Manifestations of Fasting-Induced Fatty Liver and Rapid Recovery from Steatosis in Voles Fed Lard or Flaxseed Oil Lipids
Abstract
:1. Introduction
2. Experimental Section
| Formula (g/kg) | Lard Diet | Flaxseed Oil Diet |
|---|---|---|
| Casein | 150 | 150 |
| l-Cystine | 2.25 | 2.25 |
| Corn starch | 260.2 | 260.2 |
| Maltodextrin | 80 | 80 |
| Sucrose | 100 | 100 |
| Lard | 65 | – |
| Flaxseed oil | – | 65 |
| Soybean oil | 15 | 15 |
| Cellulose | 270 | 270 |
| Mineral mix, w/o Ca & P | 23 | 23 |
| Calcium carbonate | 7.5 | 7.5 |
| Calcium phosphate | 17 | 17 |
| Vitamin mix | 10 | 10 |
| Antioxidant (TBHQ) | 0.014 | 0.014 |
| Nutrient Information (wt-%) | ||
| Protein | 13.7 | 13.7 |
| Carbohydrate | 41.4 | 41.4 |
| Fat | 8.0 | 8.0 |
| Kcal/g | 2.9 | 2.9 |
| Principal Fat Source in Feed | Assignment of Voles to Study Groups (n = 5) | Group Abbreviation |
|---|---|---|
| Lard (n = 20) | Fed controls | Lard C |
| Fasted for 18 h | Lard F | |
| Fasted for 18 h and refed for 1 day | Lard F+RF1d | |
| Fasted for 18 h and refed for 7 days | Lard F+RF7d | |
| Flaxseed oil (n = 20) | Fed controls | Flax C |
| Fasted for 18 h | Flax F | |
| Fasted for 18 h and refed for 1 day | Flax F+RF1d | |
| Fasted for 18 h and refed for 7 days | Flax F+RF7d |
3. Results
3.1. BM Changes and Food Intake
3.2. Liver Weights, Fat-%, TAG and Cholesterol Concentrations
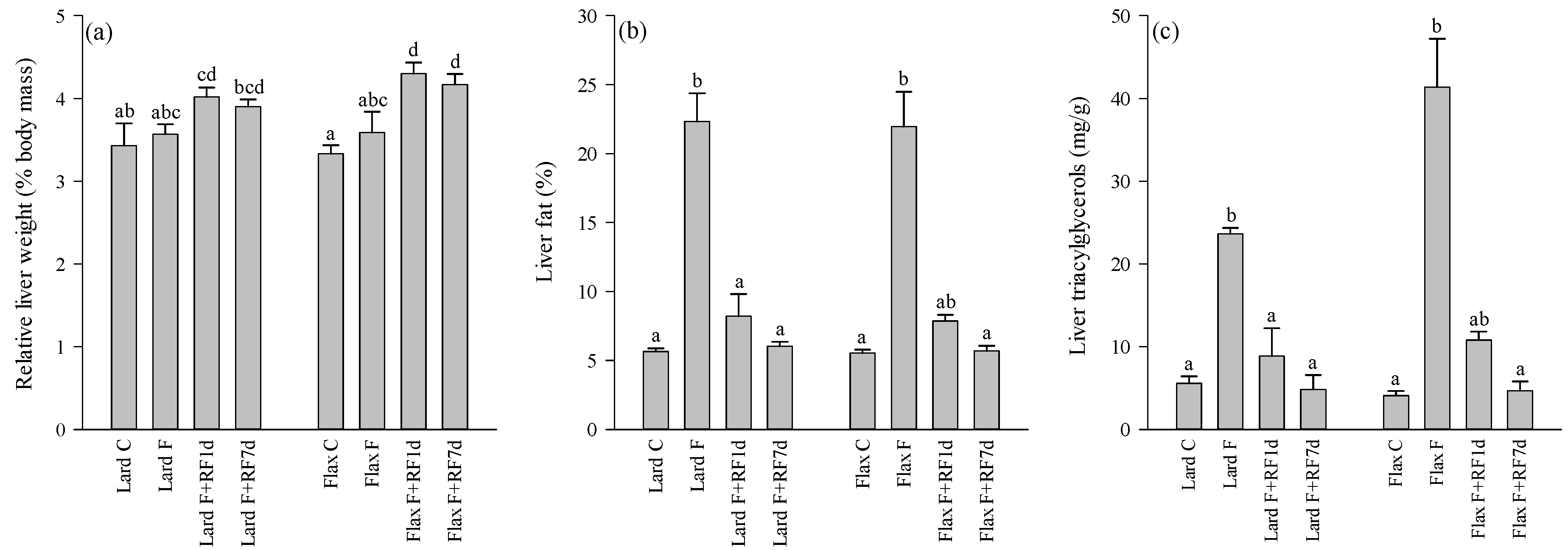
3.3. Liver Histology
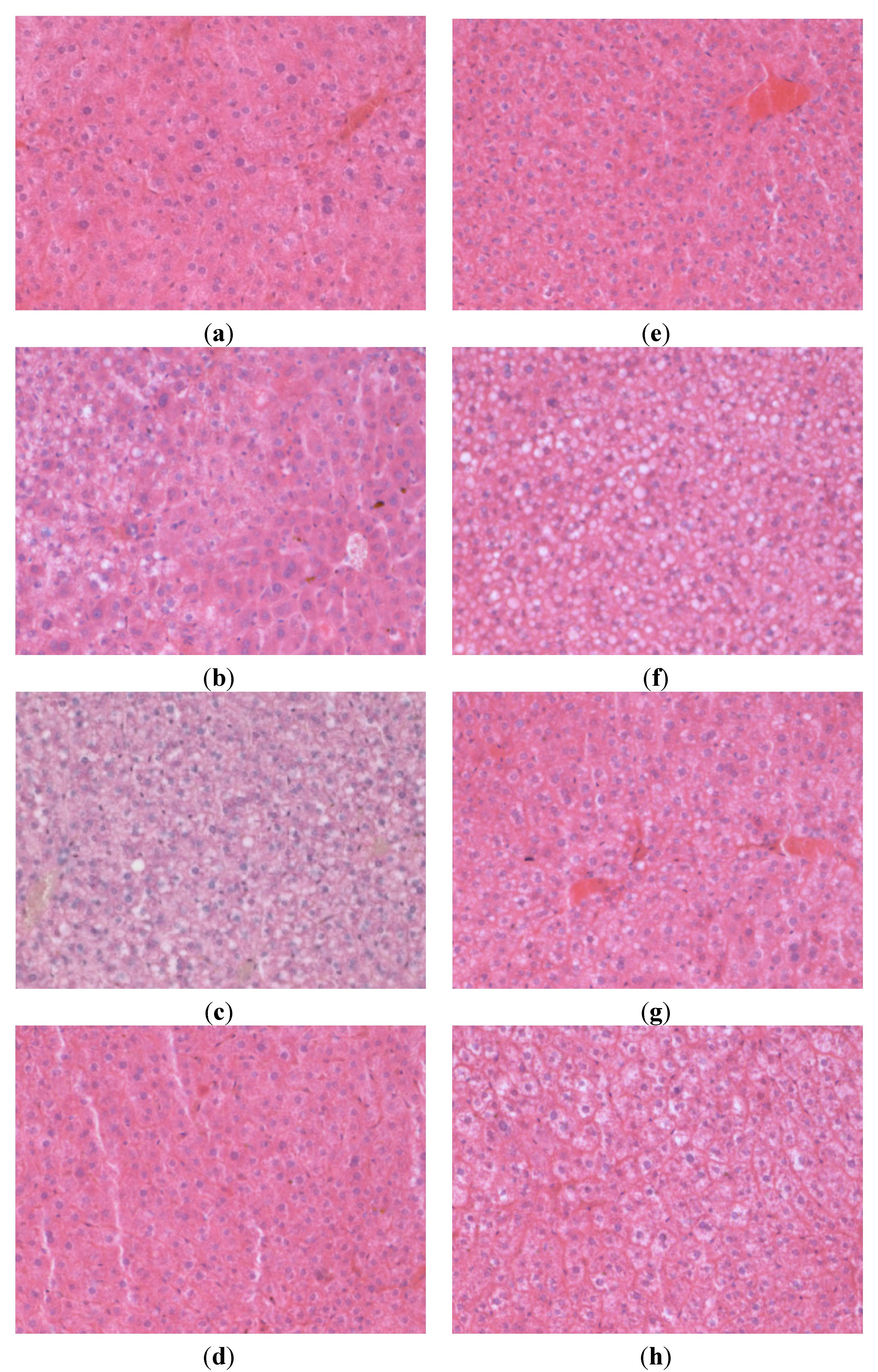
