Lipophilic Compound-Mediated Gene Expression and Implication for Intervention in Reactive Oxygen Species (ROS)-Related Diseases: Mini-review
Abstract
:1. Antioxidants and Implications of their Interventions in Reactive Oxygen Species- (ROS) Related Diseases
2. Conjugated Linoleic Acid (CLA)
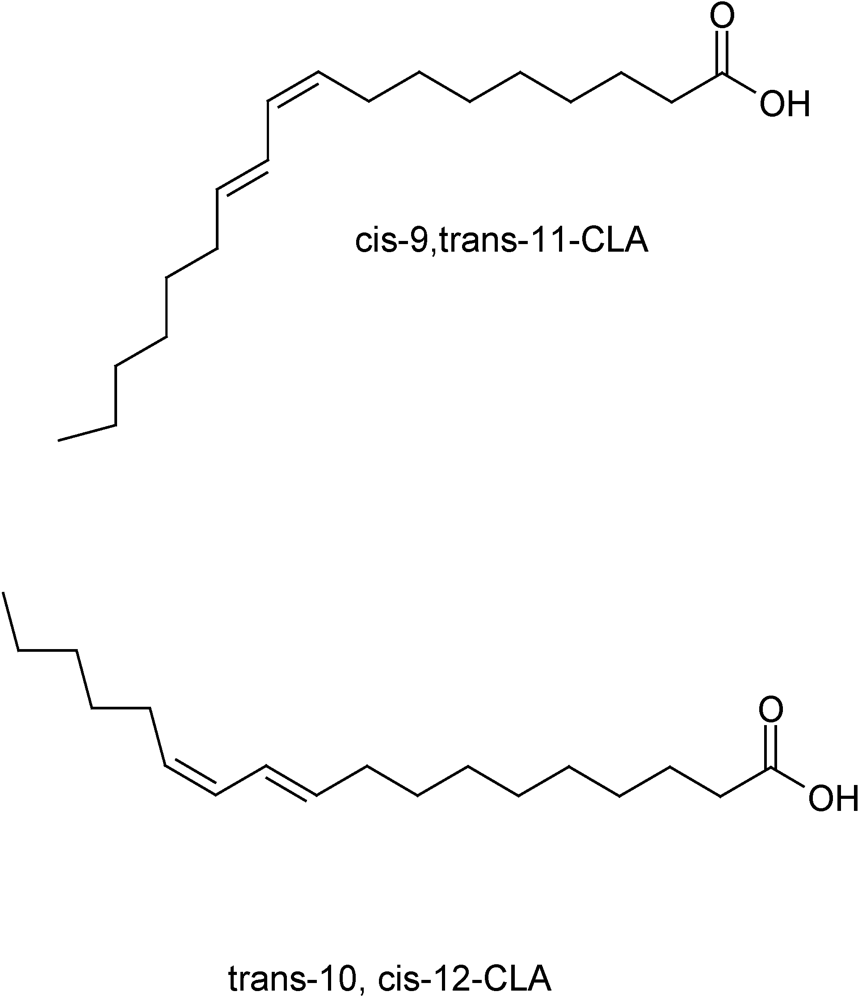
3. Vitamin E
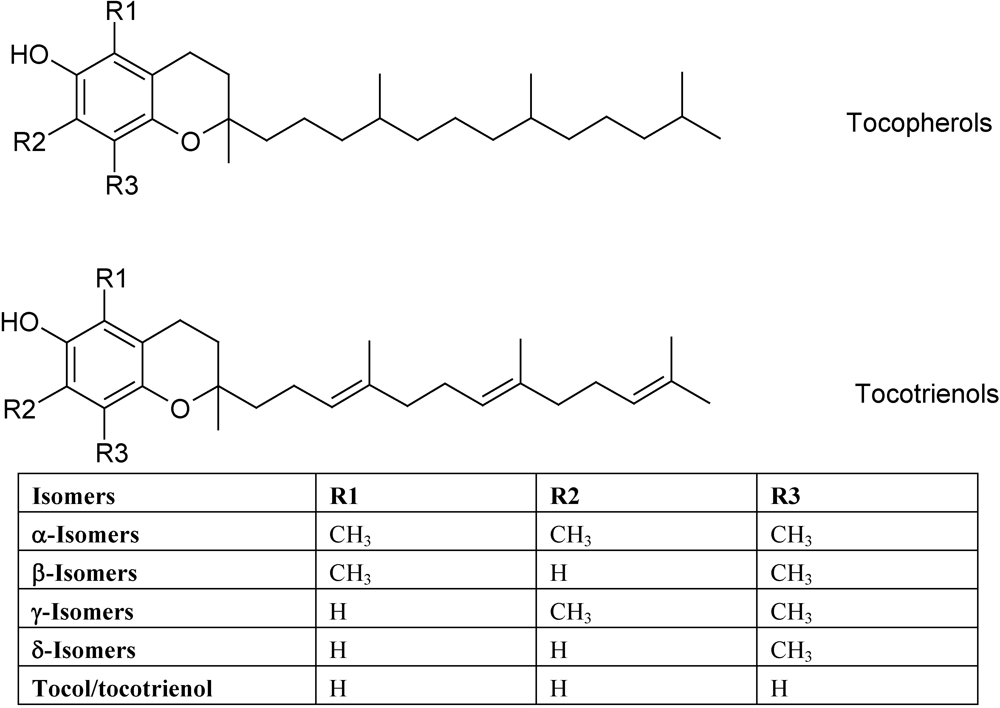
4. Cellular Micro-Environmental Factors Influence Actions of Compound and Implications for Molecular Mechanisms of Action of Cla and Vitamin E
5. Conclusions
References
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar]
- Madamanchi, N.R.; Hakim, Z.S.; Runge, M.S. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between celluar studies and clinical outcomes. J. Thromb. Haemost. 2005, 3, 254–267. [Google Scholar]
- Stocker, R.; Keaney, J.F.J. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar]
- Klipstein-Grobusch, K.; den Breeijen, J.H.; Grobbee, D.E.; Boeing, H.; Hofman, A.; Witteman, J.C. Dietary antioxidants and peripheral arterial disease: the Rotterdam Study. Am. J. Epidemiol. 2001, 154, 145–149. [Google Scholar]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.S.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar]
- Meydani, M. Vitamin E modulation of cardiovascular diseases. Ann. N Y Acad. Sci. 2004, 1031, 271–279. [Google Scholar]
- Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Albert, C.M.; Grodstein, F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women's Antioxidant and Cardiovascular Study. Circulation 2009, 119, 2772–2780. [Google Scholar]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R. J.; Gaziano, M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians' Health Study II Randomized Controlled Trial. JAMA 2008, 300, 2123–2133. [Google Scholar]
- Dietrich, M.; Jacques, P.F.; Pencina, M.J.; Lanier, K.; Keyes, M.J.; Kaur, G.; Wolf, P.A.; D'Agostino, R.B.; Vasan, R.S. VItamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham Heart Study: Does the underlying health status play a role? Atherosclerosis 2009, 205, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; LaBree, L.; Mahrer, P.R.; Sevanian, A.; Liu, C.R.; Liu, C.H.; Hwang, J.; Selzer, R.H.; Azen, S.P. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 2002, 106, 1453–1459. [Google Scholar]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 10, 14–19. [Google Scholar] [CrossRef]
- Linchtenstein, A.H. Nutrient supplements and cardiovascular disease: a heartbreaking story. J. Lipid Res. 2009, 50, S429–S433. [Google Scholar]
- Nakamura, Y.K.; Omaye, S.T. Vitamin E-modulated gene expression associated with ROS generation. J. Funct. Foods 2009, 1, 241–252. [Google Scholar]
- Bhattacharya, A.; Banu, J.; Rahman, M.; Causey, J.; Fernandes, G. Biological effects of conjugated linoleic acids in health and disease. J. Nutr. Biochem. 2006, 17, 789–810. [Google Scholar]
