Regulation of Inflammation by Short Chain Fatty Acids
Abstract
:Abbreviations
1. Introduction

2. Effects of SCFA on Leukocyte Recruitment
2.1. Leukocyte Chemotaxis
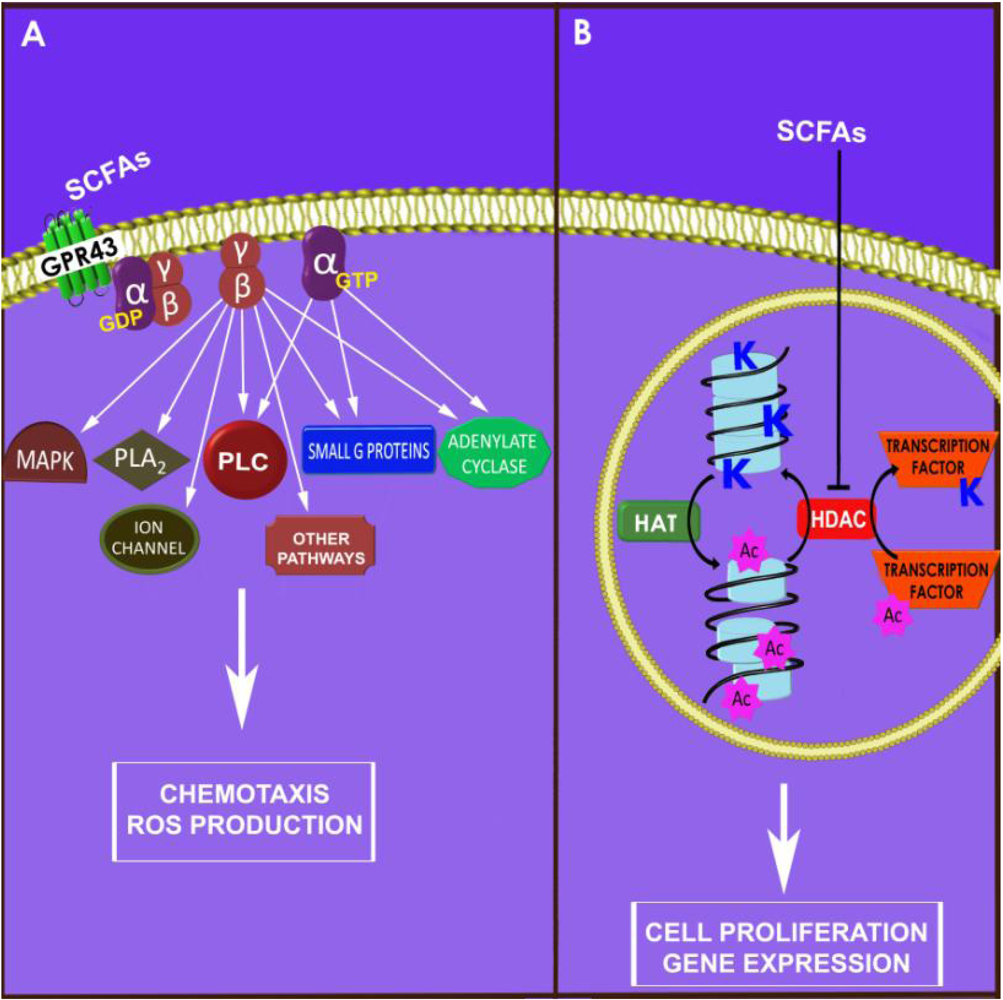
2.2. Chemokine Production
2.3. Expression of Adhesion Molecules
3. Modulation of Leukocyte Effector Mechanisms by SCFAs
3.1. Production of Inflammatory Mediators by Immune Cells
| Cell type | Effect observed | Effective fatty acid | Reference |
|---|---|---|---|
| Raw 264.7 cells | ↓ TNF-α, IL-6, NO, ↑ IL-10 | Bt | [23,48] |
| Mononuclear cells of the blood | ↓ TNF-α, ↑ PGE2 | Bt | [49] |
| Monocytes and macrophages | ↓ TNF-α | Bt | [50] |
| Monocytes | ↓ TNF-α, IL-12, IFN-γ, | Bt | [51] |
| ↑ IL-10 | |||
| ↓ MCP-1, IL-10, ↑ PGE2 | Ac, Pr and Bt | [34] | |
| Microglial cells | |||
| -N9 cells | ↑ IL-6, NO | Pr and Bt | [52] |
| -Rat primary microglia | ↓ TNF-α, IL-6, NO | Bt | [52] |
| -Murine BV2 cell | ↓ NO | Bt | [53] |
| Mesencephalic neuron-glia cultures | ↓ TNF-α, NO | Bt | [54] |
| Kupffer cells | ↓ TNF-α, ↑ PGE2 | Bt | [55] |
3.2. Effectors Mechanisms of Phagocytes
3.3. Lymphocyte Activation and Response
- Production of cytokines: incubation of lymphocytes with butyrate reduces the production of interleukin-2; this cytokine stimulates growth, differentiation and survival of antigen-selected T-lymphocytes, and interferon-γ (IFN-γ) after stimulation with concanavalin-A or anti-CD3 and anti-CD8 [76,77]. This latter cytokine is particularly important in response to viral infection, tumor cells and in auto-immune conditions. On the other hand, butyrate presents an opposite effect on the production of IL-10 by lymphocytes [75].
