Effects of Fermented Artemisia annua L. and Salicornia herbacea L. on Inhibition of Obesity In Vitro and In Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Medicinal Plants
2.2. Fermentation of Medicinal Plants
2.3. Extraction of Fermented Plants
2.4. Chemicals and Reagents
2.5. Preparation of Metabolic Extracts
2.6. UHPLC–LTQ–Orbitrap–MS Profiling
2.7. Cell Viability Assay
2.8. Oil Red O Staining
2.9. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.10. Animals and Diet
2.11. Sample Preparation and Treatment
2.12. Histological Analysis
2.13. Serum and Hepatic Triglyceride Analysis
2.14. Data Processing and Statistical Analysis
3. Results
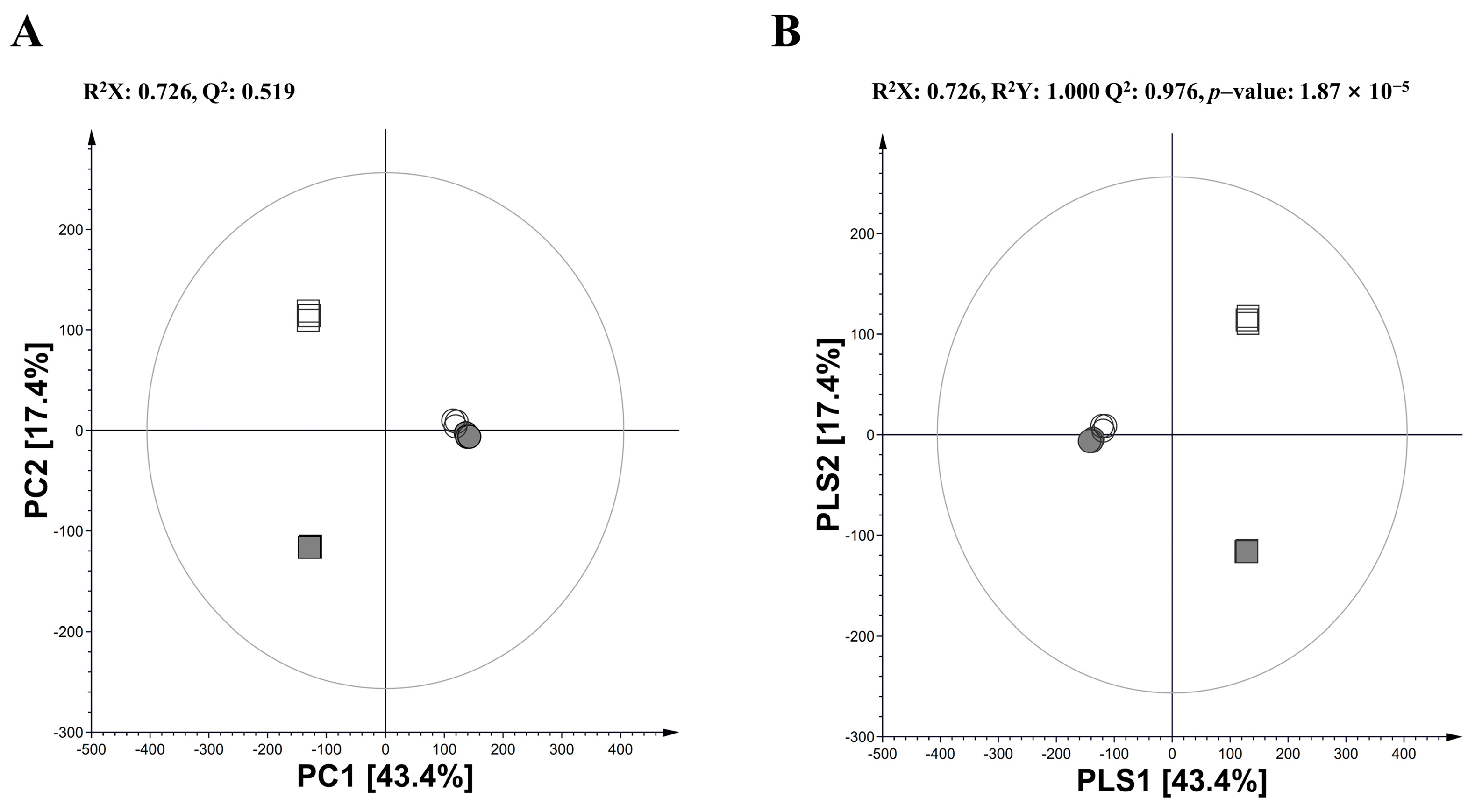
3.1. Multivariate Analysis in Annual Wormwood and Glasswort with LAB-Mediated Fermentation
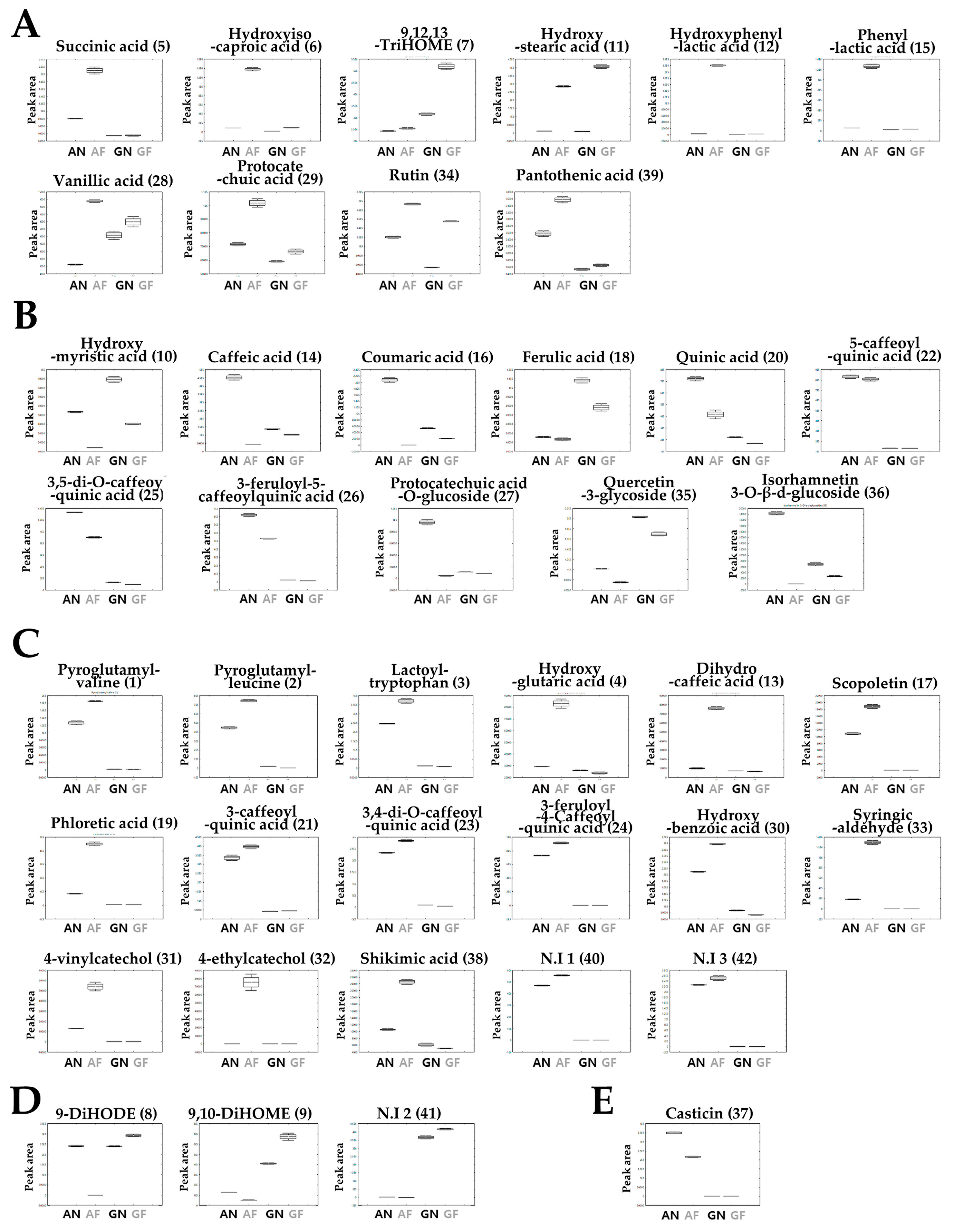
3.2. Relative Metabolite Abundance in Annual Wormwood and Glasswort with LAB-Mediated Fermentation
3.3. Phenolic Acid Degradation Pathways by Biotransformation of Plant Substrates Using LAB
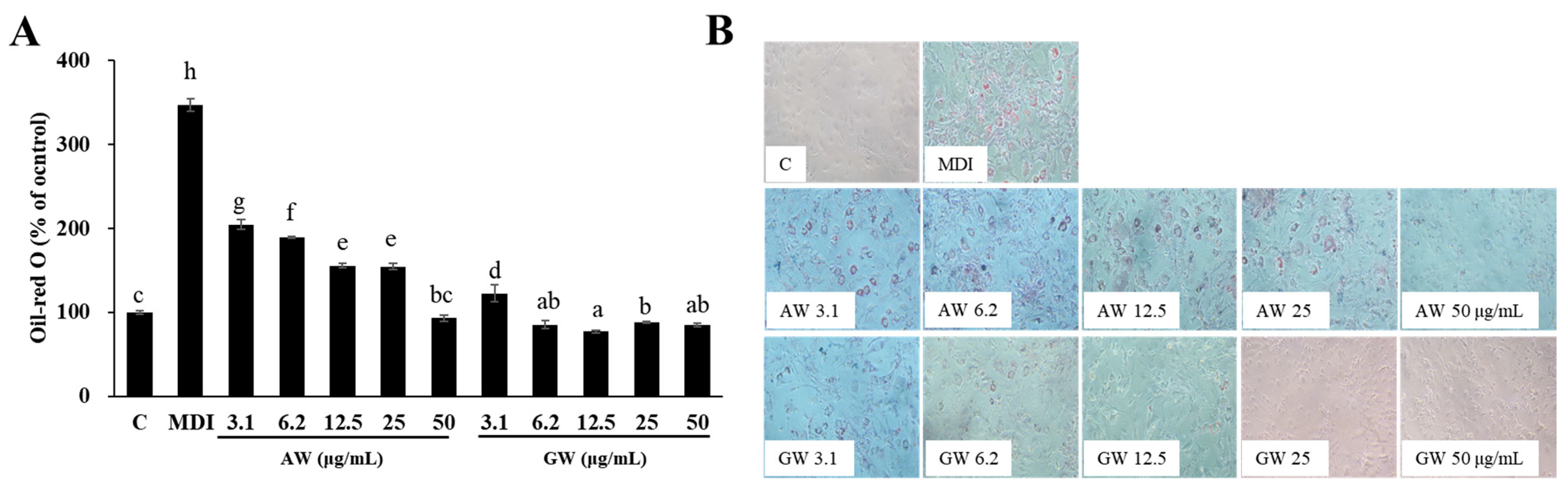
3.4. Inhibition of 3T3-L1 Adipocyte Differentiation
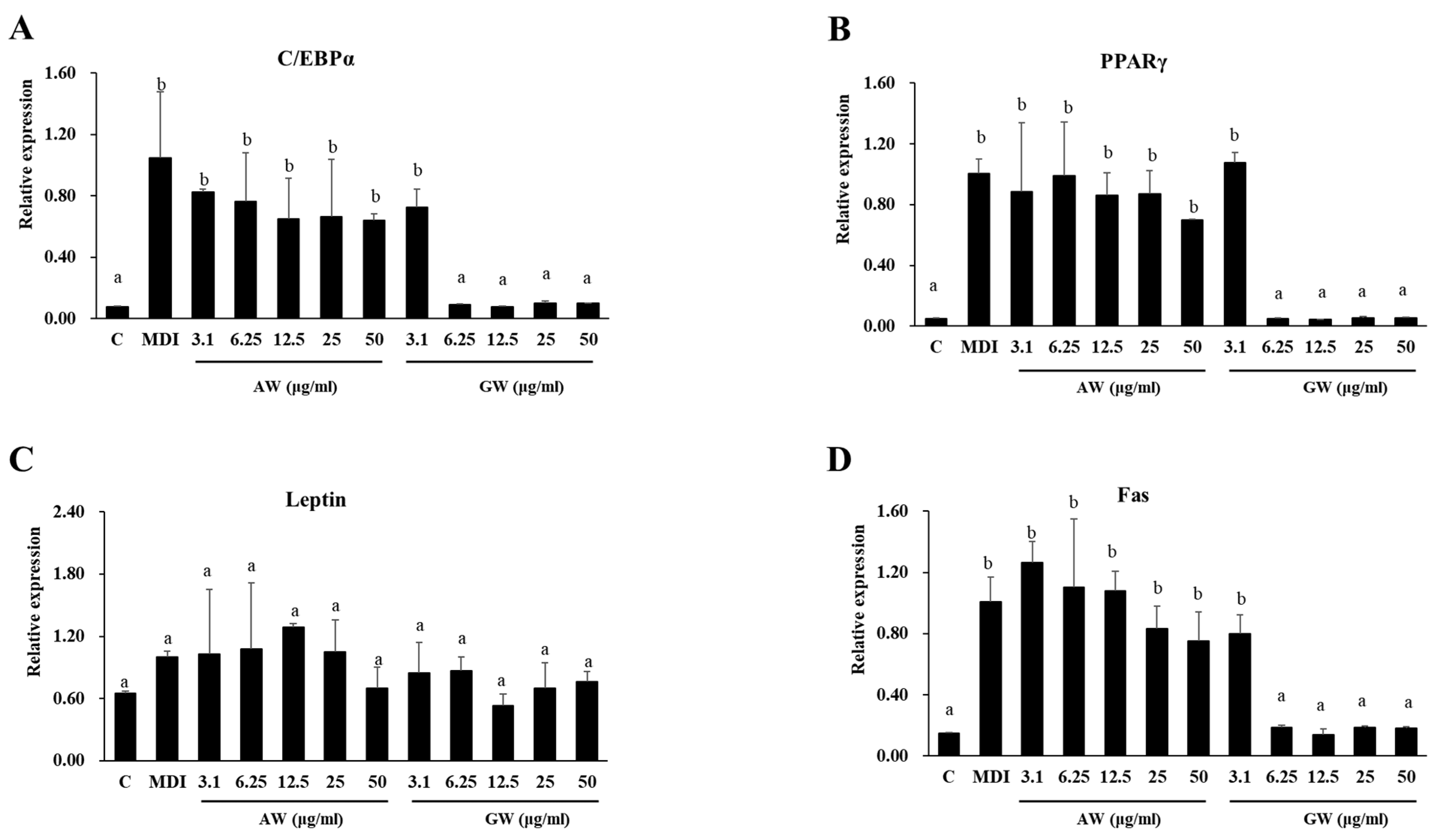
3.5. Gene Expression Analyzed Using Real-Time PCR (RT-PCR)
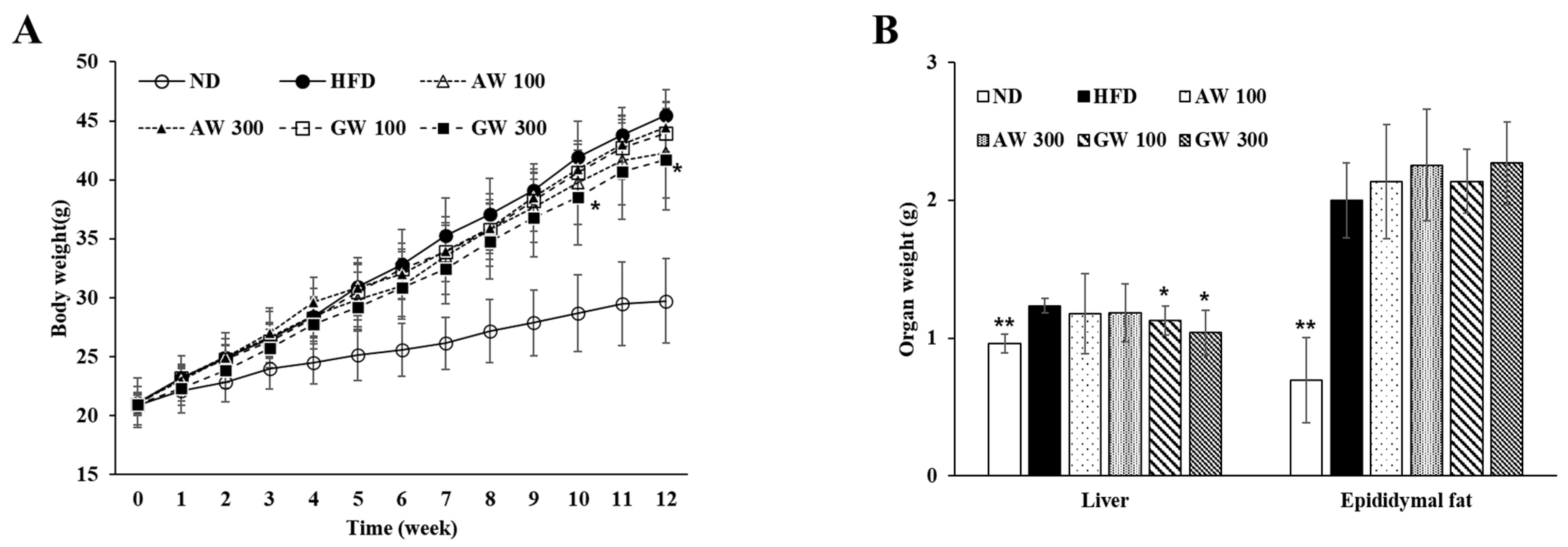
3.6. Changes in Body and Organ Weight in C57BL/6 Mice
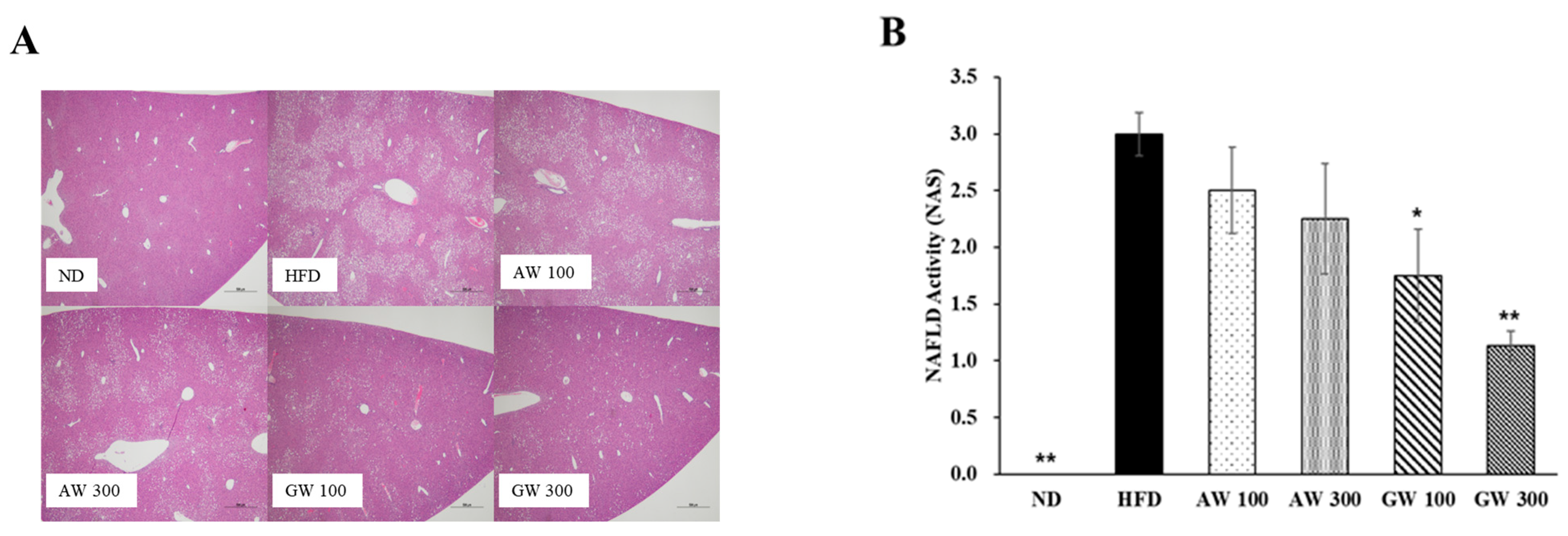
3.7. Histological Analysis