- Kuniyasu, H. The roles of dietary PPARgamma ligands for metastasis in colorectal cancer. PPAR Res. 2008, 2008, 529720. [Google Scholar]
- Wendel, A.A.; Purushotham, A.; Liu, L.F.; Belury, M.A. Conjugated linoleic acid induces uncoupling protein 1 in white adipose tissue of ob/ob mice. Lipids 2009, 44, 975–982. [Google Scholar]
- Lee, Y.; Thompson, J.T.; Vanden Heuvel, J.P.V. 9E, 11E-Conjugated linoleic acid increases expression of the endogenous antiinflammatory factor, interleukin-1 receptor antagonist, in RAW 264.7 cells. J. Nutr. 2009, 139, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Martinez, K.; Schmidt, S.; Mandrup, S.; Lapoint, K.; McIntosh, M.K. Antiobesity mechanisms of action of conjugated linoleic acid. J. Nutr. Biochem. 2010, 21, 171–179. [Google Scholar]
- Lee, Y.; Vanden Heuvel, J.P.V. Inihibition of macrophage adhesion activity by 9trans, 11trans-conjugated linoleic acid. J. Nutr. Biochem. 2010, 21, 490–497. [Google Scholar]
- Halade, G.V.; Rahman, M.M.; Fernandes, G. Effect of CLA isomers and their mixture on aging C57Bl/6J mice. Eur. J. Nutr. 2009, 48, 409–418. [Google Scholar]
- Belury, M.A. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu. Rev. Nutr. 2002, 22, 505–531. [Google Scholar]
- Bassaganya-Riera, J.; Hontecillas, R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin. Nutr. 2006, 25, 454–465. [Google Scholar]
- Eder, K.; Ringseis, R. Metabolism and actions of conjugated linoleic acids on atherosclerosis-related events in vascular endothelial cells and smooth muscle cells. Mol. Nutr. Food Res. 2010, 54, 17–36. [Google Scholar]
- Reynolds, C.M.; Draper, E.; Keogh, B.; Rahman, A.; Moloney, A.P.; Mills, K.H.; Loscher, C.E.; Roche, H.M. A conjugated linoleic acid-enriched beef diet attenuates lipopolysaccharide-induced inflammation in mice in part through PPARgamma-mediated suppression of toll-like receptor 4. J. Nutr. 2009, 139, 2351–2357. [Google Scholar]
- Wang, L.S.; Huang, Y.M.; Liu, S.; Yan, P.; Lin, Y.C. Conjugated linoleic acid induces apoptosis through estrogen receptor alpha in human breast tissue. BMC Cancer 2008, 8, 208. [Google Scholar]
- Sikorski, A.M.; Hebert, N.; Swain, R.A. Coujugated linoleic acid (CLA) inhibits new vessel growth in the mammalian brain. Brain Res. 2008, 1213, 35–40. [Google Scholar]
- Lawson, R.E.; Moss, A.R.; Givens, D.I. The role of dairy products in supplying conjugated linoleic acid to man’s diet: a review. Nutr. Res. Rev. 2001, 14, 153–172. [Google Scholar]
- Campbell, W.; Drake, M.A.; Larick, D.K. The impact of fortification with conjugated linoleic acid (CLA) on the quality of fluid milk. J. Dairy Sci. 2003, 86, 43–51. [Google Scholar]
