Antinociceptive and Anti-Inflammatory Activities of the Sesame Oil and Sesamin
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Animals
2.3. Acute Toxicity
2.4. Acetic Acid-Induced Writhing Response in Mice
2.5. Formalin-Induced Nociception in Mice
2.6. Hot Plate Latency Assay in Mice
2.7. Tail Immersion Test in Mice
2.8. Carrageenan-Induced Edema in Rats
2.9. Carrageenan-Induced Pleurisy in Rats
2.10. Statistical Analysis
3. Results
3.1. Acute Toxicity
3.2. Acetic Acid-Induced Writhing Response in Mice
3.3. Effects on the Nociception Induced by Formalin in Mice

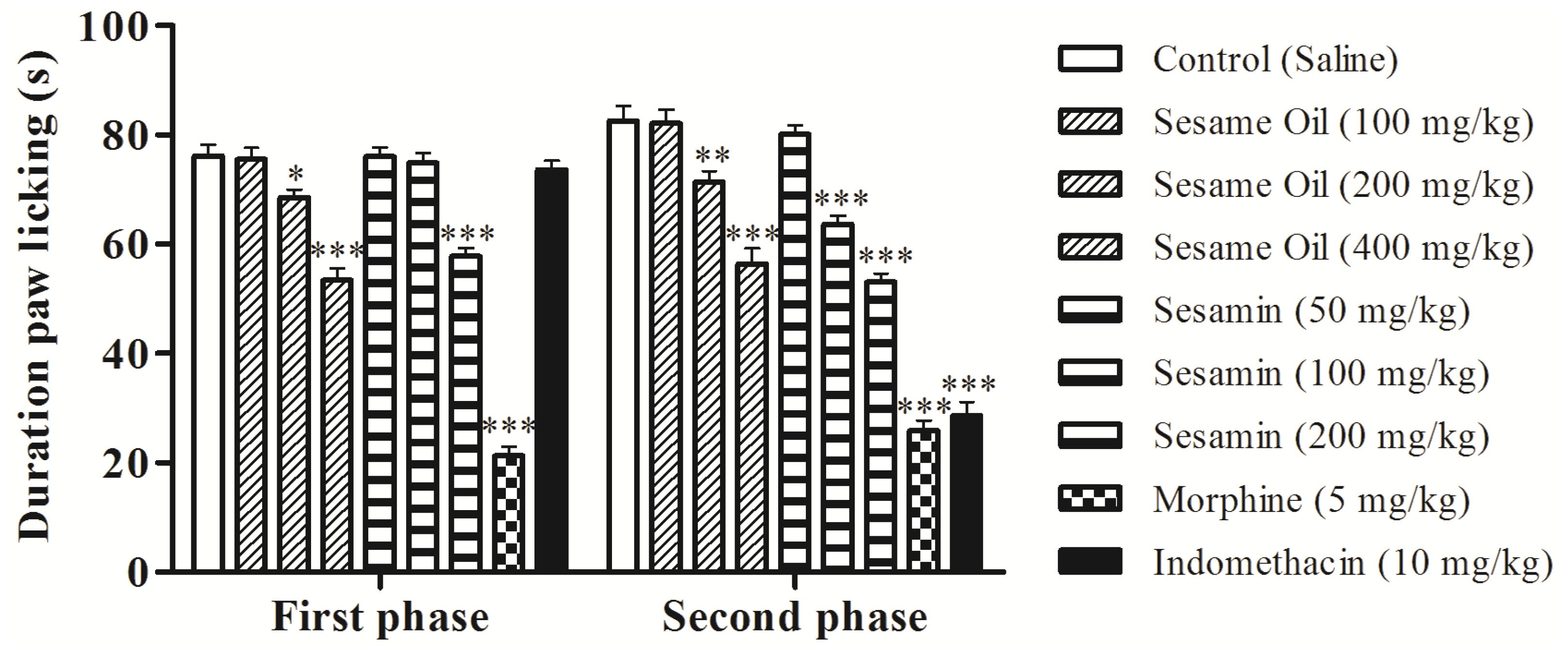
3.4. Effects on Hot-Plate Latency Assay in Mice

3.5. Effects on Tail Immersion Test in Mice
| Group | Dose (mg/kg) | Reaction time (s) | ||||
|---|---|---|---|---|---|---|
| 0′ | 30′ | 60′ | 90′ | 120′ | ||
| Control | Saline | 2.37 ± 0.42 | 2.75 ± 0.45 | 2.87 ± 0.35 | 3.00 ± 0.33 | 3.12 ± 0.29 |
| 100 | 2.50 ± 0.38 | 2.75 ± 0.56 | 3.37 ± 0.42 | 3.62 ± 0.37 | 3.75 ± 0.45 | |
| Oil | 200 | 2.37 ± 0.37 | 3.37 ± 0.46 | 4.50 ± 0.33 * | 4.87 ± 0.40 ** | 5.50 ± 0.46 ** |
| 400 | 2.62 ± 0.42 | 4.12 ± 0.48 | 4.87 ± 0.35 ** | 5.62 ± 0.37 *** | 6.87 ± 0.40 *** | |
| 50 | 2.37 ± 0.42 | 3.00 ± 0.50 | 3.12 ± 0.40 | 3.50 ± 0.33 | 3.62 ± 0.26 | |
| Sesamin | 100 | 2.12 ± 0.40 | 3.62 ± 0.50 | 3.75 ± 0.45 | 4.12 ± 0.29 | 5.00 ± 0.38 ** |
| 200 | 2.25 ± 0.37 | 4.37 ± 0.53 | 3.87 ± 0.29 | 5.25 ± 0.37 *** | 6.50 ± 0.27 *** | |
| Morphine | 5 | 2.75 ± 0.49 | 4.50 ± 0.46 | 5.75 ± 0.45 *** | 6.75 ± 0.45 *** | 8.12 ± 0.40 *** |
3.6. Effects on Edema Induced by Carrageenan in Rats
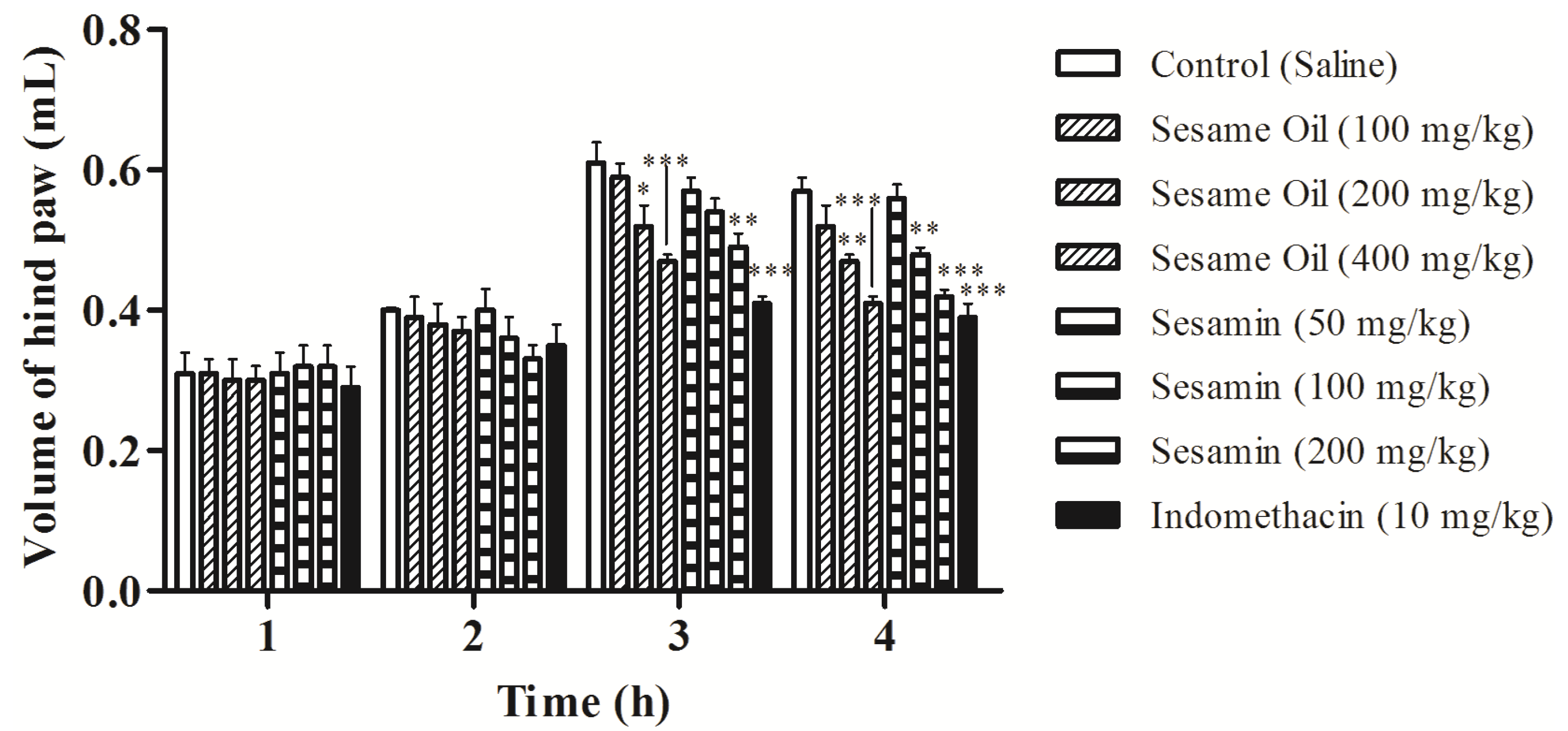
3.7. Effects on Carrageenan-Induced Pleurisy in Rats
| Group | Dose (mg/kg) | Exudate volume (mL) | Inhibition (%) | N° Leucocytes (×103 cells/mm3) | Inhibition (%) |
|---|---|---|---|---|---|
| Control | Saline | 1.13 ± 0.04 | - | 13.07 ± 0.31 | - |
| 100 | 1.07 ± 0.03 | 5.31 | 13.03 ± 0.36 | - | |
| Sesame Oil | 200 | 0.88 ± 0.05 ** | 22.12 | 10.67 ± 0.24 *** | 18.36 |
| 400 | 0.80 ± 0.06 *** | 29.20 | 9.47 ± 0.44 *** | 27.54 | |
| 50 | 1.02 ± 0.04 | 11.50 | 13.00 ± 0.27 | - | |
| Sesamin | 100 | 0.90 ± 0.04 ** | 26.55 | 11.43 ± 0.25 ** | 12.55 |
| 200 | 0.83 ± 0.03 *** | 31.86 | 10.03 ± 0.21 *** | 23.26 | |