3.8. Serum and Hepatic Triglycerie Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loos, R.J.; Yeo, G.S. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Lee, S.Y.; hye Jang, D.; Lee, S.J.; Cho, J.-Y.; Kim, S.-H. Inhibitory effects of Porphyra dentata extract on 3T3-L1 adipocyte differentiation. J. Anim. Sci. Technol. 2020, 62, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Kajikawa, M.; Higashi, Y. Obesity and Endothelial Function. Biomedicines 2022, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Krempler, F.; Breban, D.; Oberkofler, H.; Esterbauer, H.; Hell, E.; Paulweber, B.; Patsch, W. Leptin, peroxisome proliferator-activated receptor-γ, and CCAAT/enhancer binding protein-α mRNA expression in adipose tissue of humans and their relation to cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 443–449. [Google Scholar] [CrossRef]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Ferroni, P.; Gaudagni, F. Obesity and colorectal cancer role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Duquenne, M.; Folgueira, C.; Bourouh, C.; Millet, M.; Dam, J.; Prevot, V. Leptin brain entry via a tanycyclic LepR : EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 2021, 3, 1071–1090. [Google Scholar] [CrossRef]
- Ryan, D.H. Next generation antiobesity medications: Setmelanotide, semaglutide, tirzepatide and bimagrumab: What do they mean for clinical practice? J. Obes. Metab. Syndr. 2021, 30, 196–208. [Google Scholar] [CrossRef]
- Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; de Lourdes Pereira, M. The role of medicinal and aromatic plants against obesity and arthritis: A review. Nutrients 2022, 14, 985. [Google Scholar] [CrossRef]