- Subbaiah, P.V.; Sircar, D.; Aizezi, B.; Mintzer, E. Differential effects of conjugated linoleic acid isomers on the biophysical and biochemical properties of model membranes. Biochem. Biophys. Acta 2010, 1798, 506–514. [Google Scholar]
- Whigham, L.D.; Watras, A.C.; Schoeller, D.A. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am. J. Clin. Nutr. 2007, 85, 1203–1211. [Google Scholar]
- Schoeller, D.A.; Watras, A.C.; Whigham, L.D. A meta-analysis of the effects f conjugated linoleic acid on fat-free mass in humans. Appl. Physiol. Nutr. Metab. 2009, 34, 975–978. [Google Scholar]
- Kritchevsky, D.; Tepper, S.A.; Wright, S.; Tso, P.; Czarnecki, S.K. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J. Am. Coll. Nutr. 2000, 19, 472S–477S. [Google Scholar]
- Gaullier, J.M.; Halse, J.; Hoye, K.; Kristiansen, K.; Fagertun, H.; Vik, H.; Gudmundsen, O. Conjugated linoleic acid supplementation for 1y reduces body fat mass in healthy overweight humans. Am. J. Clin. Nutr. 2004, 79, 1118–1125. [Google Scholar]
- Gaullier, J.M.; Halse, J.; Hoye, K.; Kristiansen, K.; Fagertun, H.; Vik, H.; Gudmundsen, O. Supplementation with conjugated linoleic acid for 24 month is well tolerated by and reduces body fat mass in healthy, overweight human. J. Nutr. 2005, 135, 778–784. [Google Scholar]
- Mullen, A.; Moloney, F.; Nugent, A.P.; Doyle, L.; Cashman, K.D.; Roche, H.M. Conjugated linoleic acid supplementation reduces peripheral blood mononuclear cell interleukin-2 production in healthy middle-aged males. J. Nutr. Biochem. 2007, 8, 658–666. [Google Scholar]
- Moloney, F.; Yeow, T.P.; Mullen, A.; Nolan, J.J.; Roche, H.M. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2004, 80, 887–895. [Google Scholar]
- Tricon, S.; Burdge, G.C.; Kew, S.; Banerjee, T.; Russell, J.J.; Grimble, R.F.; Williams, C.M.; Calder, P.C.; Yaqoob, P. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid on immune cell function in healthy human. Am. J. Clin. Nutr. 2004, 80, 1626–1633. [Google Scholar]
- Tricon, S.; Burdge, G.C.; Kew, S.; Banerjee, T.; Russell, J.J.; Jones, E.L.; Grimble, R.F.; Williams, C.M.; Yaqoob, P.; Calader, P.C. Opposing effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid on blood lipids in healthy human. Am. J. Clin. Nutr. 2004, 80, 614–620. [Google Scholar]
- Riserus, U.; Vessby, B.; Arner, P.; Zethelius, B. Supplemetation with trans10cis12-conjugated linoleic acid induces hyperproinsulinaemia in obese men: close association with impaired insulin sensitivity. Diabetologia 2004, 47, 1016–1019. [Google Scholar]
- Nakamura, Y.K.; Omaye, S.T. Conjugated linoleic acid isomers' roles in the regulation of PPARγ and NF-κB DNA binding and subsequent expression of antioxidant enzymes in human umbilical vein endothelial cells. Nutrition 2009, 25, 800–811. [Google Scholar]
- Lass, A.; Sohal, R.S. Effect of coenzymes Q10 and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. FASEB J. 2000, 14, 87–94. [Google Scholar]
