Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health
Abstract
:1. Introduction
2. Methods
3. Atherosclerosis Surrogate and Vascular Epidemiology
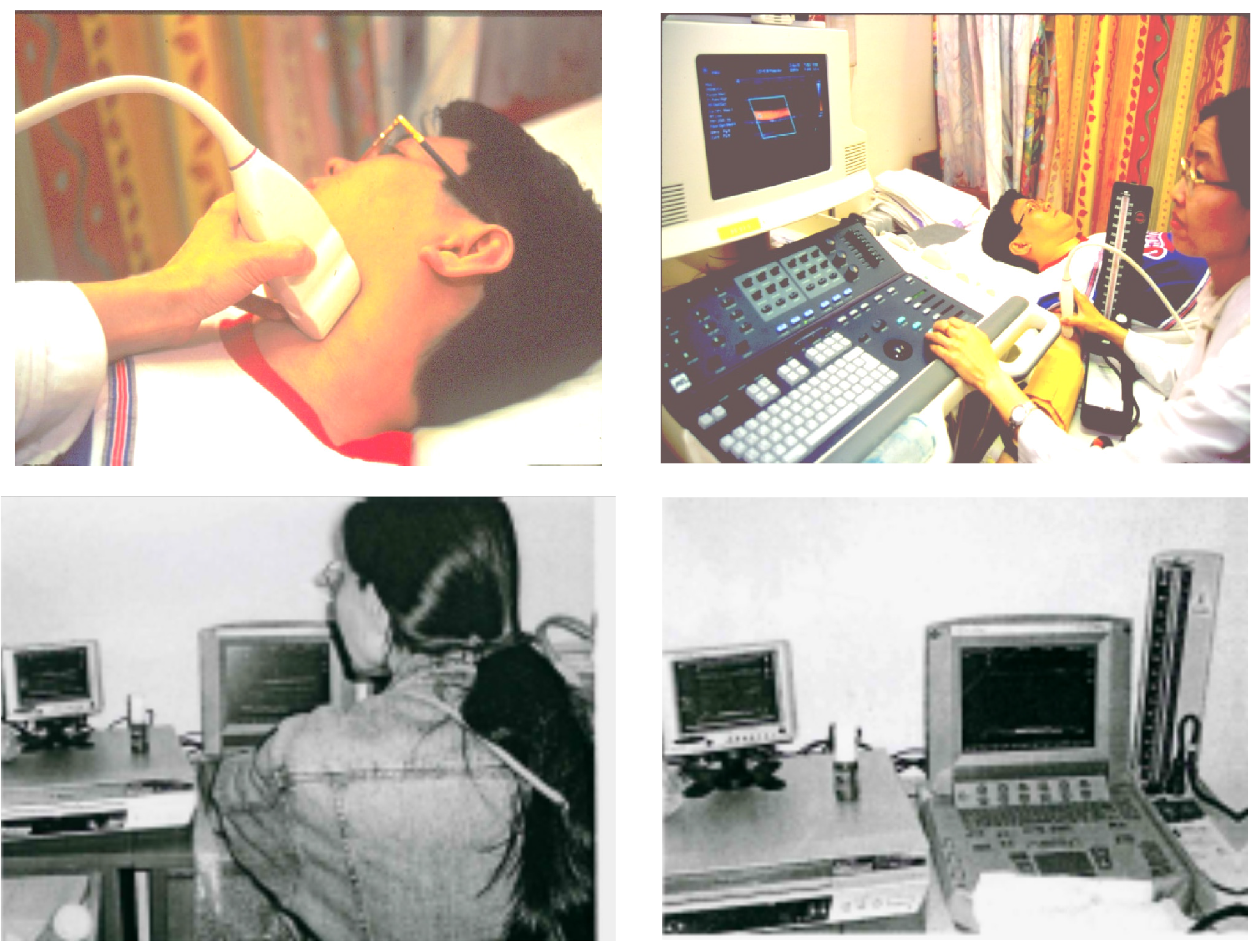
4. Metabolic Vitamin B-12 Deficiency with Vegan or Related Diets
5. Atherosclerosis Surrogates and Subnormal Vitamin B-12 Status
6. Impact of Vitamin B-12 Supplementation
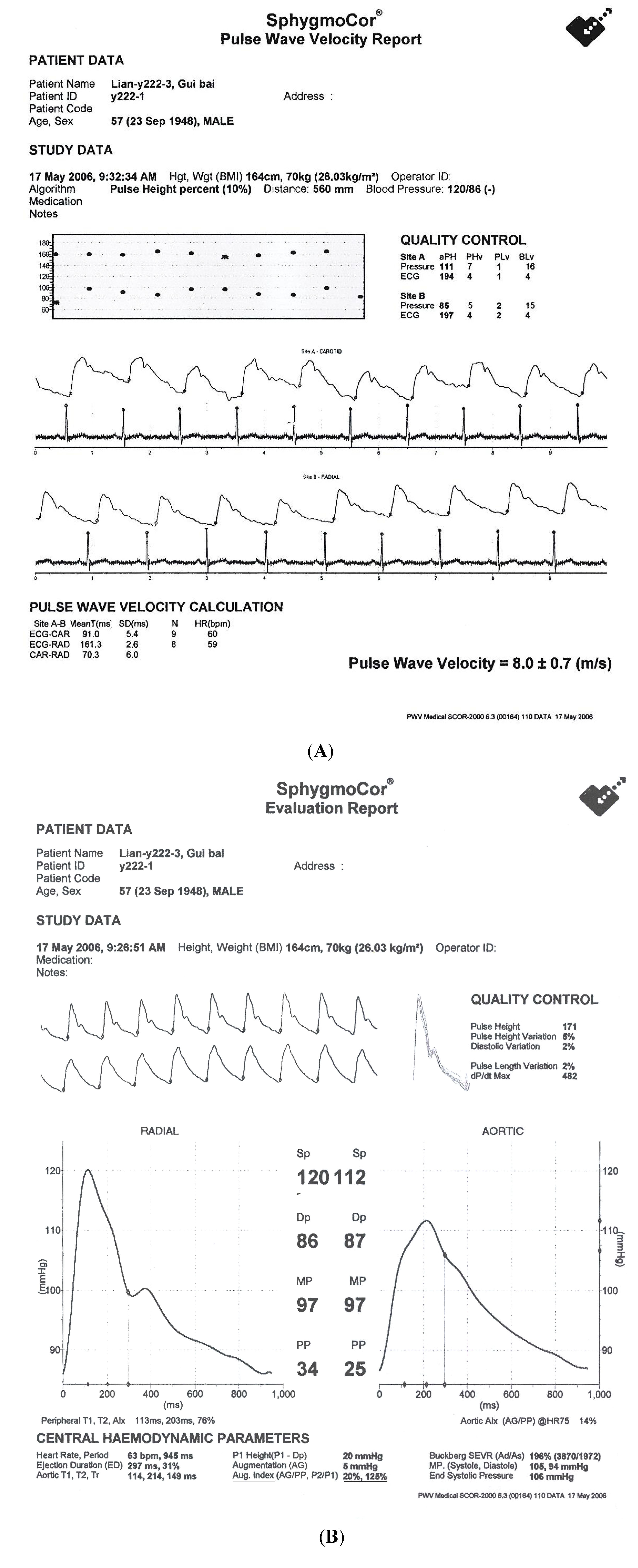
7. General Remarks
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first national health and nutrition examination survey epidemiologic follow-up study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar]
- Larsson, C.L.; Johansson, G.K. Young Swedish vegans have different sources of nutrients than young omnivores. J. Am. Diet. Assoc. 2005, 105, 1438–1441. [Google Scholar] [CrossRef]
- Kelly, J.H., Jr.; Sabate, J. Nuts and coronary heart disease: An epidemiological perspective. Br. J. Nutr. 2006, 96 (Suppl. 2), S61–S67. [Google Scholar] [CrossRef]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef]
- Fraser, G.E. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am. J. Clin. Nutr. 1999, 70 (Suppl. 3), 532S–538S. [Google Scholar]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89 (Suppl. 5), 1627S–1633S. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L. Cardiovascular disease mortality and cancer incidence in vegetarians: A meta-analysis and systematic review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar]
- Crowe, F.L.; Appleby, P.N.; Travis, R.C.; Key, T.J. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: Results from the EPIC-Oxford cohort study. Am. J. Clin. Nutr. 2013. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Rosell, M.S. Health effects of vegetarian and vegan diets. Proc. Nutr. Soc. 2006, 65, 35–41. [Google Scholar] [CrossRef]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607S–1612S. [Google Scholar] [CrossRef]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007, 10, 225–234. [Google Scholar] [CrossRef]
- Key, T.J.; Fraser, G.E.; Thorogood, M.; Appleby, P.N.; Beral, V.; Reeves, G.; Burr, M.L.; Chang-Claude, J.; Frentzel-Beyme, R.; Kuzma, J.W.; et al. Mortality in vegetarians and nonvegetarians: Detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 1999, 70, 516S–524S. [Google Scholar]
- Le, L.T.; Sabaté, J. Beyond meatless, the health effects of vegan diets: Findings from the Adventist cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar] [CrossRef]
- Flammer, A.N.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function from research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Celermajer, D.S. Flow-mediated dilatation. Br. J. Clin. Pharmacol. 2000, 50, 397–404. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 457–467. [Google Scholar] [CrossRef]