- Production of regulatory T (Treg) cells: this subpopulation of T cells actively suppresses immune function and is considered an attractive target for the treatment of immunological and inflammatory pathologies. HDAC inhibitors enhance the production and suppressive function of regulatory T cells [77]. Considering that SCFAs, as previously described, also suppress the activity of HDAC, we hypothesize that these fatty acids may also exert their effects on inflammation and immune responses through regulation of this subset of T cells.
4. Therapeutical Application of SCFAs in Inflammatory Conditions
| Disease | Route of administration and dosage | Effect | Reference |
|---|---|---|---|
| Inflammatory bowel disease | Diet with RS (1.53 kg/10 kg of diet) | Improvement of symptoms; epithelial cell proliferation; regeneration of laminin; growth of intestinal bacteria | [78] |
| Diet supplemented with cellobiose (9%) | Reduction of weight loss; diminished tissue edema; attenuation of inflammatory cytokine concentrations | [79] | |
| Fiber supplementation (5%) before and after TNBS colitis | Reduction in MPO and NO synthase activities; restoration of colonic glutathione levels; diminished TNF-α concentrations | [80] | |
| SB enemas (100 mM) | Increased the duration of pain in rats with colitis | [81] | |
| Improves clinical symptoms and inflammatory scores | [82] | ||
| SB enemas (100 mM) | Minor effects on colonic inflammation and oxidative stress; increased IL-10/IL-12 ratio and CCL5 concentrations | [83] | |
| 5-ASA (2 g) + SB (80 mM) enemas | Improvement versus the baseline; only one remission | [84] | |
| Oral SB (10 mg/kg) | Improvement of mucosa lesion and attenuation of the inflammatory profile of intestinal mucosa and local lymph nodes in a model of DSS-induced colitis | [85] | |
| Sepsis and ALI | SB (500 mg/kg) intravenous (i.v.) injection | Reduced serum alanine aminotransferase, MPO activity and creatinine concentrations; improved survival rates | [86] |
| Oral butyrate (10 mg/kg) | Attenuation of lung histopathological changes, alveolar hemorrhage and neutrophil infiltration | [87] | |
| TSA (2 mg/kg) or SB (200 mg/kg) intraperitoneal (i.p.) injection | Reduced neutrophil infiltration, inhibited ICAM-1 and E-selectin expression in lung | [88] | |
| Ischemia induced injury | SB (100 or 300 mg/kg, i.p.) | Reduced infarct size | [89] |
| Diminished brain infarct volume and microglial activation | [64] |
5. Conclusions
Acknowledgments
References
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010, 328, 228–231. [Google Scholar]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [PubMed]
- Uronis, J.M.; Muhlbauer, M.; Herfarth, H.H.; Rubinas, T.C.; Jones, G.S.; Jobin, C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One 2009, 4, e6026. [Google Scholar]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [PubMed]
- Atarashi, K.; Nishimura, J.; Shima, T.; Umesaki, Y.; Yamamoto, M.; Onoue, M.; Yagita, H.; Ishii, N.; Evans, R.; Honda, K.; et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008, 455, 808–812. [Google Scholar] [PubMed]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [PubMed]
- McIntyre, A.; Gibson, P.R.; Young, G.P. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 1993, 34, 386–391. [Google Scholar]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar]
- Plaisancie, P.; Dumoulin, V.; Chayvialle, J.A.; Cuber, J.C. Luminal peptide YY-releasing factors in the isolated vascularly perfused rat colon. J. Endocrinol. 1996, 151, 421–429. [Google Scholar]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [PubMed]