- Sen, C.K.; Khanna, S.; Rink, C.; Roy, S. Tocotrienols: the emerging face of natural vitamin E. Vitam. Horm. 2007, 76, 203–261. [Google Scholar]
- Khanna, S.K.; Roy, S.; Ryu, H.; Bahadduri, P.; Swaan, P.W.; Ratan, R.R.; Sen, C.K. Molecular basis of vitamin E action: Tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J. Biol. Chem. 2003, 278, 43508–43515. [Google Scholar]
- Kinlay, S.; Behrendt, D.; Fang, J.C.; Delagrange, D.; Morrow, J.; Witztum, J.L.; Rifai, N.; Selwyn, A.P.; Creager, M. A.; Ganz, P. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J. Am. Coll. Nutr. 2004, 43, 629–634. [Google Scholar]
- Burnett-Hartman, A.N.; Fitzpatrick, A.L.; Gao, K.; Jackson, S.A.; Schreiner, P.J. Supplement use contributes to meeting recommended dietary intakes for calcium, magnesium, and vitamin C in four ethnicities of middle-aged and older Americans: the multi-ethnic study of atheroslerosis. J. Am. Diet. Assoc. 2008, 109, 422–429. [Google Scholar]
- Omaye, S.T. Safety facets of antioxidant supplements. Top. Clin. Nutr. 1998, 14, 26–41. [Google Scholar]
- Nakamura, Y.K.; Omaye, S.T. Age-related changes of serum lipoprotein oxidation in rats. Life Sci. 2004, 74, 1265–1275. [Google Scholar]
- Nakamura, Y.K.; Read, M.H.; Elias, J.W.; Omaye, S.T. Oxidation of serum low-density lipoprotein (LDL) and antioxidant status in young and elderly humans. Arch. Gerontol. Geriatr. 2006, 42, 265–276. [Google Scholar]
- Campbell, S.E.; Stone, W.L.; Whaley, S.G.; Qui, M.; Krishnan, K. Gamma tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma expression in SW 480 human colon cancer cell lines. BMC Cancer 2003, 3, 25. [Google Scholar]
- Nakamura, Y.K.; Omaye, S.T. Alpha-tocopherol modulates human umbilical vein endothelial cell expression of Cu/Zn superoxide dismutase and catalse and lipid peroxidation in a concentration dependent manner. Nutr. Res. 2008, 28, 671–680. [Google Scholar]
- Okuno, Y.; Matsuda, M.; Miyata, Y.; Fukuhara, A.; Komoro, R.; Shimabukuro, M.; Shimomura, I. Human catalase gene is regulated by peroxisome proliferator-activated receptor-gamma through a response element distinct from that of mouse. Endocr. J. 2010, 57, 303–309. [Google Scholar]
- Albright, C.D.; Klem, E.; Shah, A.A.; Gallagher, P. Breast cancer cell-targeted oxidative stress: Enhancement of cancer cell upake of conjugated linoleic acid, activation of p53, and inhibition of proliferation. Exp. Mol. Pathol. 2005, 79, 118–125. [Google Scholar]
- O'Shea, M.; Stanton, C.; Devery, R. Antioxidant enzyme defense responses of human MCF-7 and SW480 cancer cells to conjugated linoleic acid. Anticancer Res. 1999, 19, 1953–1960. [Google Scholar]