| Indomethacin | 10 | 0.72 ± 0.04 *** | 36.28 | 8.43 ± 0.30 *** | 35.50 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, S.; Majumdar, D.K. Anti-inflammatory and antipyretic activities of Ocimum Sanctum fixed oil. Pharm. Biol. 1995, 33, 288–292. [Google Scholar] [CrossRef]
- Küpeli, E.; Erdemoğlu, N.; Yeşilada, E.; Sener, B. Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J. Ethnopharmacol. 2003, 89, 265–270. [Google Scholar] [CrossRef]
- Singh, S.; Taneja, M.; Majumdar, D.K. Biological activities of Ocimum sanctum L. fixed oil—An overview. Indian J. Exp. Biol. 2007, 45, 403–412. [Google Scholar]
- Singh, S.; Majumdar, D.K.; Rehan, H.M. Evaluation of anti-inflammatory potential of fixed oil of Ocimum sanctum (Holybasil) and its possible mechanism of action. J. Ethnopharmacol. 1996, 54, 19–26. [Google Scholar] [CrossRef]
- Singh, S. Mechanism of action of anti-inflammatory effect of fixed oil of Ocimum basilicum Linn. Indian J. Exp. Biol. 1999, 37, 248–252. [Google Scholar]
- Favacho, H.A.S.; Oliveira, B.R.; Santos, K.C.; Medeiros, B.J.L.; Sousa, P.J.C.; Perazzo, F.F.; Carvalho, J.C.T. Anti-inflammatory and antinociceptive activities of Euterpe oleracea oil. Braz. J. Pharmacogn. 2011, 21, 105–114. [Google Scholar]
- Déciga-Campos, M.; Montiel-Ruiz, R.M.; Navarrete-Vázquez, G.; ópez-Muñoz, F.J. Palmitic acid analogues exhibiting antinociceptive activity in mice. Proc. West. Pharmacol. Soc. 2007, 50, 75–77. [Google Scholar]
- Kim, J.Y.; Lim, H.J.; Lee, D.Y.; Kim, J.S.; Kim, D.H.; Lee, H.J.; Kim, H.D.; Jeon, R.; Ryu, J.-H. In vitro anti-inflammatory activity of lignans isolated from Magnolia fargesii. Bioorg. Med. Chem. Lett. 2009, 19, 937–940. [Google Scholar] [CrossRef]
- Lim, H.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Kim, H.P. Anti-inflammatory activity of phylligenin, a lignan from the fruits of Forsythia koreana, and its cellular mechanism of action. J. Ethnopharmacol. 2008, 118, 113–117. [Google Scholar] [CrossRef]
- Bastos, J.K.; Carvalho, J.C.; de Souza, G.H.; Pedrazzi, A.H.; Sarti, S.J. Anti-inflammatory activity of cubebin, a lignan from the leaves of Zanthoxyllum naranjillo Griseb. J. Ethnopharmacol. 2001, 75, 279–282. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Liu, K. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J. Ethnopharmacol. 2009, 122, 457–462. [Google Scholar] [CrossRef]
