Daily Consumption of α-Linolenic Acid Increases Conversion Efficiency to Eicosapentaenoic Acid in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Experiments
2.2.1. Institutional Approval of the Study Protocols
2.2.2. Animals
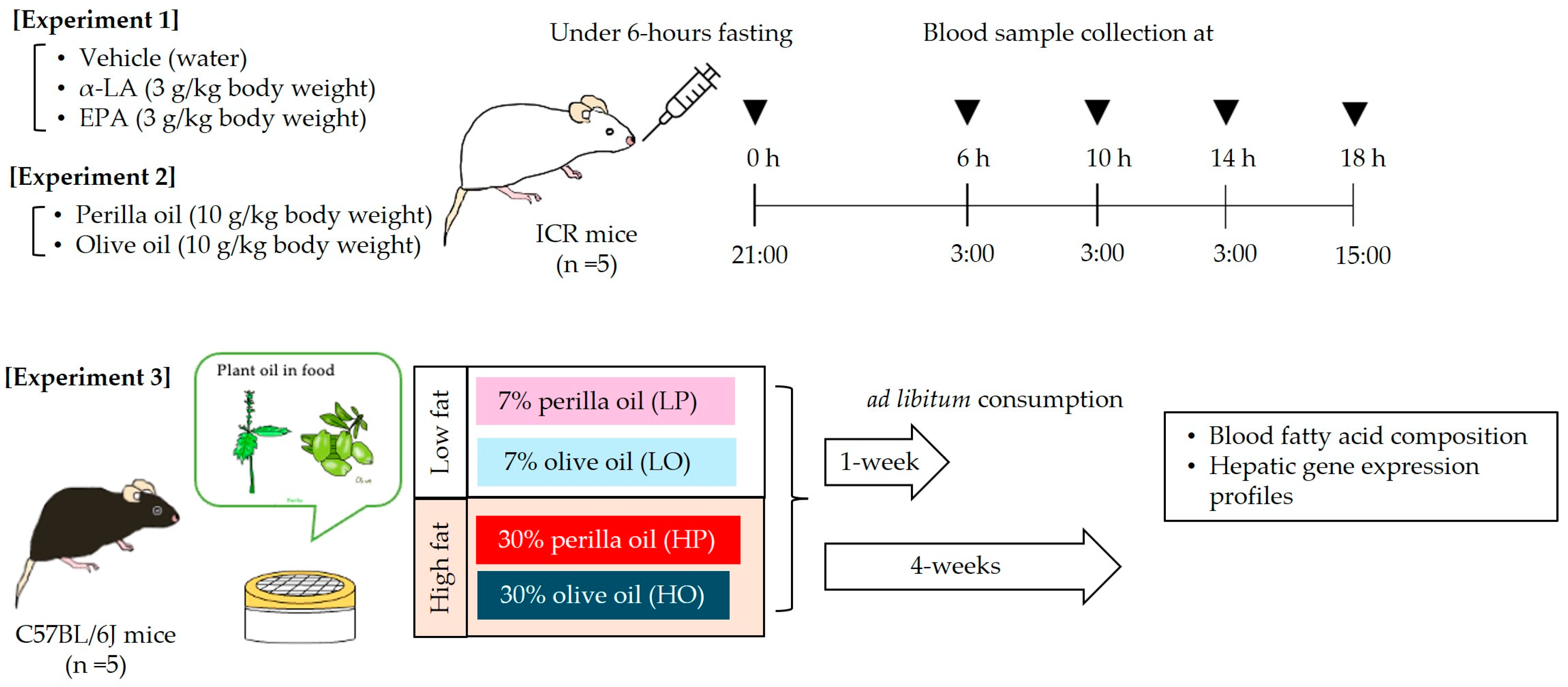
2.2.3. Experimental Design
2.3. Biochemical Parameters
2.3.1. Circulating Fatty Acids
2.3.2. Quantitative Reverse Transcription (RT)-PCR
2.4. Statistical Analysis
3. Results
3.1. Pharmacodynamics of α-LA and EPA after a Single Administration
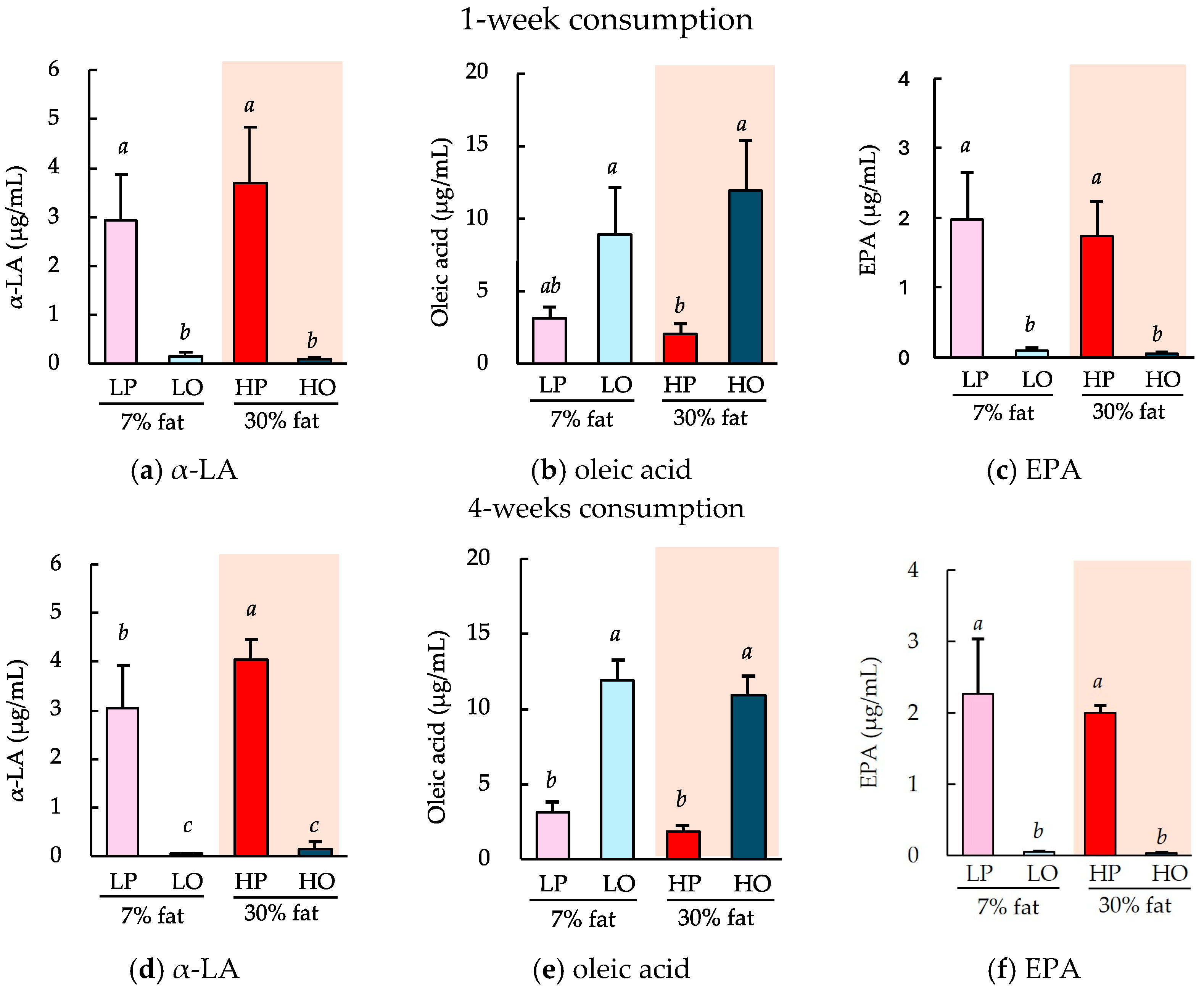
3.2. Effects of a Single Administration of Perilla Oil or Olive Oil on EPA Biosynthesis
3.3. Effect of the Daily Consumption of Perilla Oil or Olive Oil on EPA Biosynthesis
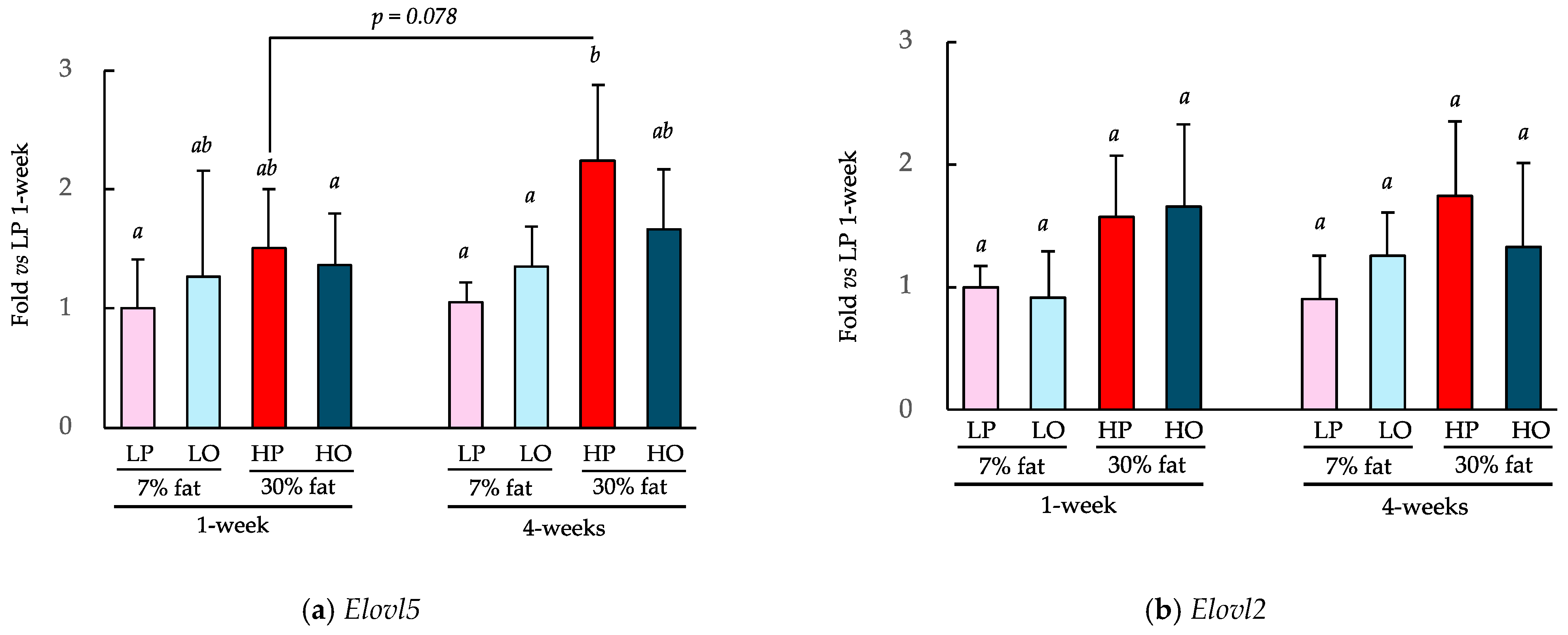
3.4. Effect of Daily Consumption of Each Oil on the Expression of Genes Encoding Fatty Acid Synthase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ciccone, L.; Nencetti, S.; Rossello, A.; Barlettani, L.; Tonali, N.; Nieri, P.; Orlandini, E. Omega-3 PUFAs as a dietary supplement in senile systemic amyloidosis. Nutrients 2023, 15, 749. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58, 25145. [Google Scholar] [CrossRef] [PubMed]
- Michaeloudes, C.; Christodoulides, S.; Christodoulou, P.; Kyriakou, T.C.; Patrikios, I.; Stephanou, A. Variability in the clinical effects of the omega-3 polyunsaturated fatty acids DHA and EPA in cardiovascular disease-possible causes and future considerations. Nutrients 2023, 15, 4830. [Google Scholar] [CrossRef] [PubMed]