- Wang, H.-N.; Xiang, J.-Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- El-Shiekh, R.; Al-Mahdy, D.; Hifnawy, M.; Abdel-Sattar, E. In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. S. Afr. J. Bot. 2019, 123, 43–50. [Google Scholar] [CrossRef]
- Redha, A.A.; Perna, S.; Riva, A.; Petrangolini, G.; Peroni, G.; Nichetti, M.; Iannello, G.; Naso, M.; Faliva, M.A.; Rondanelli, M. Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 2021, 84, 104600. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Radzi, C.W.J.W.M.; Cordell, G.A.; Yaze, I. Potential of traditional medicinal plants for treating obesity: A review. arXiv 2012, arXiv:1208.1923. [Google Scholar]
- Choi, E.-Y.; Park, C.Y.; Ho, S.H.; Park, S.-J.; Kim, D.; Han, B.; Kim, S.-H. Anti-Obesity Effects of Artemisia annua Extract in Zucker Fatty Rats and High-Fat Diet Sprague Dawley Rats through Upregulation of Uncoupling Protein 1. J. Obes. Metab. Syndr. 2021, 30, 32–43. [Google Scholar] [CrossRef]
- Şenkal, B.C.; Kiralan, M.; Yaman, C. The effect of different harvest stages on chemical composition and antioxidant capacity of essential oil from Artemisia annua L. J. Agric. Sci. 2015, 21, 71–77. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential oil and acetone extract composition and antioxidant capacity. Ind. Crops Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Baek, H.K.; Shim, H.; Lim, H.; Shim, M.; Kim, C.-K.; Park, S.-K.; Lee, Y.S.; Song, K.-D.; Kim, S.-J.; Yi, S.S. Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model. J. Vet. Sci. 2015, 16, 389–396. [Google Scholar] [CrossRef]