- Park, J.S.; Lee, E.J.; Lee, J.C.; Kim, W.K.; Kim, H.S. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-kappaB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sina, C.; Gavrilova, O.; Forster, M.; Till, A.; Derer, S.; Hildebrand, F.; Raabe, B.; Chalaris, A.; Scheller, J.; Rehmann, A.; et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009, 183, 7514–7522. [Google Scholar] [PubMed]
- Vinolo, M.A.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly, Y.M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs Induce Mouse Neutrophil Chemotaxis through the GPR43 Receptor. PLoS One 2011, 6, e21205. [Google Scholar]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [PubMed]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acid. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [PubMed]
- Maa, M.C.; Chang, M.Y.; Hsieh, M.Y.; Chen, Y.J.; Yang, C.J.; Chen, Z.C.; Li, Y.K.; Yen, C.K.; Wu, R.R.; Leu, T.H. Butyrate reduced lipopolysaccharide-mediated macrophage migration by suppression of Src enhancement and focal adhesion kinase activity. J. Nutr. Biochem. 2010, 21, 1186–1192. [Google Scholar]
- Owen, K.A.; Pixley, F.J.; Thomas, K.S.; Vicente-Manzanares, M.; Ray, B.J.; Horwitz, A.F.; Parsons, J.T.; Beggs, H.E.; Stanley, E.R.; Bouton, A.H. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 2007, 179, 1275–1287. [Google Scholar] [PubMed]
- Zapolska-Downar, D.; Naruszewicz, M. Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J. Physiol. Pharmacol. 2009, 60, 123–131. [Google Scholar]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kolodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-kappaB and PPARalpha. J. Nutr. Biochem. 2004, 15, 220–228. [Google Scholar]
- Miller, S.J.; Zaloga, G.P.; Hoggatt, A.M.; Labarrere, C.; Faulk, W.P. Short-chain fatty acids modulate gene expression for vascular endothelial cell adhesion molecules. Nutrition 2005, 21, 740–748. [Google Scholar]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [PubMed]
- Blais, M.; Seidman, E.G.; Asselin, C. Dual effect of butyrate on IL-1beta-mediated intestinal epithelial cell inflammatory response. DNA Cell Biol. 2007, 26, 133–147. [Google Scholar]
- Leung, C.H.; Lam, W.; Ma, D.L.; Gullen, E.A.; Cheng, Y.C. Butyrate mediates nucleotide-binding and oligomerisation domain (NOD) 2-dependent mucosal immune responses against peptidoglycan. Eur. J. Immunol. 2009, 39, 3529–3537. [Google Scholar]
- Bocker, U.; Nebe, T.; Herweck, F.; Holt, L.; Panja, A.; Jobin, C.; Rossol, S.; Sartor, R.B.; Singer, M.V. Butyrate modulates intestinal epithelial cell-mediated neutrophil migration. Clin. Exp. Immunol. 2003, 131, 53–60. [Google Scholar]
- Fusunyan, R.D.; Quinn, J.J.; Fujimoto, M.; MacDermott, R.P.; Sanderson, I.R. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol. Med. 1999, 5, 631–640. [Google Scholar]
- Inatomi, O.; Andoh, A.; Kitamura, K.; Yasui, H.; Zhang, Z.; Fujiyama, Y. Butyrate blocks interferon-gamma-inducible protein-10 release in human intestinal subepithelial myofibroblasts. J. Gastroenterol. 2005, 40, 483–489. [Google Scholar]
- Menzel, T.; Luhrs, H.; Zirlik, S.; Schauber, J.; Kudlich, T.; Gerke, T.; Gostner, A.; Neumann, M.; Melcher, R.; Scheppach, W. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm. Bowel Dis. 2004, 10, 122–128. [Google Scholar]
- Bohmig, G.A.; Krieger, P.M.; Saemann, M.D.; Wenhardt, C.; Pohanka, E.; Zlabinger, G.J. n-Butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: A potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology 1997, 92, 234–243. [Google Scholar]