| Steatosis Grading | Distribution | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Zone 1 | Zone 3 | Panacinar | Azonal | |
| Lard C | 3 | 1 | 1 | 1 | 1 | |||
| Lard F | 2 | 3 | 1 | 2 | 2 | |||
| Lard F+RF1d | 3 | 2 | 2 | |||||
| Lard F+RF7d | 5 | |||||||
| Flax C | 5 | |||||||
| Flax F | 5 | 1 | 4 | |||||
| Flax F+RF1d | 3 | 1 | 1 | 2 | ||||
| Flax F+RF7d | 5 | |||||||
| Study Group | Lard C | Lard F | Lard F+RF1d | Lard F+RF7d | Flax C | Flax F | Flax F+RF1d | Flax F+RF7d |
|---|---|---|---|---|---|---|---|---|
| HA Bile Ducts | ||||||||
| 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 1 | 4 | 3 | 3 | 3 | 3 | 4 | 2 | 4 |
| 2 | 0 | 2 | 2 | 1 | 1 | 1 | 2 | 0 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| HA Venous | ||||||||
| 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| 1 | 3 | 3 | 3 | 3 | 3 | 4 | 2 | 4 |
| 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
3.4. Fatty Acid Profiles of the Diets and Control Livers
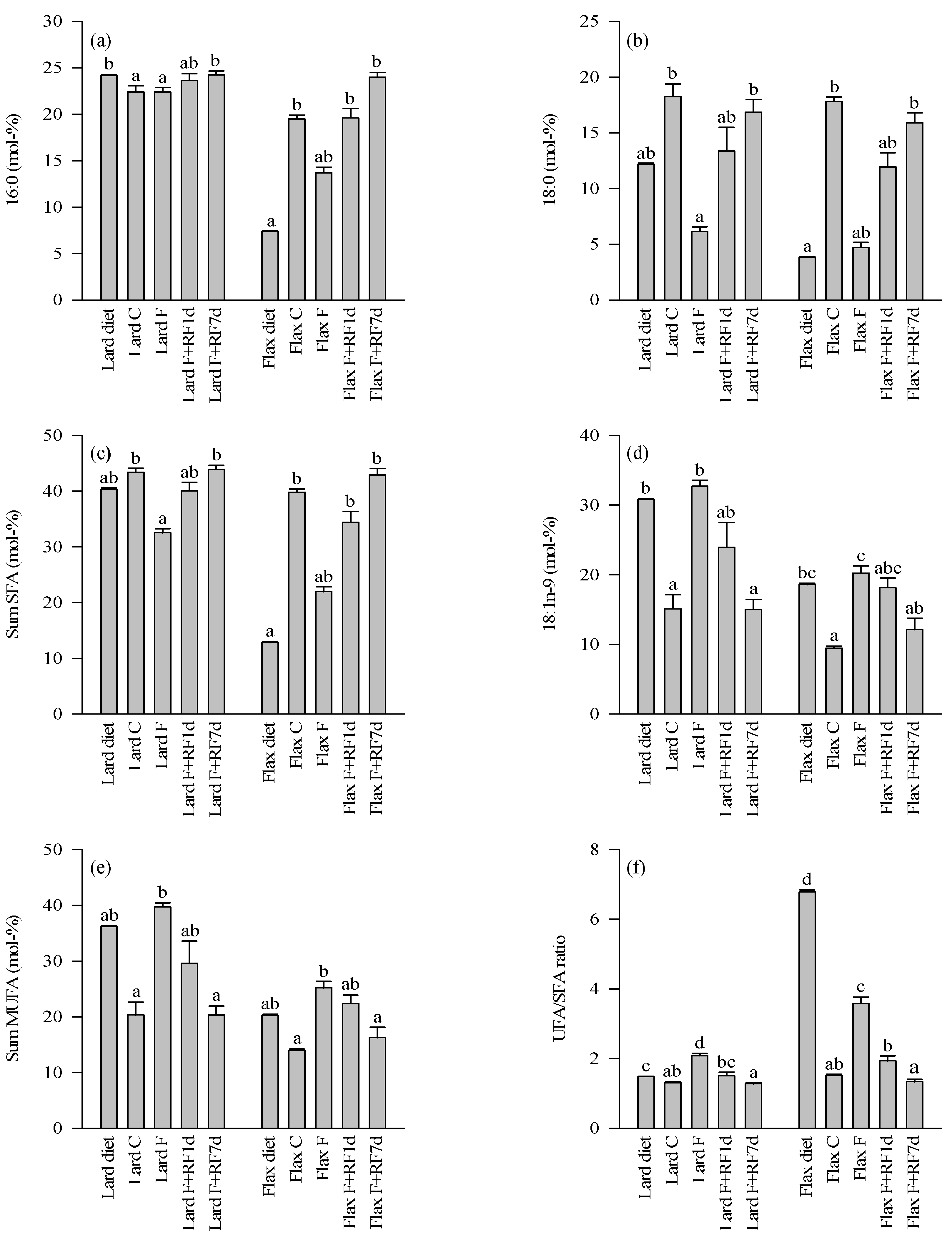
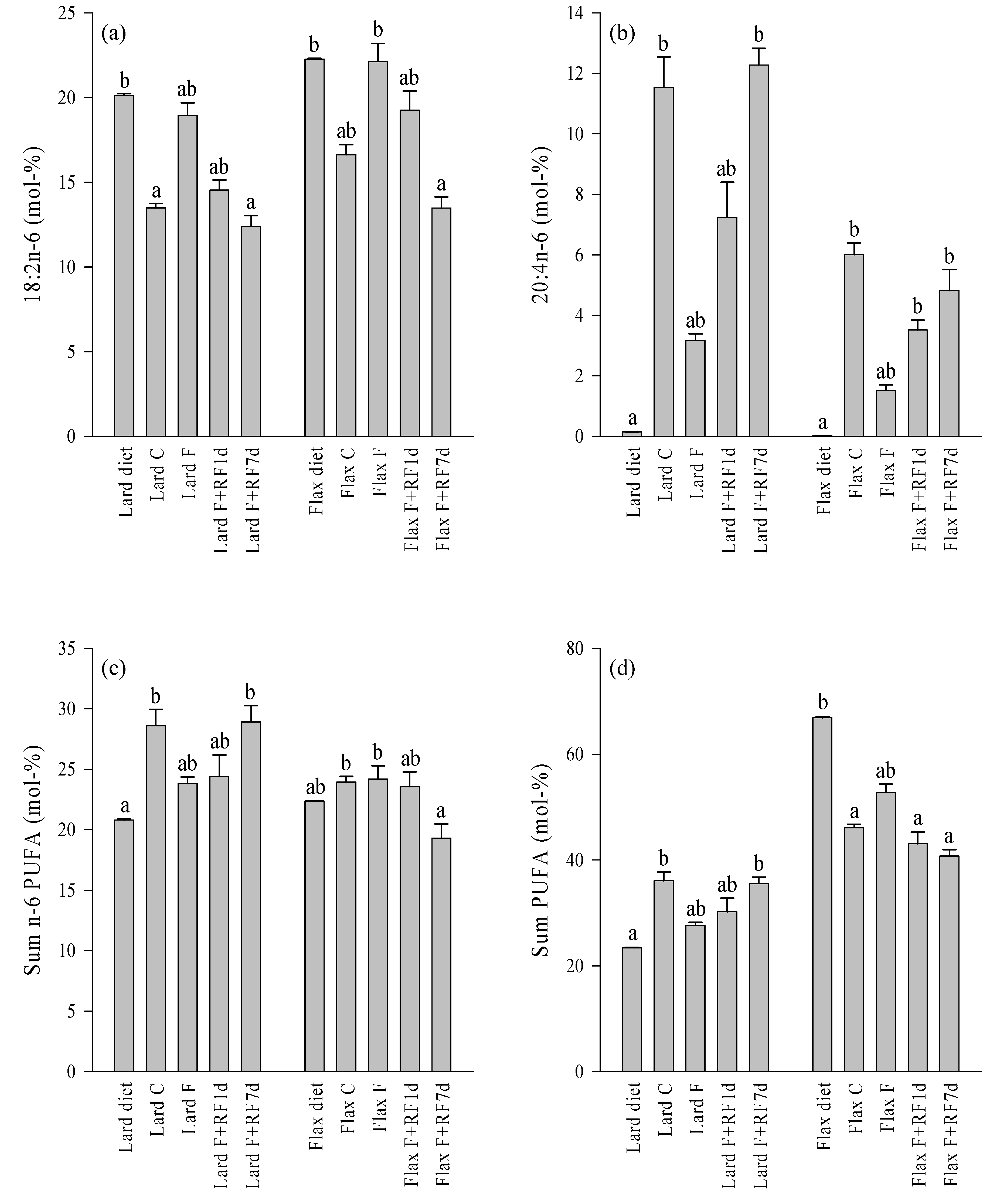
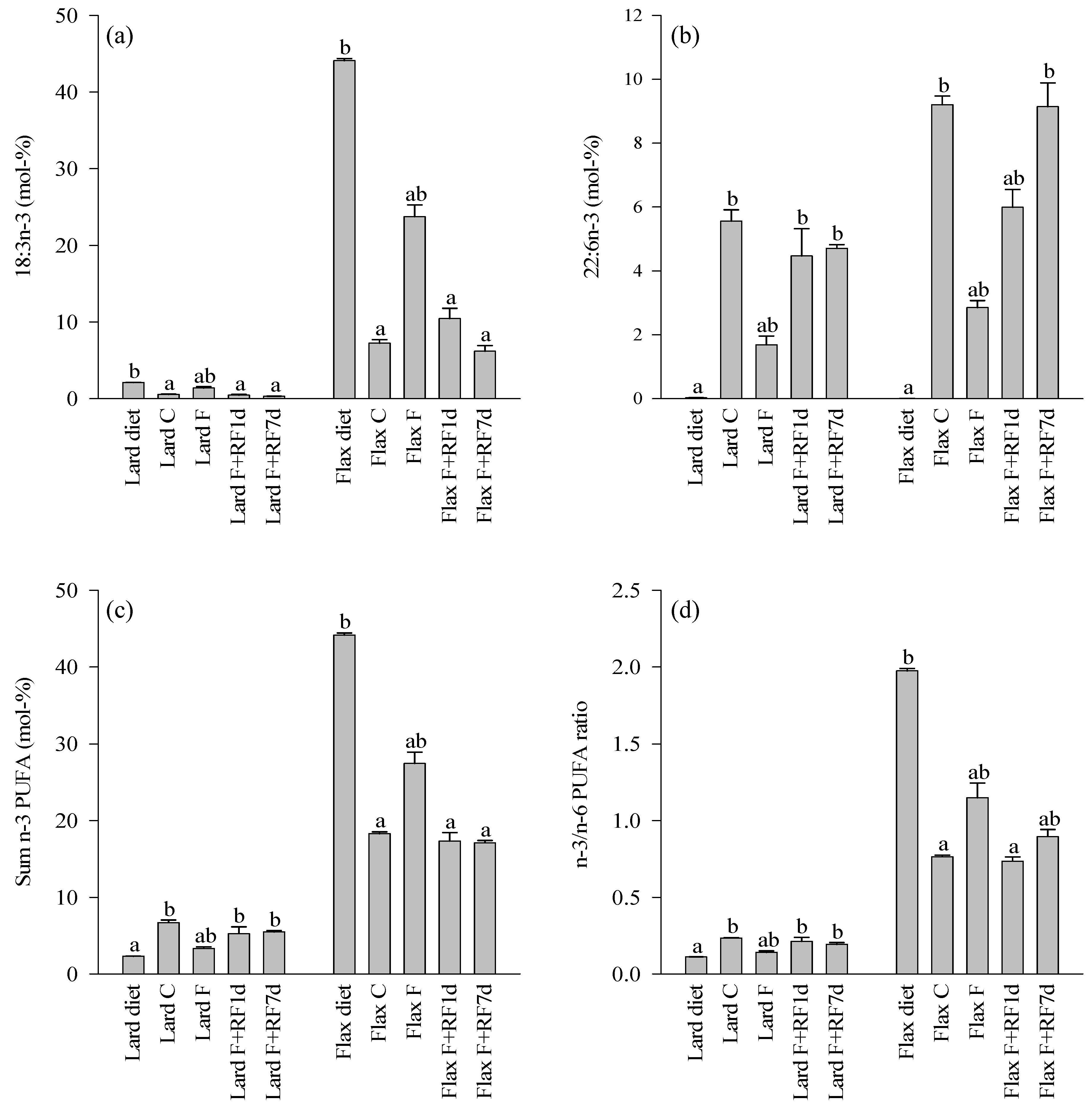
3.5. Effects of Fasting on the Liver FA Profiles
3.6. Effects of Refeeding on the Liver FA Profiles
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 155–161. [Google Scholar] [CrossRef]
- Masterton, G.S.; Plevris, J.N.; Hayes, P.C. Review article: Omega-3 fatty acids—A promising novel therapy for non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2010, 31, 679–692. [Google Scholar] [CrossRef]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Pathogenesis of non-alcoholic fatty liver disease. Q. J. Med. 2010, 103, 71–83. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef]
