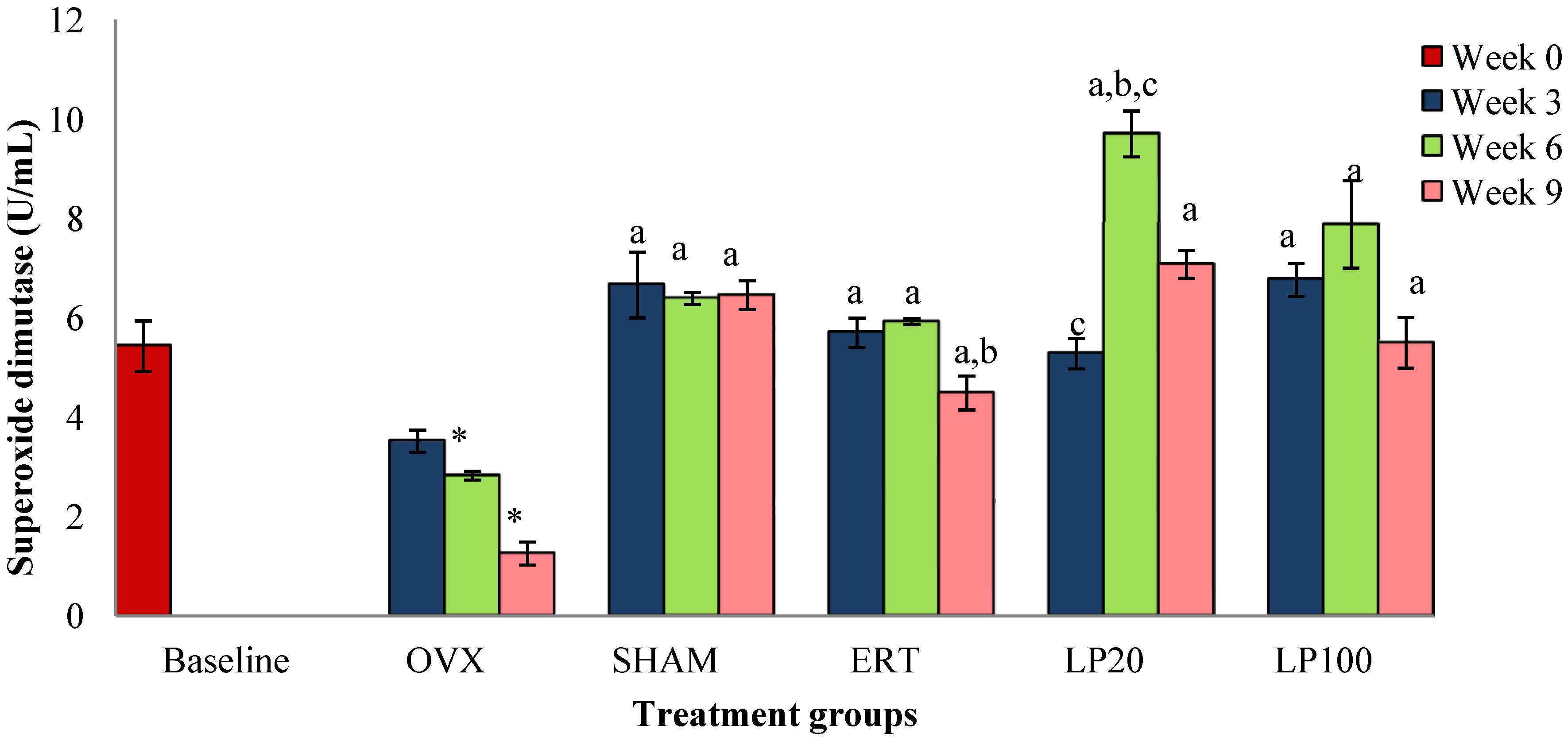
3.1. Superoxide Dismutase (SOD)
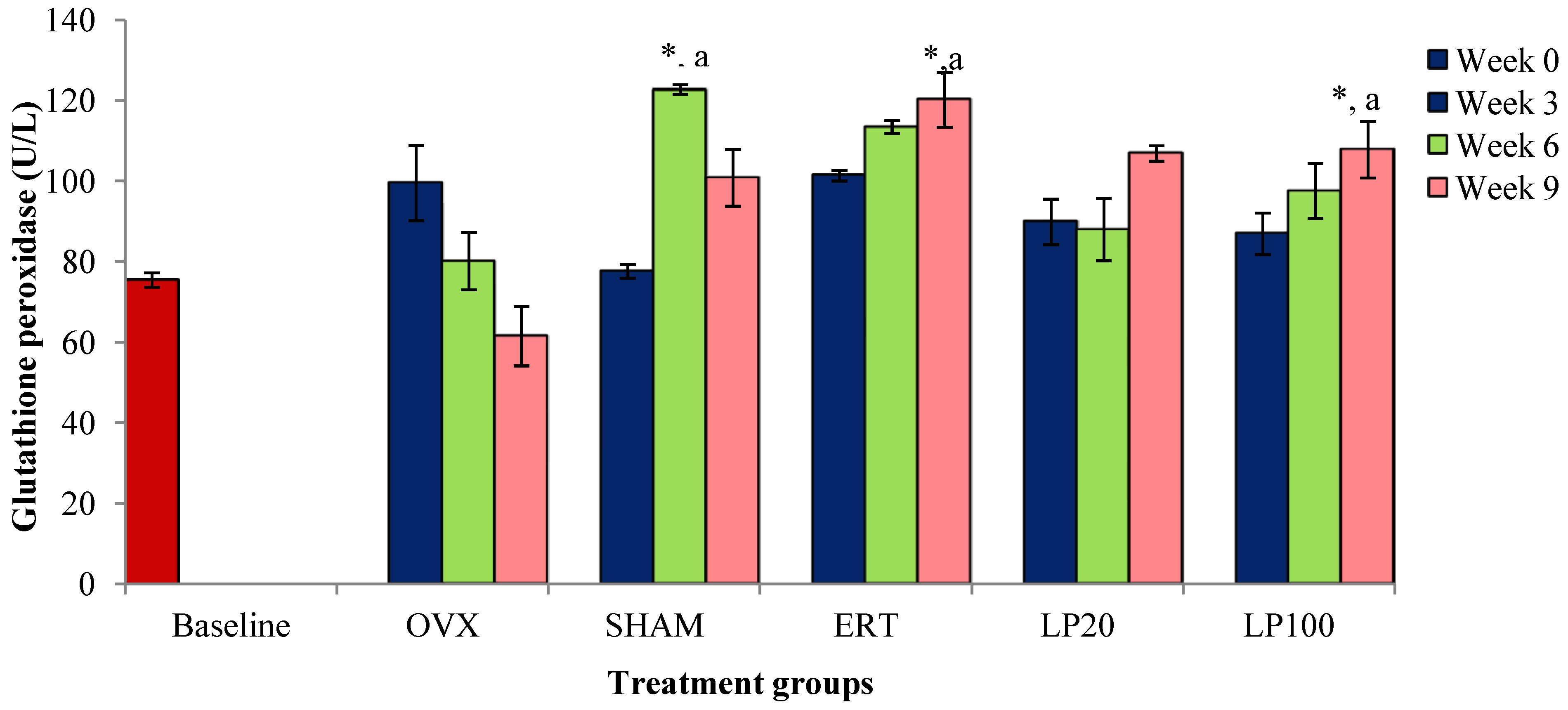
3.2. Glutathione Peroxidase (GPx)
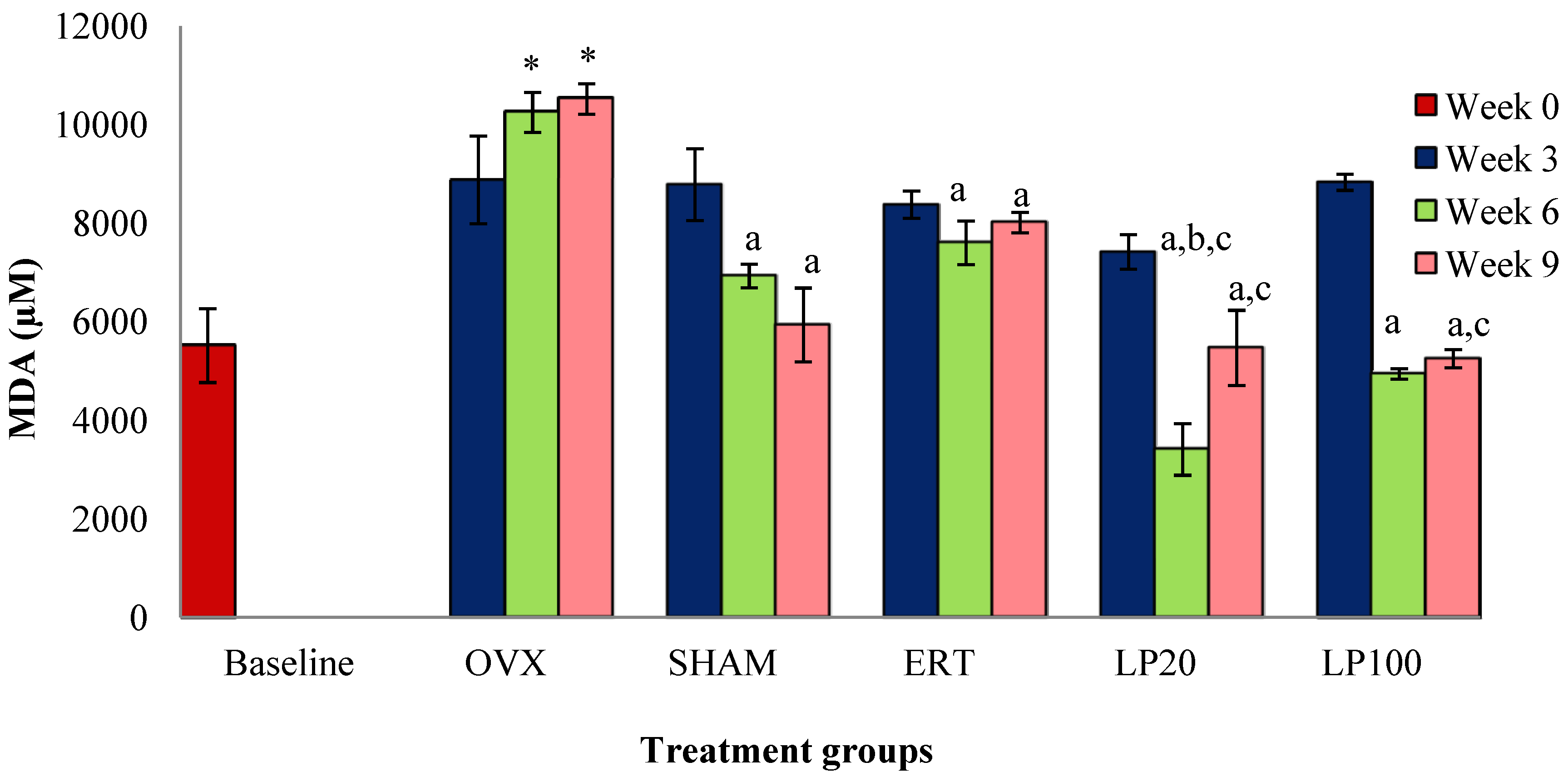
3.3. Lipid Peroxidation (MDA)a
4. Discussion
5. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Christiansen, C. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Helfand, M.; Woolf, S.H.; Allan, J.D. Screening for postmenopausal osteoporosis: A review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2002, 137, 529–541. [Google Scholar]
- Laura, A.G.; Robert, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Neri, S.; Sciacchitano, S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas 2006, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, M.A.; Zacarias-Flores, M.; Arronte-Rosales, A.; Correa-Munoz, E.; Mendoza-Nunez, V.M. Menopause as risk factor for oxidative stress. Menopause 2011, 19, 361–367. [Google Scholar]
- Rao, A.V.; Rao, L.G. Carotenoids and human health (Review). Pharmacol. Res. 2007, 55, 207–216. [Google Scholar]
- Zhang, Y.B.; Zhong, Z.M.; Hou, G.; Jiang, H.; Chen, J.T. Involvement of oxidative stress in age-related bone loss. J. Surg. Res. 2011, 169, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Jagger, C.J.; Lean, J.M.; Davies, J.T.; Chambers, T.J. Tumor