- Dianzani, C.; Cavalli, R.; Zara, G.P.; Gallicchio, M.; Lombardi, G.; Gasco, M.R.; Panzanelli, P.; Fantozzi, R. Cholesteryl butyrate solid lipid nanoparticles inhibit adhesion of human neutrophils to endothelial cells. Br. J. Pharmacol. 2006, 148, 648–656. [Google Scholar]
- Allport, J.R.; Ding, H.T.; Ager, A.; Steeber, D.A.; Tedder, T.F.; Luscinskas, F.W. L-selectin shedding does not regulate human neutrophil attachment, rolling, or transmigration across human vascular endothelium in vitro. J. Immunol. 1997, 158, 4365–4372. [Google Scholar] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar]
- Kinne, R.W.; Brauer, R.; Stuhlmuller, B.; Palombo-Kinne, E.; Burmester, G.R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000, 2, 189–202. [Google Scholar]
- Griffin, W.S. Inflammation and neurodegenerative diseases. Am. J. Clin. Nutr. 2006, 83, 470S–474S. [Google Scholar]
- Boyle, J.J. Macrophage activation in atherosclerosis: Pathogenesis and pharmacology of plaque rupture. Curr. Vasc. Pharmacol. 2005, 3, 63–68. [Google Scholar]
- Chakravortty, D.; Koide, N.; Kato, Y.; Sugiyama, T.; Mu, M.M.; Yoshida, T.; Yokochi, T. The inhibitory action of butyrate on lipopolysaccharide-induced nitric oxide production in RAW 264.7 murine macrophage cells. J. Endotoxin Res. 2000, 6, 243–247. [Google Scholar] [PubMed]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar]
- Fukae, J.; Amasaki, Y.; Yamashita, Y.; Bohgaki, T.; Yasuda, S.; Jodo, S.; Atsumi, T.; Koike, T. Butyrate suppresses tumor necrosis factor alpha production by regulating specific messenger RNA degradation mediated through a cis-acting AU-rich element. ArthritisRheum. 2005, 52, 2697–2707. [Google Scholar]
- Saemann, M.D.; Bohmig, G.A.; Osterreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stockl, J.; Horl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar]
- Huuskonen, J.; Suuronen, T.; Nuutinen, T.; Kyrylenko, S.; Salminen, A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br. J. Pharmacol. 2004, 141, 874–880. [Google Scholar]
- Park, J.S.; Woo, M.S.; Kim, S.Y.; Kim, W.K.; Kim, H.S. Repression of interferon-gamma-induced inducible nitric oxide synthase (iNOS) gene expression in microglia by sodium butyrate is mediated through specific inhibition of ERK signaling pathways. J. Neuroimmunol. 2005, 168, 56–64. [Google Scholar]
- Chen, P.S.; Wang, C.C.; Bortner, C.D.; Peng, G.S.; Wu, X.; Pang, H.; Lu, R.B.; Gean, P.W.; Chuang, D.M.; Hong, J.S. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 2007, 149, 203–212. [Google Scholar]
- Perez, R.; Stevenson, F.; Johnson, J.; Morgan, M.; Erickson, K.; Hubbard, N.E.; Morand, L.; Rudich, S.; Katznelson, S.; German, J.B. Sodium butyrate upregulates Kupffer cell PGE2 production and modulates immune function. J. Surg. Res. 1998, 78, 1–6. [Google Scholar]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar]
- Cox, H.M.; Tough, I.R.; Woolston, A.M.; Zhang, L.; Nguyen, A.D.; Sainsbury, A.; Herzog, H. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010, 11, 532–542. [Google Scholar]
- Yao, C.; Sakata, D.; Esaki, Y.; Li, Y.; Matsuoka, T.; Kuroiwa, K.; Sugimoto, Y.; Narumiya, S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 2009, 15, 633–640. [Google Scholar]
- Sakata, D.; Yao, C.; Narumiya, S. Prostaglandin E2, an immunoactivator. J. Pharmacol. Sci. 2010, 112, 1–5. [Google Scholar]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar]
- Nakamura, Y. Regulating factors for microglial activation. Biol. Pharm. Bull. 2002, 25, 945–953. [Google Scholar]
- Kim, H.J.; Rowe, M.; Ren, M.; Hong, J.S.; Chen, P.S.; Chuang, D.M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007, 321, 892–901. [Google Scholar]
- Vinolo, M.A.; Hatanaka, E.; Lambertucci, R.H.; Newsholme, P.; Curi, R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem. Funct. 2009, 27, 48–55. [Google Scholar]