- Herrington, D.M.; Fan, L.; Drum, M.; Riley, W.A.; Pusser, B.E.; Crouse, J.R.; Burke, G.L.; McBurnie, M.A.; Morgan, T.M.; Espeland, M.A. Brachial flow-mediated vasodilator responses in population-based research: Methods, reproducibility and effects of age, gender and baseline diameter. J. Cardiovasc. Risk 2001, 8, 319–328. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodiation of the brachial artery. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar]
- Teragawa, H.; Ueda, K.; Matsuda, K.; Kimura, M.; Higashi, Y.; Oshima, T.; Yoshizumi, M.; Chayama, K. Relationship between endothelial function in the coronary and brachial arteries. Clin. Cardiol. 2005, 28, 460–466. [Google Scholar] [CrossRef]
- Donald, A.E.; Charakida, M.; Cole, T.J.; Friberg, P.; Chowienczyk, P.J.; Millasseau, S.C.; Deanfield, J.E.; Halcox, J.P. Non-invasive assessment of endothelial function which technique? J. Am. Coll. Cardiol. 2006, 48, 1846–1850. [Google Scholar] [CrossRef]
- Donald, A.E.; Halcox, J.P.; Charakida, M.; Storry, C.; Wallace Sharon, M.L.; Cole, T.J.; Friberg, P.; Deanfield, J.E. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J. Am. Coll. Cardiol. 2008, 51, 1959–1964. [Google Scholar] [CrossRef]
- Charakida, M.; de Groot, E.; Loukogeorgakis, S.T.; Khan, T.; Lüscher, T.; Kastelein, J.J.; Gasser, T.; Deanfield, J.E. Variability and reproducibility of flow-mediated dilatation in a multicentre clinical trial. Eur. Heart J. 2013, 34, 3501–3507. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in childeren and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Georgakopoulos, D.; Bull, C.; Thomas, O.; Robinson, J.; Deanfield, J.E. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993, 88, 2149–2155. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Adams, M.R.; Clarkson, P.; Robinson, J.; McCredie, R.; Donald, A.; Deanfield, J.E. Passive smoking and impaired endothelium-dependent arterial dilation in healthy young adults. N. Engl. J. Med. 1999, 130, 578–581. [Google Scholar]
- Clarkson, P.; Celermajer, D.S.; Donald, A.E.; Sampson, M.; Sorensen, K.E.; Adams, M.; Yue, D.K.; Betteridge, D.J.; Deanfield, J.E. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and LDL-cholesterol levels. J. Am. Coll. Cardiol. 1996, 28, 573–579. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Lolin, Y.I.; Cheung, A.S.; Chan, L.T.; Sun, Y.Y.; Sanderson, J.E.; Metreweli, C.; Celermajer, D.S. Hyperhomocysteinemia is a risk factor for arterial dysfunction in humans. Circulation 1997, 96, 2642–2644. [Google Scholar]
- Woo, K.S.; Chook, P.; Yu, C.W.; Sung, R.Y.; Qiao, M.; Leung, S.S.; Lam, C.W.; Metreweli, C.; Celermajer, D.S. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 852–857. [Google Scholar] [CrossRef]