- Zailani, H.; Satyanarayanan, S.K.; Liao, W.C.; Hsu, Y.T.; Huang, S.Y.; Galecki, P.; Su, K.P.; Chang, J.P. Roles of omega-3 polyunsaturated fatty acids in managing cognitive ompairment in chronic obstructive pulmonary disease: A review. Nutrients 2023, 15, 4363. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. omega3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Tomczyk, M.; Heileson, J.L.; Babiarz, M.; Calder, P.C. Athletes can benefit from increased Iintake of EPA and DHA-evaluating the evidence. Nutrients 2023, 15, 4925. [Google Scholar] [CrossRef]
- Neff, L.M.; Culiner, J.; Cunningham-Rundles, S.; Seidman, C.; Meehan, D.; Maturi, J.; Wittkowski, K.M.; Levine, B.; Breslow, J.L. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J. Nutr. 2011, 141, 207–213. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing omega-3 long-chain polyunsaturated fatty acid content of dairy-derived foods for human consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 2002, 27, 467. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Julien, P.; Vohl, M.C. Effects of FADS and ELOVL polymorphisms on indexes of desaturase and elongase activities: Results from a pre-post fish oil supplementation. Genes Nutr. 2014, 9, 437. [Google Scholar] [CrossRef]
- Chiu, C.C.; Su, K.P.; Cheng, T.C.; Liu, H.C.; Chang, C.J.; Dewey, M.E.; Stewart, R.; Huang, S.Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar]
- Yang, J.E.; Choe, E.; Chung, L. A cross-cultural comparison of the sensory characteristics of perilla oil by American, Chinese, and Korean Panels. Food Sci. Biotechnol. 2012, 21, 399–407. [Google Scholar] [CrossRef]