necrosis factor mediates osteopenia caused by depletion of antioxidants. Endocrinology 2005, 146, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; O’Brien, C.A. Basic biology of skeletal aging: Role of stress response pathways. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1197–1208. [Google Scholar] [CrossRef]
- Bellanti, F.; Matteo, M.; Rollo, T.; de Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex hormone modulate circulating antioxidant enzyme: Impact of estrogen therapy. Redox. Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Kousteni, S.; Jilka, R.L. Sex steroids and bone. Recent. Prog. Horm. Res. 2002, 57, 385–409. [Google Scholar] [CrossRef] [PubMed]
- Wassertheil-Smoller, S.; Hendrix, S.L.; Limacher, M.; Heiss, G.; Kooperberg, C.; Baird, A.; Kotchen, T.; Curb, J.D.; Black, H.; Rossouw, J.E.; et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. JAMA 2003, 289, 2673–2684. [Google Scholar]
- Reiner, B.; Bertha, F.; Emmo, V.T.; Christoph, B. Biphosphonates in Medical Practice; Springer: New York, NY, USA, 2007. [Google Scholar]
- Delmas, P.D.; Ensrud, K.E.; Adachi, J.D.; Harper, K.D.; Sarkar, S.; Gennari, C.; Reginster, J. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: Four-year results from a randomized clinical trial. J. Clin. Endocr. Metab. 2002, 87, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Marcea, M.; Jia, G.; Theresa, K.; George, B. Biphosphonates for Osteoporosis—Where do we go from here? NEJM 2012, 366, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Norliza, M.; Douglas, A.L.; Nazrun, A.S.; Norazlina, M.; Ima-Nirwana, S. Tocotrienol supplementation in postmenopausal osteoporosis: Evidence from a laboratory study. Clinics 2013, 68, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Lucas, E.A.; Khalil, D.A.; Devareddy, L.; Smith, B.J.; McDonalds, J.; Arquitt, A.B.; Payton, M.E.; Mason, C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr. J. 2005, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devareddy, L.; Hooshmad, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Jamia, A.J.; Houghton, P.J.; Milligan, S.R.; Ibrahim, J. The Oestrogenic and Cytotoxic Effects of the Extracts of Labisia pumila var. alata and Labisia pumila var. pumila in Vitro. MJMS 1988, 1, 53–60. [Google Scholar]
- Zakaria, M.; Mohd, M.A. Traditional Malay MedicinalPlants; Penerbit Fajar Bakti: Kuala Lumpur, Malaysia, 1994; Volume 8. [Google Scholar]