- Neunteufl, T.; Katzenschlager, R.; Hassan, A.; Klaar, U.; Schwarzacher, S.; Glogar, D.; Bauer, P.; Weidinger, F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 1997, 129, 111–118. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Lolin, W.I.; Sanderson, J.E.; Metreweli, C.; Celermajer, D.S. Folic acid improves arterial endothelial function in adults with hyperhomocysteinemia. J. Am. Coll. Cardiol. 1999, 34, 2002–2006. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Yu, C.W.; Sung, R.Y.; Qiao, M.; Leung, S.S.; Lam, C.W.; Metreweli, C.; Celermajer, D.S. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 2004, 109, 1981–1986. [Google Scholar] [CrossRef]
- Woo, K.S.; McCrohon, J.A.; Chook, P.; Adams, M.R.; Robinson, J.T.; McCredie, R.J.; Lam, C.W.; Feng, J.Z.; Celermajer, D.S. Chinese adults are less susceptible than whites to age-related endothelial dysfunction. J. Am. Coll. Cardiol. 1997, 30, 113–118. [Google Scholar] [CrossRef]
- Woo, K.S.; Robinson, J.T.; Chook, P.; Adams, M.R.; Yip, G.; Mai, Z.J.; Lam, C.W.; Sorensen, K.E.; Deanfield, J.E.; Celermajer, D.S. Differences in the effect of cigarette smoking on endothelial function in Chinese and white adults. Ann. Intern. Med. 1997, 127, 372–375. [Google Scholar]
- Woo, K.S.; Chook, P.; Raitakari, O.T.; McQuillan, B.; Feng, J.Z.; Celermajer, D.S. Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2487–2493. [Google Scholar] [CrossRef]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef]
- Bots, M.L.; Baldassarre, D.; Simon, A.; de Groot, E.; O’Leary, D.H.; Riley, W.; Kastelein, J.J.; Grobbee, D.E. Carotid intima-media thickness and coronary atherosclerosis: Weak or strong relations? Eur. Heart J. 2007, 28, 398–406. [Google Scholar] [CrossRef]
- Bots, M.L.; Sutton-Tyrrell, K. Lessons from the past and promises for the future for carotid intima-media thickness. J. Am. Coll. Cardiol. 2012, 60, 1599–1604. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular Disease in the development world. Prevalence, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef]
- Anand, S.S.; Yusuf, S.; Vuksan, V.; Devanesen, S.; Teo, K.K.; Montague, P.A.; Kelemen, L.; Yi, C.; Lonn, E.; Gerstein, H.; et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic Groups (SHARE). Lancet 2000, 356, 279–284. [Google Scholar] [CrossRef]
- Tam, W.Y.; Chook, P.; Qiao, M.; Chan, L.T.; Chan, T.Y.K.; Poon, Y.K.; Fung, K.P.; Leung, P.C.; Woo, K.S. The efficacy and tolerability of adjunctive alternative herbal medicine (Salvia miltiorrhia and Pueraria lobata) on vascular function and structure in coronary patients. J. Altern. Complement. Med. 2009, 15, 415–421. [Google Scholar] [CrossRef]
- Chook, P.; Qiao, M.; Kum, C.C.; Szeto, C.C.; Chan, L.L.T.; Ho, C.P.; Yu, A.W.Y.; Celermajer, D.S.; Woo, K.S. High-dose folic supplementation improves atherogenic process in predialysing chronic renal failure independent of homocysteine-lowering. J. Am. Coll. Cardiol. 2006, 47 (Suppl. A), 345A. [Google Scholar] [CrossRef]