- Abou-Gharbia, H.A.; Shahidi, F.; Shehata, A.A.Y.; Youssef, M.M. Effect of processing on oxidative stability of sesame oil extracted from intact and dehulled seed. J. Am. Oil Chem. Soc. 1997, 74, 215–221. [Google Scholar] [CrossRef]
- Abou-Gharbia, H.A.; Shehata, A.A.Y.; Shahidi, F. Effect of processing on oxidative stability and lipid classes of sesame oil. Food Res. Int. 2000, 33, 331–340. [Google Scholar] [CrossRef]
- Carvalho, R.H.R.; Galvão, E.L.; Barros, J.Â.C.; Conceição, M.M.; Sousa, E.M.B.D. Extraction, fatty acid profile and antioxidant activity of sesame extract (Sesamum Indicum L.). Braz. J. Chem. Eng. 2012, 29, 409–420. [Google Scholar] [CrossRef]
- Isha, D.; Milind, P. Eat til and protect dil. Int. Res. J. Pharm. 2012, 3, 54–57. [Google Scholar]
- Xu, J.; Chen, S.; Hu, Q. Antioxidant activity of brown pigment and extracts from black sesame seed (Sesamum indicum L). Food Chem. 2005, 91, 79–83. [Google Scholar] [CrossRef]
- Doker, O.; Salgin, U.; Yieldiz, N.; Aydognus, M.; Calimi, A. Extraction of sesame seed oil using supercritical CO2 and mathematical modeling. J. Food Eng. 2010, 97, 360–366. [Google Scholar] [CrossRef]
- Corso, M.P.; Klen, M.F.; Silva, E.A.; Filho, L.C.; Santos, J.N.; Freitas, L.S.; Dariva, C. Extraction of sesame seed (Sesamun indicum L) oil using compressed propane and supercritical carbon dioxide. J. Supercrit. Fluids 2010, 52, 56–61. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Pholphana, N.; Mahidol, C.; Wongyai, W.; Saengsooksree, K.; Nookabkaew, S.; Satayavivad, J. Variation of sesamin, sesamolin and tocopherols in sesame (Sesamum indicum L.) seeds and oil products in Thailand. Food Chem. 2010, 122, 724–730. [Google Scholar] [CrossRef]
- Saleem, T.S.M.; Basha, S.D.; Mahesh, G.; Rani, P.V.S. Analgesic, anti-pyretic and anti-inflammatory activity of dietary sesame oil in experimental animal models. Pharmacologia 2011, 2, 172–177. [Google Scholar] [CrossRef]
- Schwertner, H.A.; Rios, D.C. Analysis of sesamin, asarinin, and sesamolin by HPLC with photodiode and fluorescent detection and by GC/MS: Application to sesame oil and serum samples. J. Am. Oil Chem. Soc. 2012, 89, 1943–1950. [Google Scholar] [CrossRef]
- Wu, W.-H. The contents of lignans in commercial sesame oils of Taiwan and their changes during heating. Food Chem. 2007, 104, 341–344. [Google Scholar] [CrossRef]