- Darusman, L.; Batubara, I.; Utami, M. Fractionation of active components from Piper cf. fragile essential oil as aromatherapy for anti-obesity. In Proceedings of the International Symposium on Medicinal and Aromatic Plants 1023, Chiang Mai, Thailand, 15 December 2011; pp. 23–28. [Google Scholar]
- Kim, M.J.; Jeon, D.; Kwak, C.; Ryoo, S.; Kim, Y. Rhamnetin Exhibits Anti-Tuberculosis Activity and Protects against Lung Inflammation. Bull. Korean Chem. Soc. 2016, 37, 1703–1709. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M. Potential of wormwood (Artemisia absinthium Linn.) herb for use as additive in livestock feeding: A review. Pharma Innov. 2017, 6, 176–187. [Google Scholar]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia species with high biological values as a potential source of medicinal and cosmetic raw materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Ozawa, T.; Wu, J.; Fujii, S. Effect of inoculation with a strain of Pseudomonas pseudoalcaligenes isolated from the endorhizosphere of Salicornia europea on salt tolerance of the glasswort. Soil Sci. Plant Nutr. 2007, 53, 12–16. [Google Scholar] [CrossRef]
- Altay, A.; Celep, G.S.; Yaprak, A.E.; Baskose, I.; Bozoglu, F. Glassworts as possible anticancer agents against human colorectal adenocarcinoma cells with their nutritive, antioxidant and phytochemical profiles. Chem. Biodivers. 2017, 14, e1600290. [Google Scholar] [CrossRef]