- Torres-Duarte, A.P.; Vanderhoek, J.Y. Conjugated linoleic acid exhibits stimulatory and inhibitory effects on prostanoid production in human endothelial cells and platelet. Biochem. Biophys. Acta 2003, 1640, 69–76. [Google Scholar]
- Smedman, A.; Vessby, B.; Basu, S. Isomer-specific effects of conjugated linoleic acid on lipid peroxidation in humans: regulation by alpha-tocopherol and cyclo-oxigenase-2 inhibitor. Clin. Sci. (Lond) 2004, 106, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Barnes, D.; Butz, D.; Bjorling, D.; Cook, M.E. 10t, 12c-conjugated linoleic acid inhibits lipopolysaccharide-induced cyclooxygenase expression in vitro and in vivo. J. Lipid Res. 2005, 46, 2134–2142. [Google Scholar]
- Winterbone, M.S.; Sampson, M.J.; Saha, S.; Hughes, J.C.; Hughes, D.A. Pro-oxidant effect of α-tocopherol in patients with type 2 diabetes after an oral glucoese tolelance test - a randomised controlled trial. Cardiovasc. Diabetol. 2007, 6, 8. [Google Scholar]
- Wu, D.; Liu, L.; Meydani, M.; Meydani, S.N. Vitamin E increases production of vasodilator prostanoids in human aortic endothelial cells through opposing effects on cycloxygenase-2 and phopholipase A2. J. Nutr. 2005, 135, 1847–1853. [Google Scholar]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cell. Proc. Natl. Acad. Sci. USA. 2000, 97, 11494–11499. [Google Scholar]
- Jiang, Q.; Ames, B.N. Tocopherol, but not α-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rat. FASEB J. 2003, 17, 816–822. [Google Scholar]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. Tocotrienol inhibits nuclear factor-κB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar]
- Shah, S.J.; Sylvester, P.W. γ-Tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor κB activity. Exp. Biol. Med. (Maywood) 2005, 230, 235–241. [Google Scholar] [PubMed]
- Matthews, J.R.; Kaszubska, W.; Turcatti, G.; Wells, T.N.C.; Hay, R.T. Role of cycteine62 in DNA recognition by the p50 subunit of NF-kappaB. Nucleic Acids Res. 1993, 21, 1727–1734. [Google Scholar]
- Muller, C.W.; Rey, F.A.; Sodeoka, M.; Verdine, G.L.; Harrison, S.C. Structure of the NF-kappaB p50 homodimer bound to DNA. Nature 1995, 373, 311–317. [Google Scholar]
- Cao, S.; Zhang, X.; Edwards, J.P.; Mosser, D.M. NF-kB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 2006, 281, 26041–26050. [Google Scholar]
- Yashin, A.I. Hormesis against aging and diseases: using properties of biological adaptation for health and survival improvement. Dose Response 2010, 8, 41–47. [Google Scholar]
- Calabrese, E.J. Hormesis and medicine. Br. J. Clin. Pharmacol. 2008, 66, 594–617. [Google Scholar]
- Hayes, D.P. Nutrition hormesis. European Journal of Clinical Nutrition 2007, 61, 147–159. [Google Scholar]
- Kritchevsky, D. Diet and atherosclerosis. J. Nutr. Health Aging 2001, 5, 155–159. [Google Scholar]