- Jamal, J.A.; Houghton, P.J.; Milligan, S.R. Testing of labisia pumila for oestrogenic activity using a recombinant yeast screen. J. Pharm. Pharmacol. 1998, 50, 79. [Google Scholar] [CrossRef]
- Nadia, M.E.; Nazrun, A.S.; Norazlina, M.; Isa, N.M.; Norliza, M.; Ima-Nirwana, S. The anti-inflammatory, phytoestrogenic and antioxidative role of Labisia pumila in prevention of postmenopausal osteoporosis. Adv. Pharmacol. Sci. 2012, 2012, 1–7. [Google Scholar]
- Nazrun, A.S.; Lee, P.L.; Norliza, M.; Norazlina, M.; Ima-Nirwana, S. The effects of Labisia pumila var. alata on bone markers and bone calcium in a rat model of post-menopausal osteoporosis. J. Ethnopharmacol. 2011, 133, 538–542. [Google Scholar]
- Mlakar, S.J.; Osredkar, J.; Prezeli, J.; Marc, J. Antioxidant enzymes GSR, SOD1, SOD2 and Cat gene variants and bone mineral density values in postmenopausal women: A genetic association analysis. Menopause 2012, 19, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Method. Mol. Biol. 1998, 108, 101–106. [Google Scholar]
- Cayman Chemical Company. Available online: http://www.caymanchem.com/ (accessed on 21 April 2014).
- BioAssay Systems. EnzyChrom™ Glutathione Peroxidase Assay Kit. Available online: http://www.bioassaysys.com/ (accessed on 21 April 2014).