- Larsson, C.L.; Johansson, G.K. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am. J. Clin. Nutr. 2002, 76, 100–106. [Google Scholar]
- Woo, J.; Kwok, T.; Ho, S.C.; Sham, A.; Lau, E. Nutritional status of elderly Chinese vegetarians. Age Ageing 1998, 27, 455–461. [Google Scholar] [CrossRef]
- Majchrzak, D.; Singer, I.; Mӓnner, M.; Rust, P.; Genser, D.; Wagner, K.H.; Elmadfa, I. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann. Nutr. Metab. 2006, 50, 485–491. [Google Scholar]
- Bissoli, L.; di Francesco, V.; Ballarin, A.; Mandragona, R.; Trespidi, R.; Brocco, G.; Caruso, B.; Bosello, O.; Zamboni, M. Effect of vegetarian diet on homocysteine levels. Ann. Nutr. Metab. 2002, 46, 73–79. [Google Scholar] [CrossRef]
- Koebnick, C.; Hoffmann, I.; Dagnelie, P.C.; Heins, U.A.; Wickramasinghe, S.N.; Ratnayaka, I.D.; Gruendel, S.; Lindemans, J.; Leitzmann, C. Long-term ovo-lactovegetarian diet impairs vitamin B-12 status in pregnant women. J. Nutr. 2004, 134, 3319–3326. [Google Scholar]
- Hokin, B.D.; Butler, T. Cyanocobalamin (vitamin B-12) status in the seventh-day advenstist ministers in Australia. Am. J. Clin. Nutr. 1999, 70 (Suppl. 3), 576S–578S. [Google Scholar]
- Yajnik, C.S.; Deshpande, S.S.; Lubree, H.G.; Naik, S.S.; Bhat, D.S.; Uradey, B.S.; Deshpande, J.A.; Rege, S.S.; Refsum, H.; Yudkin, J.S. Vitamin B12 deficiency and hyperhomocysteinemia in rural nad urban Indians. J. Assoc. Physicians India 2006, 54, 775–782. [Google Scholar]
- Refsum, H.; Yajnik, C.S.; Gadkari, M.; Schneede, J.; Vollset, S.E.; Orning, L.; Guttormsen, A.B.; Joglekar, A.; Sayyad, M.G.; Ulvik, A.; et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin dificiecy in Asian Indians. Am. J. Clin. Nutr. 2001, 74, 233–241. [Google Scholar]
- Huang, Y.C.; Chang, S.J.; Chiu, Y.T.; Chang, H.H.; Cheng, C.H. The status of plasma homocysteine and related B-vitamins in healthy young vegetarians and nonvegetarians. Eur. J. Nutr. 2003, 42, 84–90. [Google Scholar] [CrossRef]
- Kwok, T.; Chook, P.; Tam, L.; Qiao, M.; Woo, J.L.F.; Celermajer, D.S.; Woo, K.S. Vascular dysfunction in Chinese vegetarians: An apparent paradox? J. Am. Coll. Cardiol. 2005, 46, 1957–1958. [Google Scholar] [CrossRef]
- Smith, D.; Refsum, H. Do we need to reconsider the desirable blood level of vitamin B12? J. Int. Med. 2011, 271, 179–182. [Google Scholar] [CrossRef]
- Madry, E.; Lisowska, A.; Grebowiec, P.; Walkowiak, J. The impact of vegan diet on B-12 status in healthy omnivores: Five-year prospective study. Acta Sci. Pol. Technol. Aliment. 2012, 11, 209–213. [Google Scholar]