- Bloedon, L.T.; Szapary, P.O. Flaxseed and cardiovascular risk. Nutr. Rev. 2004, 62, 18–27. [Google Scholar] [CrossRef]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef]
- Kajikawa, S.; Harada, T.; Kawashima, A.; Imada, K.; Mizuguchi, K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 229–238. [Google Scholar] [CrossRef]
- Kelley, D.S.; Vemuri, M.; Adkins, Y.; Gill, S.H.S.; Fedor, D.; Mackey, B.E. Flaxseed oil prevents trans-10, cis-12-conjugated linoleic acid-induced insulin resistance in mice. Br. J. Nutr. 2009, 101, 701–708. [Google Scholar] [CrossRef]
- Fraulob, J.C.; Ogg-Diamantino, R.; Fernandes-Santos, C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J. Clin. Biochem. Nutr. 2010, 46, 212–223. [Google Scholar] [CrossRef]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005, 19, 136–138. [Google Scholar]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; de Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.C.; Camilo, M.E. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin. Nutr. 2006, 25, 816–823. [Google Scholar] [CrossRef]
- Montazeri, G.; Estakhri, A.; Mohamadnejad, M.; Nouri, N.; Montazeri, F.; Mohammadkani, A.; Derakhshan, M.H.; Zamani, F.; Samiee, S.; Malekzadeh, R. Serum hyaluronate as a non-invasive marker of hepatic fibrosis and inflammation in HBeAg-negative chronic hepatitis B. BMC Gastroenterol. 2005, 5, 32. [Google Scholar] [CrossRef]