- Park, Y.-H.; Lee, J.-J.; Son, H.-K.; Kim, B.-H.; Byun, J.; Ha, J.-H. Antiobesity effects of extract from Spergularia marina Griseb in adipocytes and high-fat diet-induced obese rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, J.Y.; Lee, Y.G.; Lee, H.J.; Shim, H.J.; Lee, J.H.; Kim, S.-J.; Ham, K.-S.; Moon, J.-H. Four new dicaffeoylquinic acid derivatives from glasswort (Salicornia herbacea L.) and their antioxidative activity. Molecules 2016, 21, 1097. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea. Immunopharm. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, M.-J.; Kim, J.-H.; Kim, S.-H.; Go, H.-K.; Kweon, M.-H.; Kim, D.-H. Desalted Salicornia europaea powder and its active constituent, trans-ferulic acid, exert anti-obesity effects by suppressing adipogenic-related factors. Pharm. Biol. 2018, 56, 183–191. [Google Scholar] [CrossRef]
- Kim, H.-W.; Hwang, K.-E.; Song, D.-H.; Kim, Y.-J.; Lim, Y.-B.; Ham, Y.-K.; Yeo, E.-J.; Chang, S.-J.; Choi, Y.-S.; Kim, C.-J. Effect of glasswort (Salicornia herbacea L.) on the texture of frankfurters. Meat Sci. 2014, 97, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Cavaleiro, C.; Ramos, F. Sodium reduction in bread: A role for glasswort (Salicornia ramosissima J. Woods). Compr. Rev. Food Sci. Food Saf. 2017, 16, 1056–1071. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-H.; Jin, J.-S. Fermentation of traditional medicine: Present and future. Orient. Pharm. Exp. Med. 2012, 12, 163–165. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kothari, D.; Niu, K.-M.; Han, S.G.; Yoon, J.E.; Lee, H.-G.; Kim, S.-K. Effect of fermented medicinal plants as dietary additives on food preference and fecal microbial quality in dogs. Animals 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-R.; Niu, K.-M.; Kang, S.-K.; Han, S.-G.; Lee, B.-J.; Kim, S.-K. Antioxidant and antibacterial activities of Lactobacillus-fermented Artemisia annua L. as a potential fish feed additive. J. Life Sci. 2017, 27, 652–660. [Google Scholar]