- Armstrong, D.; Browne, R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds to oxidative stress as applied to the clinical chemistry laboratory. Adv. Exp. Med. Biol. 1994, 366, 43–58. [Google Scholar] [PubMed]
- Bednarek-Tupikowska, G.; Bohdanowicz-Pawlak, B.; Bidzinska, A.; Melewicz, J.; Antonowicz-Juchniewicz, R.; Andrzejak, R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecol. Endocrinol. 2001, 15, 298–303. [Google Scholar]
- Serviddio, G.; Loverro, G.; Vicino, M.; Prigigallo, F.; Grattagliano, I.; Altomare, E.; Vendermiale, G. Modulation of endometrial redox balance during the menstrual cycle: Relation with sex hormones. J. Clin. Endocrinol. Metab. 2002, 87, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.P.; Yang, W.S.; Lee, S.K.; Min, W.K.; Park, J.S.; Kim, S.B. Effects of hormonal replacement therapy on oxidative stress and total antioxidant capacity in postmenopausal hemodialysis patients. Ren Fail. 2002, 24, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Larrea, M.B.; Leal, A.M.; Martin, C.; Martinez, R.; Lacort, M. Antioxidant action of estrogens in rat hepatoctes. Rev. Esp Fisiol. 1997, 53, 225–229. [Google Scholar]
- Borras, C.; Gambini, J.; Gomez-Cabrera, M.C.; Sastre, J.; Pallardó, F.V.; Mann, G.E.; Viña, J. 17 beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFκB cascade. Aging Cell 2005, 4, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Pavlos, P.L.; Theodoros, T.X.; Sofia, E.T.; George, P.L.; Ismene, A.D. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 2005, 146, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Smietana, M.J.; Arruda, E.M.; Faulkner, J.A.; Brooks, S.V.; Larkin, L.M. Reactive oxygen species on bone mineral density and mechanics in Cu, Zn superokxide dismutase (SOD1) knockout mice. Biochem. Biophys. Res. Commun. 2010, 401, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Joint Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E.; Aydogan, R.; Yilmaz, Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell Biochem. 2007, 295, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fathilah, S.N.; Nazrun, A.S.; Norazlina, M.; Norliza, M.; Ima-Nirwana, S. Labisia pumila protects the bone of estrogen-deficient rat model: A histomorphometric study. J. Ethnopharmacol. 2012, 142, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Wan Ezumi, M.F.; Siti Amrah, S.; Suhaimi, A.W.M.; Mohsin, S.S.J. Evaluation of the female reproductive toxicity of aqueous extract of Labisia pumila var. alata in rats. Indian J. Pharmacol. 2007, 39, 30–32. [Google Scholar]
- Singh, G.D.; Ganjoo, M.; Youssouf, M.S.; Koul, A.; Sharma, R.; Singh, S.; Sangwan, P.L.; Koul, S.; Ahamad, D.B.; Johri, R.K. Sub-acute toxicity evaluation of an aqueous extract of Labisia pumila, a Malaysian herb. Food Chem. Toxicol. 2009, 47, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.C. Sub-Chronic (90 days) Oral Toxicity Studies of Aqueous Extract of Labisia pumila in Wistar Rats (250,500 & 1000 mg/kg b. wt. only); Indian Institute of Integrative Medicine: Jammu, India, 2004. [Google Scholar]
- Nadia, M.E.; Fadhli, M.K.; Ima Nirwana, S.; Nazrun, A.S. The fffects of Labisia pumila on postmenopausal osteoporotic rat model: Dose and time-dependent micro-CT analysis. J. X-Ray Sci. Technol. 2014, 22, 503–518. [Google Scholar]
- Muthusami, S.; Ramachandran, I.; Muthusamy, B.; Vasudevan, G.; Prabhu, V.; Subramaniam, V.; Jagadeesan, A.; Narasimhan, S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta 2005, 360, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fazliana, M. Studies on Labisia pumila var. alata Extract with Phytoestrogenic Effects: Impact on Biological Activities and Gene Expression; Karolinska Institutet: Stockholm, Sweden, 2010; pp. 1–4. [Google Scholar]
- Norhaiza, M.; Maziah, M.; Hakiman, M. Antioxidative properties of leaf extracts of a popular Malaysian herb, Labisia pumila. J. Med. Plants Res. 2009, 3, 217–223. [Google Scholar]