- Sharma, S.; Barrett, F.; Adamson, J.; Todd, A.; Megson, I.L.; Zentler-Munro, P.L.; MacRury, S.M. Diabetic fatty liver disease is associated with specific changes in blood-borne markers. Diabetes Metab. Res. Rev. 2012, 28, 343–348. [Google Scholar] [CrossRef]
- Ichida, T.; Sugitani, S.; Satoh, T.; Matsuda, Y.; Sugiyama, M.; Yonekura, K.; Ishikawa, T.; Asakura, H. Localization of hyaluronan in human liver sinusoids: A histochemical study using hyaluronan-binding protein. Liver 1996, 16, 365–371. [Google Scholar]
- Gerdin, B.; Hällgren, R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J. Intern. Med. 1997, 242, 49–55. [Google Scholar] [CrossRef]
- Oikari, S.; Jokela, T.A.; Tammi, R.H.; Tammi, M.I. Multiple Roles of Hyaluronan as a Target and Modifier of the Inflammatory Response. In Extracellular Matrix: Pathobiology and Signaling; Karamanos, N.K., Ed.; Walter de Gruyter: Berlin, Germany, 2012; pp. 39–65. [Google Scholar]
- Clarke, S.D. Polyunsaturated fatty acid regulation of gene transcription: A molecular mechanism to improve the metabolic syndrome. J. Nutr. 2001, 131, 1129–1132. [Google Scholar]
- Shapiro, H.; Tehilla, M.; Attal-Singer, J.; Bruck, R.; Luzzatti, R.; Singer, P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin. Nutr. 2011, 30, 6–19. [Google Scholar] [CrossRef]
- Capanni, M.; Calella, F.; Biagini, M.R.; Genise, S.; Raimondi, L.; Bedogni, G.; Svegliati-Baroni, G.; Sofi, F.; Milani, S.; Abbate, R.; et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: A pilot study. Aliment. Pharmacol. Ther. 2006, 23, 1143–1151. [Google Scholar] [CrossRef]