- Mills, S.W.; Montgomery, S.H.; Morck, D.W. Evaluation of the effects of short-chain fatty acids and extracellular pH on bovine neutrophil function in vitro. Am. J. Vet. Res. 2006, 67, 1901–1907. [Google Scholar]
- Eftimiadi, C.; Buzzi, E.; Tonetti, M.; Buffa, P.; Buffa, D.; van Steenbergen, M.T.; de Graaff, J.; Botta, G.A. Short-chain fatty acids produced by anaerobic bacteria alter the physiological responses of human neutrophils to chemotactic peptide. J. Infect. 1987, 14, 43–53. [Google Scholar]
- Eftimiadi, C.; Tonetti, M.; Cavallero, A.; Sacco, O.; Rossi, G.A. Short-chain fatty acids produced by anaerobic bacteria inhibit phagocytosis by human lung phagocytes. J. Infect. Dis. 1990, 161, 138–142. [Google Scholar]
- Millard, A.L.; Mertes, P.M.; Ittelet, D.; Villard, F.; Jeannesson, P.; Bernard, J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin. Exp. Immunol. 2002, 130, 245–255. [Google Scholar]
- Stringer, R.E.; Hart, C.A.; Edwards, S.W. Sodium butyrate delays neutrophil apoptosis: Role of protein biosynthesis in neutrophil survival. Br. J. Haematol. 1996, 92, 169–175. [Google Scholar]
- Nakao, S.; Moriya, Y.; Furuyama, S.; Niederman, R.; Sugiya, H. Propionic acid stimulates superoxide generation in human neutrophils. Cell Biol. Int. 1998, 22, 331–337. [Google Scholar]
- Tonetti, M.; Cavallero, A.; Botta, G.A.; Niederman, R.; Eftimiadi, C. Intracellular pH regulates the production of different oxygen metabolites in neutrophils: Effects of organic acids produced by anaerobic bacteria. J. Leukoc. Biol. 1991, 49, 180–188. [Google Scholar]
- Liu, Q.; Shimoyama, T.; Suzuki, K.; Umeda, T.; Nakaji, S.; Sugawara, K. Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand. J. Gastroenterol. 2001, 36, 744–750. [Google Scholar]
- Sandoval, A.; Trivinos, F.; Sanhueza, A.; Carretta, D.; Hidalgo, M.A.; Hancke, J.L.; Burgos, R.A. Propionate induces pH(i) changes through calcium flux, ERK1/2, p38, and PKC in bovine neutrophils. Vet. Immunol. Immunopathol. 2007, 115, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar]
- Dagtas, A.S.; Edens, R.E.; Gilbert, K.M. Histone deacetylase inhibitor uses p21(Cip1) to maintain anergy in CD4+ T cells. Int. Immunopharmacol. 2009, 9, 1289–1297. [Google Scholar]
- Tao, R.; de Zoeten, E.F.; Ozkaynak, E.; Chen, C.; Wang, L.; Porrett, P.M.; Li, B.; Turka, L.A.; Olson, E.N.; Greene, M.I.; Wells, A.D.; Hancock, W.W. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007, 13, 1299–1307. [Google Scholar]
- Jacobasch, G.; Schmiedl, D.; Kruschewski, M.; Schmehl, K. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Colorectal Dis. 1999, 14, 201–211. [Google Scholar]
- Nishimura, T.; Andoh, A.; Hashimoto, T.; Kobori, A.; Tsujikawa, T.; Fujiyama, Y. Cellobiose prevents the development of dextran sulfate sodium (DSS)-induced experimental colitis. J. Clin. Biochem. Nutr. 2010, 46, 105–110. [Google Scholar]
- Rodriguez-Cabezas, M.E.; Galvez, J.; Lorente, M.D.; Concha, A.; Camuesco, D.; Azzouz, S.; Osuna, A.; Redondo, L.; Zarzuelo, A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J. Nutr. 2002, 132, 3263–3271. [Google Scholar]
- Tarrerias, A.L.; Millecamps, M.; Alloui, A.; Beaughard, C.; Kemeny, J.L.; Bourdu, S.; Bommelaer, G.; Eschalier, A.; Dapoigny, M.; Ardid, D. Short-chain fatty acid enemas fail to decrease colonic hypersensitivity and inflammation in TNBS-induced colonic inflammation in rats. Pain 2002, 100, 91–97. [Google Scholar]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar]