- Naik, S.; Bhide, V.; Babhulkar, A.; Mahalle, N.; Parab, S.; Thakre, R.; Kulkarni, M. Daily milk intake improves vitamin B-12 status in young vegetarian Indians: An intervention trial. Nutr. J. 2013, 12, 136–144. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Yip, T.W.C.; Kwong, S.K.; Hu, Y.J.; Huang, X.S.; Wang, G.G.; Wu, M.J.; Liu, Y.M.; Lam, C.W.K.; et al. Folic acid and vitamin B-12 supplementation improves arterial function and structure in subjects with subnormal intake. Heart Lung Circ. 2008, 17, S201–202. [Google Scholar]
- Woo, K.S.; Chook, P.; Chiu, M.L.; Feng, X.H.; Evora, M.L.; Koon, K.V.; Zhang, X.M.; Chu, C.L.; Leong, H.C.; Yip, T.W.C.; et al. Vitamins B12 and C supplementation improves arterial reactivity and structure in passive smokers. Heart Lung Circ. 2012, 21, 524. [Google Scholar]
- Toohey, M.L.; Harris, M.A.; DeWitt, W.; Foster, G.; Schmidt, W.D.; Melby, C.L. Cardiovascular disease risk factors are lower in African-American vegans compared to lacto-ovo-vegetarians. J. Am. Coll. Nutr. 1998, 17, 425–434. [Google Scholar] [CrossRef]
- Su, T.C.; Jeng, J.S.; Wang, J.D.; Torng, P.L.; Chang, S.J.; Chen, C.F.; Liau, C.S. Homocysteine, circulating vascular cell adhesion molecule and carotid atherosclerosis in postmenopausal vegetarian women and omnivores. Atherosclerosis 2006, 184, 356–362. [Google Scholar] [CrossRef]
- Su, T.C.; Torng, P.L.; Jeng, J.S.; Chen, M.F.; Liau, C.S. Arterial function of carotid and brachial arteries in postmenopausal vegetarians. Vasc. Health Risk Manag. 2011, 7, 517–523. [Google Scholar]
- Yang, S.Y.; Li, X.J.; Zhang, W.; Liu, C.Q.; Zhang, H.J.; Lin, J.R.; Yan, B.; Yu, Y.X.; Shi, X.L.; Li, C.D.; et al. Chinese lacto-vegetarian diet exerts favorable effects on metabolic parameters, intima-media thickness, and cardiovascular risks in healthy men. Nutr. Clin. Pract. 2012, 27, 392–398. [Google Scholar] [CrossRef]
- Ferreira-Sae, M.C.S.; Cipolli, J.A.; Cornelio, M.E.; Matos-Souza, J.R.; Fernandes, M.N.; Schreiber, R.; Costa, F.O.; Franchini, K.G.; Rodrigues, R.C.; Gallani, M.C.; et al. Sodium intake is associated with carotid artery structure alterations and plasma matrix metalloproteinase-9 upregulation in hypertensive adults. J. Nutr. 2011. [Google Scholar] [CrossRef]
- Njoroge, J.N.; EI Khoudary, S.R.; Fried, L.F.; Barinas-Mitchell, E.; Sutton-Tyrrell, K. High urinary sodium is associated with increased carotid intima-media thickness in normotensive overweight and obese adults. Am. J. Hypertens. 2011, 24, 70–76. [Google Scholar] [CrossRef]
- Kwok, T.; Chook, P.; Qiao, M.; Tam, L.; Poon, Y.K.P.; Ahuja, A.T.; Woo, J.; Celermajer, D.S.; Woo, K.S. Vitamin B-12 supplementation improves arterial function in vegetarians with subnormal vitamin B-12 status. J. Nutr. Health Aging 2012, 16, 569–573. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Chan, L.L.T.; Celermajer, D.S. The impact of folic acid and vitamin B12 supplementation on blood pressure and arterial stiffness in subjects with subnormal micronutrient intake. Heart Lung Circ. 2008, 17, S84. [Google Scholar]