- Spadaro, L.; Magliocco, O.; Spampinato, D.; Piro, S.; Oliveri, C.; Alagona, C.; Papa, G.; Rabuazzo, A.M.; Purrello, F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig. Liver Dis. 2008, 40, 194–199. [Google Scholar] [CrossRef]
- Nobili, V.; Bedogni, G.; Alisi, A.; Pietrobattista, A.; Risé, P.; Galli, C.; Agostoni, C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: Double-blind randomized controlled clinical trial. Arch. Dis. Child. 2011, 96, 350–353. [Google Scholar] [CrossRef]
- Mustonen, A.-M.; Saarela, S.; Nieminen, P. Food deprivation in the common vole (Microtus arvalis) and the tundra vole (Microtus oeconomus). J. Comp. Physiol. 2008, 178, 199–208. [Google Scholar]
- Mustonen, A.-M.; Käkelä, R.; Halonen, T.; Kärjä, V.; Vartiainen, E.; Nieminen, P. Fatty acid mobilization in voles—Model species for rapid fasting response and fatty liver. Comp. Biochem. Physiol. 2012, 163A, 152–160. [Google Scholar]
- Dieterich, R.A.; Preston, D.J. The tundra vole (Microtus oeconomus) as a laboratory animal. Lab. Anim. Sci. 1977, 27, 500–506. [Google Scholar]
- Dieterich, R.A.; Preston, D.J. Atherosclerosis in lemmings and voles fed a high fat, high cholesterol diet. Atherosclerosis 1979, 33, 181–189. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Gobejishvili, L.N.; Bon Homme, M.; Waigel, S.; Cave, M.; Arteel, G.; Barve, S.S.; McClain, C.J.; Deaciuc, I.V. Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J. Nutr. Biochem. 2011, 22, 38–45. [Google Scholar] [CrossRef]