- Revelou, P.K.; Xagoraris, M.; Alexandropoulou, A.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Chemometric study of fatty acid composition of virgin olive oil from four widespread Greek cultivars. Molecules 2021, 26, 4151. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M. Evaluating the appropriate oral lipid tolerance test model for investigating plasma triglyceride elevation in mice. PLoS ONE 2020, 15, e0235875. [Google Scholar] [CrossRef]
- Matsuzaka, H.; Matsuyama, H.; Tanaka, W.; Tajiri, H.; Sakakibara, H. Selective consumption of fish oil at end of the day increases the physiological fatty acid compositions of eicosapentaenoic acid and docosahexaenoic acid in mice. Molecules 2022, 27, 1271. [Google Scholar] [CrossRef]
- Tanaka, W.; Matsuyama, H.; Yokoyama, D.; Yamashita, Y.; Ashida, H.; Sakono, M.; Sakakibara, H. Daily consumption of black soybean (Glycine max L.) seed coat polyphenols T attenuates dyslipidemia in apolipoprotein E-deficient mice. J. Funct. Food 2020, 72, 104054. [Google Scholar] [CrossRef]
- Ichi, T.; Fujiwara, Y. Essential fatty acid and nutrition. Vitamins 2020, 94, 197–202. [Google Scholar]
- Robichaud, P.P.; Munganyiki, J.E.; Boilard, E.; Surette, M.E. Polyunsaturated fatty acid elongation and desaturation in activated human T-cells: ELOVL5 is the key elongase. J. Lipid Res. 2018, 59, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Compartmental modeling to quantify alpha-linolenic acid conversion after longer term intake of multiple tracer boluses. J. Lipid Res. 2005, 46, 1474–1483. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Harris, W.S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J. Lipid Res. 2003, 44, 455–463. [Google Scholar] [CrossRef]
- Morentin Gutierrez, P.; Yates, J.; Nilsson, C.; Birtles, S. Evolving data analysis of an Oral Lipid Tolerance Test toward the standard for the Oral Glucose Tolerance Test: Cross species modeling effects of AZD7687 on plasma triacylglycerol. Pharmacol. Res. Perspect. 2019, 7, e00465. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lim, S.J.; Ryu, Y.M.; Park, H.R.; Suh, H.J.; Han, S.H. Absorption rate of krill oil and fish oil in blood and brain of rats. Lipids Health Dis. 2018, 17, 162. [Google Scholar] [CrossRef]
- el Boustani, S.; Colette, C.; Monnier, L.; Descomps, B.; Crastes de Paulet, A.; Mendy, F. Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids 1987, 22, 711–714. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Hwang, H.J.; Shin, K.O.; Jeon, W.M.; Choi, K.S. Effects of perilla oil on plasma concentrations of cardioprotective (n-3) fatty acids and lipid profiles in mice. Nutr. Res. Pract. 2013, 7, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men*. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C.; International Society for the Study of Fatty, A.; Lipids, I. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Wada, S. Metabolism and function of icosapentaenoic and docosahexaenoic acids. J. Jap. Oil Chem. Soc. 1988, 37, 781–787. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.E.; Park, Y. Low-linoleic acid diet and oestrogen enhance the conversion of alpha-linolenic acid into DHA through modification of conversion enzymes and transcription factors. Br. J. Nutr. 2019, 121, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Torres-Gonzalez, M.; Tripathy, S.; Botolin, D.; Christian, B.; Jump, D.B. Elevated hepatic fatty acid elongase-5 activity affects multiple pathways controlling hepatic lipid and carbohydrate composition. J. Lipid Res. 2008, 49, 1538–1552. [Google Scholar] [CrossRef]


 ) or olive oil (♢). Data are mean ± SD (n = 5). Differing alphabetical superscripts indicate significant differences (p < 0.05).
) or olive oil (♢). Data are mean ± SD (n = 5). Differing alphabetical superscripts indicate significant differences (p < 0.05).
 ) or olive oil (♢). Data are mean ± SD (n = 5). Differing alphabetical superscripts indicate significant differences (p < 0.05).
) or olive oil (♢). Data are mean ± SD (n = 5). Differing alphabetical superscripts indicate significant differences (p < 0.05).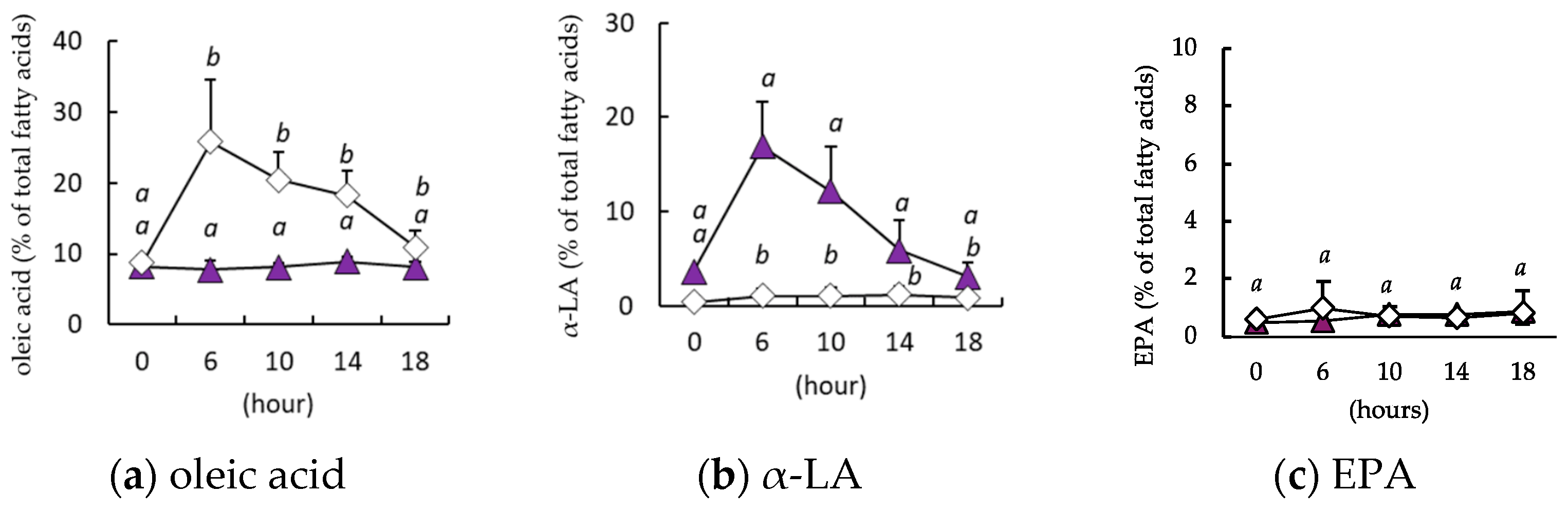
| Low-Fat Diet (7% Fat) | High-Fat Diet (30% Fat) | ||||
|---|---|---|---|---|---|
| Soybean Oil (LS) | Perilla Oil (LP) | Olive Oil (LO) | Perilla Oil (HP) | Olive Oil (HO) | |
| β-Cornstarch | 39.75 | 39.75 | 39.75 | 16.75 | 16.75 |
| α-Cornstarch | 13.2 | 13.2 | 13.2 | 13.2 | 13.2 |
| Casein | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean oil | 7.0 | – | – | – | – |
| Perilla oil | – | 7.0 | – | – | 30.0 |