- Ghanbari, M.; Lamuki, M.S.; Habibi, E.; Sadeghimahalli, F. Artemisia annua L. Extracts Improved Insulin Resistance via Changing Adiponectin, Leptin and Resistin Production in HFD/STZ Diabetic Mice. J. Pharmacopunct. 2022, 25, 130–138. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Younossi, Z.N.; Konenig, A.B.; Abdeltif, D.; Wymer, M. Global epidemiology of NAFLD meta analytic assessment of prevalence, incidence, and outcome. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Jung, E.J.; Paramanantham, A.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.-M.; Ryu, C.H.; Hong, S.C.; Chung, K.H.; Kim, C.W. Artemisia annua L. Polyphenol-Induced Cell Death Is ROS-Independently Enhanced by Inhibition of JNK in HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1366. [Google Scholar] [CrossRef]
- Kang, S.; Choi, Y.; Hong, J. Modulation of arachidonic acid metabolism and inflammatory process in macrophages by different solvent fractions of Glasswort (Salicornia herbacea L.) extract. Korean J. Food Sci. Technol. 2018, 50, 671–679. [Google Scholar]
- Na, E.-J.; Kim, D.-J.; Kim, J.-H.; Kim, G.-R. Recent trends in anti-obesity and anti-inflammatory studies in modern health care. Technol. Health Care 2019, 27, 519–530. [Google Scholar] [CrossRef]