- Till, U.; Rӧhl, P.; Jentsch, A.; Till, H.; Müller, A.; Bellstedt, K.; Plonné, D.; Fink, H.S.; Vollandt, R.; Sliwka, U.; et al. Decrease of carotid intima-media thickness in patients at risk to cerebral ischemia after supplemenation with folic acid, vitamins B6 and B12. Atherosclerosis 2005, 181, 131–135. [Google Scholar] [CrossRef]
- Bleys, J.; Miller, E.R., III.; Pastor-Barriuso, R.; Appel, L.J.; Guallar, E. Vitamin-mineral supplementation and the progression of atherosclerosis: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006, 84, 880–887. [Google Scholar]
- Potter, K.; Hankey, G.J.; Green, D.J.; Eikelboom, J.; Jamrozik, K.; Arnolda, L.F. The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: A randomized controlled trial and meta-analysis. BMC Cardiovasc. Disord. 2008, 8, 24. [Google Scholar]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Sides, E.G.; Wang, C.H.; Stampfer, M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef]
- Bonaa, K.H.; Njolstad, I.; Ueland, P.M.; Schirmer, H.; Tverdal, A.; Steigen, T.; Wang, H.; Nordrehaug, J.E.; Arnesen, E.; Rasmussen, K.; et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N. Engl. J. Med. 2006, 354, 1578–1588. [Google Scholar] [CrossRef]
- Clarke, R.; Lewington, S.; Sherliker, P.; Armitage, J. Effects of B-vitamins on plasma homocysteine concentrations and on risk of cardiovascular disease and dementia. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 32–39. [Google Scholar] [CrossRef]
- Albert, C.M.; Cook, N.R.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: A randomized trial. JAMA 2008, 299, 2027–2036. [Google Scholar] [CrossRef]
- Mearns, G.J.; Kozio-McLain, J.; Obolonkin, V.; Rush, E.C. Preventing vitamin B12 deficiency in South Asian women of childbearing age: A randomized controlled trial comparing an oral vitamin B12 supplement with B12 dietary advice. Eur. J. Clin. Nutr. 2014, 68, 870–875. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Woo, K.S.; Kwok, T.C.Y.; Celermajer, D.S. Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health. Nutrients 2014, 6, 3259-3273. https://doi.org/10.3390/nu6083259
Woo KS, Kwok TCY, Celermajer DS. Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health. Nutrients. 2014; 6(8):3259-3273. https://doi.org/10.3390/nu6083259
Chicago/Turabian StyleWoo, Kam S., Timothy C.Y. Kwok, and David S. Celermajer. 2014. "Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health" Nutrients 6, no. 8: 3259-3273. https://doi.org/10.3390/nu6083259
APA StyleWoo, K. S., Kwok, T. C. Y., & Celermajer, D. S. (2014). Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health. Nutrients, 6(8), 3259-3273. https://doi.org/10.3390/nu6083259