| Olive oil | – | – | 7.0 | 30.0 | – |
| Sucrose | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Cellulose | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Vitamin mixture | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral mixture | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| l-Cysteine | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| t-Butylhydroquinone | 0.0014 | 0.0014 | 0.0014 | 0.0014 | 0.0014 |
| Energy (kcal/g) | 3.95 | 3.96 | 3.95 | 5.13 | 5.13 |
| Primer | Sequences (5′→3′) | |
|---|---|---|
| Elovl2 | Forward | GAGAAGGTGATGTCCGGGTAG |
| Reverse | ACATGGACGCGTGGTGATAG | |
| Elovl5 | Forward | TTCCTCTTGCATCGCGGCT |
| Reverse | CCATCCTTTGACTCTTGTATCTCGG | |
| β-actin | Forward | GTGGGAATGGGTCAGAAGG |
| Reverse | GGTCATCTTTTCACGGTTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watabe, S.; Tanaka, W.; Sakakibara, H.; Yokoyama, D. Daily Consumption of α-Linolenic Acid Increases Conversion Efficiency to Eicosapentaenoic Acid in Mice. Nutrients 2024, 16, 1407. https://doi.org/10.3390/nu16101407
Watabe S, Tanaka W, Sakakibara H, Yokoyama D. Daily Consumption of α-Linolenic Acid Increases Conversion Efficiency to Eicosapentaenoic Acid in Mice. Nutrients. 2024; 16(10):1407. https://doi.org/10.3390/nu16101407
Chicago/Turabian StyleWatabe, Saori, Wataru Tanaka, Hiroyuki Sakakibara, and Daigo Yokoyama. 2024. "Daily Consumption of α-Linolenic Acid Increases Conversion Efficiency to Eicosapentaenoic Acid in Mice" Nutrients 16, no. 10: 1407. https://doi.org/10.3390/nu16101407