- Yang, S.-F.; Tseng, J.-K.; Chang, Y.-Y.; Chen, Y.-C. Flaxseed oil attenuates nonalcoholic fatty liver of hyperlipidemic hamsters. J. Agric. Food Chem. 2009, 57, 5078–5083. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, D.; Yu, B. Effect of different dietary energy sources on induction of fatty liver-hemorrhagic syndrome in laying hens. Int. J. Poult. Sci. 2008, 7, 1232–1236. [Google Scholar] [CrossRef]
- Cherian, G.; Hayat, Z. Long-term effects of feeding flaxseeds on hepatic lipid characteristics and histopathology of laying hens. Poult. Sci. 2009, 88, 2555–2561. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. In Advances in Lipid Methodology—Two; Christie, W.W., Ed.; Oily Press: Dundee, UK, 1993; pp. 69–111. [Google Scholar]
- Mustonen, A.-M.; Pyykönen, T.; Paakkonen, T.; Ryökkynen, A.; Asikainen, J.; Aho, J.; Mononen, J.; Nieminen, P. Adaptations to fasting in the American mink (Mustela vison): Carbohydrate and lipid metabolism. Comp. Biochem. Physiol. 2005, 140A, 195–202. [Google Scholar]
- Rouvinen-Watt, K.; Mustonen, A.-M.; Conway, R.; Pal, C.; Harris, L.; Saarela, S.; Strandberg, U.; Nieminen, P. Rapid development of fasting-induced hepatic lipidosis in the American mink (Neovison vison): Effects of food deprivation and re-alimentation on body fat depots, tissue fatty acid profiles, hematology and endocrinolog. Lipids 2010, 45, 111–128. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al.; for the Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Tammi, R.; Pasonen-Seppänen, S.; Kolehmainen, E.; Tammi, M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J. Investig. Dermatol. 2005, 124, 898–905. [Google Scholar] [CrossRef]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012, 10, 50. [Google Scholar] [CrossRef]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef]
- Buckley, J.D.; Howe, P.R.C. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity—A review. Nutrients 2010, 2, 1212–1230. [Google Scholar] [CrossRef]
- Alwayn, I.P.J.; Gura, K.; Nosé, V.; Zausche, B.; Javid, P.; Garza, J.; Verbesey, J.; Voss, S.; Ollero, M.; Andersson, C.; et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 2005, 57, 445–452. [Google Scholar] [CrossRef]
- Käkelä, R.; Pölönen, I.; Miettinen, M.; Asikainen, J. Effects of different fat supplements on growth and hepatic lipids and fatty acids in male mink. Acta Agric. Scand. 2001, 51, 217–223. [Google Scholar]
- Fébel, H.; Mézes, M.; Pálfy, T.; Hermán, A.; Gundel, J.; Lugasi, A.; Balogh, K.; Kocsis, I.; Blázovics, A. Effect of dietary fatty acid pattern on growth, body fat composition and antioxidant parameters in broilers. J. Anim. Physiol. Anim. Nutr. 2008, 92, 369–376. [Google Scholar] [CrossRef]
- Carter-Kent, C.; Brunt, E.M.; Yerian, L.M.; Alkhouri, N.; Angulo, P.; Kohli, R.; Ling, S.C.; Xanthakos, S.A.; Whitington, P.F.; Charatcharoenwitthaya, P.; et al. Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 190–197. [Google Scholar] [CrossRef]