- Omer, F.S.; Yasemin, T.; Engin, T.; Mukadder, S. Antioxidant status in patients with osteoporosis: A controlled study. Joint Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fujii, S.; Kosaka, H. Effect of oestrogen on reactive oxygen species production in the aortas of ovariectomized Dahl salt-sensitive rats. J. Hypertens. 2007, 25, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, M.J.; Appel, W.H., Jr.; Verhoeven, A.J.; Karnovsky, M.J. NADPH-oxidase expression and in situ production of superoxideby osteoclasts actively resorbing bone. J. Cell Biol. 1994, 126, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.N.; Tare, R.S. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin. Chim. Acta 2002, 318, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Atlindag, O.; Erel, O.; Soran, N.; Celik, H.; Selek, S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheum Int. 2008, 28, 317–321. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Daniels, J.S.; Rouzer, C.A.; Greene, R.E.; Marnett, L.J. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Itoh, K. Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radic Biol. Med. 2006, 41, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Susanne, P.; Sonja, K.; Mahmoud, K. Nrf2/ARE signaling pathway: Key mediator in oxidative stress and potential therapeutic target in ALS. Neurol. Res. Int. 2012, 2012, 1–7. [Google Scholar]
- Duke, P.; Sandy, S. Thiol antioxidants reduce bone loss in estrogen-deficient female mice. In Life Enhancement; Life Enhancement Magazine: Minden, NV, USA, 2003. [Google Scholar]
- Lee, S.C.; Norliza, A.L.; Sze, Y.L.; Chew, T.L.; Mohamad, R.S.; Ramlan, A.A. Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem. 2011, 127, 1186–1192. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Vitamins E and C, β-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [PubMed]
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A.M. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef]
- Anderson, J.J.B.; Anthony, M.; Messina, M.; Garner, S.C. Effects of phytoestrogens on tissues. Nutr. Res. Rev. 1999, 12, 75–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/beta-catenin canonical wnt signalling. J. Bone Miner Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model. PLoS One 2014, 9, e97177. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Angel, P.; Lafyatis, R.; Hattori, K.; Kim, K.Y.; Sporn, M.B.; Karin, M.; Roberts, A.B. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol. Cell Biol. 1990, 10, 1492–1497. [Google Scholar] [PubMed]
- Meyer, M.; Schreck, R.; Baeuerle, P.A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993, 12, 2005–2015. [Google Scholar] [PubMed]
- Lin, Y.L.; Lin, J.K. Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [PubMed]
- Gonzalez-Gallego, J.; Sanchez-Campoz, S.; Tunon, M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007, 22, 287–293. [Google Scholar]
- Van’t Hof, R.J.; Armour, K.J.; Smith, L.M.; Armour, K.E.; Wei, X.Q.; Liew, F.Y.; Ralston, S.H. Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proce. Natl. Acad. Sci. USA. 2000, 97, 7993–7998. [Google Scholar]
- Hao, Y.J.; Tang, Y.; Chen, F.B.; Pei, F.X. Different doses of nitric oxide donor prevent osteoporosis in ovariectomized rats. Clin. Orthop. Relat. Res. 2005, 435, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Armour, K.E.; Van’T Hof, R.J.; Grabowski, P.S.; Reid, D.M.; Ralston, S.H. Evidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosis. J. Bone Miner Res. 1999, 14, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar]
- Ehsan, K.; Hawa, Z.E.J.; Syahida, A. Antifungal, anti-inflammatory and cytotoxicity activities of three varieties of labisia pumila benth: From microwave obtained extracts. BMC Complement Altern Med. 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Effendy, N.M.; Shuid, A.N. Time and Dose-Dependent Effects of Labisia pumila on Bone Oxidative Status of Postmenopausal Osteoporosis Rat Model. Nutrients 2014, 6, 3288-3302. https://doi.org/10.3390/nu6083288
Effendy NM, Shuid AN. Time and Dose-Dependent Effects of Labisia pumila on Bone Oxidative Status of Postmenopausal Osteoporosis Rat Model. Nutrients. 2014; 6(8):3288-3302. https://doi.org/10.3390/nu6083288
Chicago/Turabian StyleEffendy, Nadia Mohd, and Ahmad Nazrun Shuid. 2014. "Time and Dose-Dependent Effects of Labisia pumila on Bone Oxidative Status of Postmenopausal Osteoporosis Rat Model" Nutrients 6, no. 8: 3288-3302. https://doi.org/10.3390/nu6083288