- Hamer, H.M.; Jonkers, D.M.; Vanhoutvin, S.A.; Troost, F.J.; Rijkers, G.; de Bruine, A.; Bast, A.; Venema, K.; Brummer, R.J. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar]
- Vernia, P.; Annese, V.; Bresci, G.; d’Albasio, G.; D’Inca, R.; Giaccari, S.; Ingrosso, M.; Mansi, C.; Riegler, G.; Valpiani, D.; et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur. J. Clin. Invest. 2003, 33, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.L; Leonel, A.J.; Sad, A.P.; Beltrao, N.R.; Costa, T.F.; Ferreira, T.M.; Gomes-Santos, A.C.; Faria, A.M.; Peluzio, M.C.; Cara, D.C.; et al. Oral admisnistration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 2011. [Google Scholar] [CrossRef]
- Zhang, L.T.; Yao, Y.M.; Lu, J.Q.; Yan, X.J.; Yu, Y.; Sheng, Z.Y. Sodium butyrate prevents lethality of severe sepsis in rats. Shock 2007, 27, 672–677. [Google Scholar]
- Ni, Y.F.; Wang, J.; Yan, X.L.; Tian, F.; Zhao, J.B.; Wang, Y.J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, S.; Wang, C.; Jiang, R.; Wan, J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J. Surg. 2010, 34, 1676–1683. [Google Scholar]
- Hu, X.; Xu, C.; Zhou, X.; He, B.; Wu, L.; Cui, B.; Lu, Z.; Jiang, H. Sodium butyrate protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Biomed. Pharmacother. 2010. [Google Scholar] [CrossRef]
- Weber, T.E.; Kerr, B.J. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs. J. Anim. Sci. 2008, 86, 442–450. [Google Scholar]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [PubMed]
- Ulloa, L.; Tracey, K.J. The “cytokine profile”: A code for sepsis. Trends Mol. Med. 2005, 11, 56–63. [Google Scholar]
- Zhang, L.; Jin, S.; Wang, C.; Jiang, R.; Wan, J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J. Surg. 2010, 34, 1676–1683. [Google Scholar]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar]
- Balibrea, J.L.; Arias-Diaz, J. Acute respiratory distress syndrome in the septic surgical patient. World J. Surg. 2003, 27, 1275–1284. [Google Scholar]
- Kurita-Ochiai, T.; Ochiai, K.; Fukushima, K. Butyric acid-induced T-cell apoptosis is mediated by caspase-8 and -9 activation in a Fas-independent manner. Clin. Diagn. Lab. Immunol. 2001, 8, 325–332. [Google Scholar]
- Bailon, E.; Cueto-Sola, M.; Utrilla, P.; Rodriguez-Cabezas, M.E.; Garrido-Mesa, N.; Zarzuelo, A.; Xaus, J.; Galvez, J.; Comalada, M. Butyrate in vitro immune-modulatory effects might be mediated through a proliferation-related induction of apoptosis. Immunobiology 2010, 215, 863–873. [Google Scholar]
- Ramos, M.G.; Rabelo, F.L.; Duarte, T.; Gazzinelli, R.T.; Alvarez-Leite, J.I. Butyrate induces apoptosis in murine macrophages via caspase-3, but independent of autocrine synthesis of tumor necrosis factor and nitric oxide. Braz. J. Med. Biol. Res. 2002, 35, 161–173. [Google Scholar]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661. [Google Scholar]
- Niederman, R.; Buyle-Bodin, Y.; Lu, B.Y.; Robinson, P.; Naleway, C. Short-chain carboxylic acid concentration in human gingival crevicular fluid. J. Dent. Res. 1997, 76, 575–579. [Google Scholar]
- Halili, M.A.; Andrews, M.R.; Labzin, L.I.; Schroder, K.; Matthias, G.; Cao, C.; Lovelace, E.; Reid, R.C.; Le, G.T.; Hume, D.A.; et al. Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. J. Leukoc. Biol. 2010, 87, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858-876. https://doi.org/10.3390/nu3100858
Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients. 2011; 3(10):858-876. https://doi.org/10.3390/nu3100858
Chicago/Turabian StyleVinolo, Marco A.R., Hosana G. Rodrigues, Renato T. Nachbar, and Rui Curi. 2011. "Regulation of Inflammation by Short Chain Fatty Acids" Nutrients 3, no. 10: 858-876. https://doi.org/10.3390/nu3100858