- Wong, S.W.; Kwon, M.; Choi, A.M.K.; Kim, H.; Nakahira, K.; Hwang, D.H. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-depedent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar]
- Ziouzenkova, O.; Plutzky, J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008, 582, 32–38. [Google Scholar]
- Wood, R.J. Vitamin D and adipogenesis: new molecular insights. Nutr. Rev. 2008, 66, 40–46. [Google Scholar]
- Deb, D.K.; Chen, Y.; Zhang, Z.; Zhang, Y.; Szeto, F.L.; Wong, K.E.; Kong, J.; Li, Y.C. 1, 25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-kappa B pathway. Am. J. Physiol. Renal Physiol. 2009, 296, F1212–F1218. [Google Scholar]
- Peehl, D.M.; Feldman, D. Interaction of nuclear receptor ligands with the vitamin D signaling pathway in prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 307–315. [Google Scholar]
- Pavan, B.; Biondi, C.; Dalpiaz, A. Nuclear retinoic acid receptor beta as a tool in chemoprevetion trials. Curr. Med. Chem. 2006, 13, 3553–3563. [Google Scholar]
- Bassaganya-Riera, J.; Reynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPARgamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004, 127, 777–791. [Google Scholar]
- Zingg, J.M.; Azzi, A.; Meydani, M. Genetic polymorphisms as determinants for disease-preventive effects of vitamin E. Nutr. Rev. 2008, 66, 406–414. [Google Scholar]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; Planells, R.; Lairon, D. Human plasma levels of vitamin E and carotinoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J. Nutr. 2007, 137, 2653–2659. [Google Scholar]
- Eck, P.; Christian Erichsen, H.; Taylor, J.G.; Corpe, C.; Chanock, S.J.; Levine, M. Genomic and fuctional analysis of the sodium-dependent vitamin C transporter SLC23A1-SVCT1. Genes Nutr. 2007, 2, 143–145. [Google Scholar]
- Malerba, G.; Schaeffer, L.; Xumerle, L.; Klopp, N.; Trabetti, E.; Biscuola, M.; Cavallari, U.; Galavotti, R.; Martinelli, N.; Guarini, P.; Girelli, D.; Olivieri, O.; Corrocher, R.; Heinrich, J.; Pignatti, P.F.; Illig, T. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 2008, 43, 289–299. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nakamura, Y.K.; Omaye, S.T. Lipophilic Compound-Mediated Gene Expression and Implication for Intervention in Reactive Oxygen Species (ROS)-Related Diseases: Mini-review. Nutrients 2010, 2, 725-736. https://doi.org/10.3390/nu2070725
Nakamura YK, Omaye ST. Lipophilic Compound-Mediated Gene Expression and Implication for Intervention in Reactive Oxygen Species (ROS)-Related Diseases: Mini-review. Nutrients. 2010; 2(7):725-736. https://doi.org/10.3390/nu2070725
Chicago/Turabian StyleNakamura, Yukiko K., and Stanley T. Omaye. 2010. "Lipophilic Compound-Mediated Gene Expression and Implication for Intervention in Reactive Oxygen Species (ROS)-Related Diseases: Mini-review" Nutrients 2, no. 7: 725-736. https://doi.org/10.3390/nu2070725