- Suja, K.P.; Jayalekshmy, A.; Arumughan, C. Free radical scavenging behavior of antioxidant compounds of sesame (Sesamum indicum L.) in DPPH˙ system. J. Agric. Food Chem. 2004, 52, 912–915. [Google Scholar] [CrossRef]
- Yokota, T.; Matsuzaki, Y.; Koyama, M.; Hitomi, T.; Kawanaka, M.; Enoki-Konishi, M.; Okuyama, Y.; Takayasu, J.; Nishino, H.; Nishikawa, A.; et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007, 98, 1447–1453. [Google Scholar] [CrossRef]
- Ghafoorunissa; Hemalatha, S.; Rao, M.V.V. Sesame lignans enhance the antioxidant activity of vitamin E in lipid peroxidation systems. Mol. Cell. Biochem. 2004, 262, 195–202. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, P.R.; Lin, S.; Tsai, S.C.; Wang, B.W.; Chen, W.W.; Tsai, C.E.; Shyu, K.G. Sesamin induces nitric oxide and decreases endothelin-1 production in HUVECs: Possible implications for its antihypertensive effect. J. Hypertens. 2004, 22, 2329–2338. [Google Scholar]
- Nakano, D.; Kurumazuka, D.; Nagai, Y.; Nishiyama, A.; Kiso, Y.; Matsumura, Y. Dietary sesamin suppresses aortic NADPH oxidase in DOCA salt hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 324–326. [Google Scholar] [CrossRef]
- Cheng, F.C.; Jinn, T.R.; Hou, R.C.; Tzen, J.T.C. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int. J. Biomed. Sci. 2006, 2, 284–288. [Google Scholar]
- Visavadiya, N.P.; Narasimhacharya, A.V.R.L. Sesame as a hypocholesteraemic and antioxidant dietary component. Food Chem. Toxicol. 2008, 46, 1889–1895. [Google Scholar] [CrossRef]
- Ashakumary, L.; Rouyer, I.; Takahashi, Y.; Ide, T.; Fukuda, N.; Aoyama, T.; Hashimoto, T.; Mizugaki, M.; Sugano, M. Sesamin, a sesame lignan, is a potent inducer of hepatic fatty acid oxidation in the rat. Metabolism 1999, 48, 1303–1313. [Google Scholar] [CrossRef]
- Williamson, K.S.; Morris, J.B.; Pye, Q.N.; Kamat, C.D.; Hensley, K. A survey of sesamin and composition of tocopherol variability from seeds of eleven diverse sesame (Sesamum indicum L.) genotypes using HPLC-PAD-ECD. Phytochem. Anal. 2008, 19, 311–322. [Google Scholar] [CrossRef]
- Hemalatha, S.; Ghafoorunissa. Lignans and tocopherols in Indian sesame cultivars. J. Am. Oil Chem. Soc. 2004, 81, 467–470. [Google Scholar] [CrossRef]