- De La Cruz, J.; Ruiz-Moreno, M.; Guerrero, A.; López-Villodres, J.; Reyes, J.; Espartero, J.; Labajos, M.; González-Correa, J. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J. Nutr. Biochem. 2015, 26, 549–555. [Google Scholar] [CrossRef]
- da Silva Sabo, S.; Vitolo, M.; González, J.M.D.; de Souza Oliveira, R.P. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Audisio, M.C.; Oliver, G.; Apella, M.C. Effect of different complex carbon sources on growth and bacteriocin synthesis of Enterococcus faecium. Int. J. Food Microbiol. 2001, 63, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, G.-M.; Cha, G.-S. Biotransformation of flavonoids by newly isolated and characterized Lactobacillus pentosus NGI01 strain from kimchi. Microorganisms 2021, 9, 1075. [Google Scholar] [CrossRef]
- Zieliński, H.; Wiczkowski, W.; Honke, J.; Piskuła, M.K. In vitro expanded bioaccessibility of quercetin-3-rutinoside and quercetin aglycone from buckwheat biscuits formulated from flours fermented by lactic acid bacteria. Antioxidants 2021, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Gullon, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Yan, X.; Zhai, Y.; Zhou, W.; Qiao, Y.; Guan, L.; Liu, H.; Jiang, J.; Peng, L. Intestinal Flora Mediates Antiobesity Effect of Rutin in High-Fat-Diet Mice. Mol. Nutr. Food Res. 2022, 66, 2100948. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Rogozinska, M.; Korsak, D.; Mroczek, J.; Biesaga, M. Catabolism of hydroxycinnamic acids in contact with probiotic Lactobacillus. J. Appl. Microbiol. 2021, 131, 1464–1473. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes over the fermentation period in phenolic compounds and antioxidant and anticancer activities of blueberries fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human EA. hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef]