- Nelson, G.J. The lipid composition of normal mouse liver. J. Lipid Res. 1962, 3, 256–262. [Google Scholar]
- Zhu, F.-S.; Liu, S.; Chen, X.-M.; Huang, Z.-G.; Zhang, D.-W. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J. Gastroenterol. 2008, 14, 6395–6400. [Google Scholar] [CrossRef]
- Anderson, B.M.; Ma, D.W.L. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef]
- Jung, U.J.; Millman, P.N.; Tall, A.R.; Deckelbaum, R.J. n-3 Fatty acids ameliorate hepatic steatosis and dysfunction after LXR agonist ingestion in mice. Biochim. Biophys. Acta 2011, 1811, 491–497. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mustonen, A.-M.; Kärjä, V.; Kilpiö, M.; Tammi, R.; Tammi, M.; Rouvinen-Watt, K.; Halonen, T.; Nieminen, P. Manifestations of Fasting-Induced Fatty Liver and Rapid Recovery from Steatosis in Voles Fed Lard or Flaxseed Oil Lipids. Nutrients 2013, 5, 4211-4230. https://doi.org/10.3390/nu5104211
Mustonen A-M, Kärjä V, Kilpiö M, Tammi R, Tammi M, Rouvinen-Watt K, Halonen T, Nieminen P. Manifestations of Fasting-Induced Fatty Liver and Rapid Recovery from Steatosis in Voles Fed Lard or Flaxseed Oil Lipids. Nutrients. 2013; 5(10):4211-4230. https://doi.org/10.3390/nu5104211
Chicago/Turabian StyleMustonen, Anne-Mari, Vesa Kärjä, Michael Kilpiö, Raija Tammi, Markku Tammi, Kirsti Rouvinen-Watt, Toivo Halonen, and Petteri Nieminen. 2013. "Manifestations of Fasting-Induced Fatty Liver and Rapid Recovery from Steatosis in Voles Fed Lard or Flaxseed Oil Lipids" Nutrients 5, no. 10: 4211-4230. https://doi.org/10.3390/nu5104211