- Lorke, D. A new approach to pratical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Schmidt, A.P.; Böhmer, A.E.; Schallenberger, C.; Antunes, C.; Tavares, R.G.; Wofchuk, S.T.; Elisabetsky, E.; Souza, D.O. Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br. J. Pharmacol. 2010, 159, 1247–1263. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: dissociation between inflammatory and noninflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl and dithienylbutilamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar]
- Ramabadran, K.; Bansinath, M.; Turndorf, H.; Puig, M.M. Tail immersion test for the evaluation of a nociceptive reaction in mice: Methodological considerations. J. Pharmacol. Methods 1989, 21, 21–31. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Vinegar, R.; Truax, J.F.; Selph, J.L. Some quantitative temporal characteristics of carrageenin-induced pleurisy in the rat. Proc. Soc. Exp. Biol. Med. 1973, 143, 711–714. [Google Scholar] [CrossRef]
- Fujimori, S.; Gudis, K.; Sakamoto, C. A review of anti-inflammatory drug-induced gastrointestinal injury: Focus on prevention of small intestinal injury. Pharmaceuticals 2010, 3, 1187–1201. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Kidd, B.L.; Urban, L.A. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef]
- Deraedt, R.; Jouquey, S.; Delevallée, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Guo, T.; Deng, Y.X.; Xie, H.; Yao, C.Y.; Cai, C.C.; Pan, S.L.; Wang, Y.L. Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia 2011, 82, 347–351. [Google Scholar] [CrossRef]
- Lima, L.M.; Perazzo, F.F.; Tavares Carvalho, J.C.; Bastos, J.K. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J. Pharm. Pharmacol. 2007, 59, 1151–1158. [Google Scholar]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified formalin test: characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Rosland, J.H.; Tjolsen, A.; Maehle, B.; Hole, K. The formalin test in mice: effect of formalin concentration. Pain 1990, 42, 235–242. [Google Scholar] [CrossRef]
- Abbott, F.V.; Melzack, R. Brainstem lesions dissociated neural mechanisms of morphine analgesia in different kinds of pain. Brain Res. 1982, 251, 149–155. [Google Scholar] [CrossRef]
- Nemirovsky, A.; Chen, L.; Zelman, V.; Jurna, I. The antinociceptive effect of the combination of spinal morphine with systemic morphine or buprenorphine. Anesth. Analg. 2011, 93, 197–203. [Google Scholar]
- Schmauss, C.; Yaksh, T.L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J. Pharmacol. Exp. Ther. 1984, 228, 1–12. [Google Scholar]
- Yaksh, T.L.; Rudy, T.A. Studies on direct spinal action of narcotics in production of analgesia in rat. J. Pharmacol. Exp. Ther. 1977, 202, 411–428. [Google Scholar]
- Zakaria, Z.A.; Mat Jais, A.M.; Goh, Y.M.; Sulaiman, M.R.; Somchit, M.N. Amino acid and fatty acid composition of an aqueous extract of Channa striatus (Haruan) that exhibits antinociceptive activity. Clin. Exp. Pharmacol. Physiol. 2007, 34, 198–204. [Google Scholar] [CrossRef]
- Eddouks, M.; Chattopadhyay, D.; Zeggwagh, N.A. Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–14. [Google Scholar]
- Posadas, I.; Bucci, M.; Roviezzo, F; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Patel, M.; Murugananthan, G.; Gowda, K.P.S. In vivo animal models in preclinical evaluation of anti-inflammatory activity—A review. Int. J. Pharm. Res. Allied Sci. 2012, 1, 1–5. [Google Scholar]
- Almeida, A.P.; Bayer, B.M.; Horakova, Z.; Beaven, M.A. Influence of indomethacin and other anti-inflammatory drugs on mobilization and production of neutrophils: Studies with carrageenan induced inflammation in rats. J. Pharmacol. Exp. Ther. 1980, 214, 74–79. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Monteiro, É.M.H.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.S.; Vilela, F.M.P.; Rodarte, M.P.; De Oliveira Pinto, M.A.; Da Penha Henriques do Amaral, M.; Silvério, M.S.; De Matos Araújo, A.L.S.; et al. Antinociceptive and Anti-Inflammatory Activities of the Sesame Oil and Sesamin. Nutrients 2014, 6, 1931-1944. https://doi.org/10.3390/nu6051931
Monteiro ÉMH, Chibli LA, Yamamoto CH, Pereira MCS, Vilela FMP, Rodarte MP, De Oliveira Pinto MA, Da Penha Henriques do Amaral M, Silvério MS, De Matos Araújo ALS, et al. Antinociceptive and Anti-Inflammatory Activities of the Sesame Oil and Sesamin. Nutrients. 2014; 6(5):1931-1944. https://doi.org/10.3390/nu6051931
Chicago/Turabian StyleMonteiro, Érika Maria Henriques, Lucas Apolinário Chibli, Célia Hitomi Yamamoto, Mônica Cecília Santana Pereira, Fernanda Maria Pinto Vilela, Mírian Pereira Rodarte, Míriam Aparecida De Oliveira Pinto, Maria Da Penha Henriques do Amaral, Marcelo Silva Silvério, Ana Lúcia Santos De Matos Araújo, and et al. 2014. "Antinociceptive and Anti-Inflammatory Activities of the Sesame Oil and Sesamin" Nutrients 6, no. 5: 1931-1944. https://doi.org/10.3390/nu6051931