- Moon, J.-H.; Terao, J. Antioxidant activity of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Cai, Y.; Luo, Y.; Shi, X. Safety assessment of desaminotyrosine: Acute, subchronic oral toxicity, and its effects on intestinal microbiota in rats. Toxicol. Appl. Pharmacol. 2021, 417, 115464. [Google Scholar] [CrossRef] [PubMed]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Son, J.H.; Kim, S.-Y.; Jang, H.H.; Lee, S.N.; Ahn, K.J. Protective effect of protocatechuic acid against inflammatory stress induced in human dermal fibroblasts. Biomed. Dermatol. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Nour, O.A.; Ghoniem, H.A.; Nader, M.A.; Suddek, G.M. Impact of protocatechuic acid on high fat diet-induced metabolic syndrome sequelae in rats. Eur. J. Pharmacol. 2021, 907, 174257. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Fernandez, M.; Zuniga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef]
- Averesch, N.J.; Krömer, J.O. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds—Present and future strain construction strategies. Front. Bioeng. Biotechnol. 2018, 6, 32–51. [Google Scholar] [CrossRef]
- Serra, S.; De Simeis, D.; Castagna, A.; Valentino, M. The fatty-acid hydratase activity of the most common probiotic microorganisms. Catalysts 2020, 10, 154. [Google Scholar] [CrossRef]
- Hirata, A.; Kishino, S.; Park, S.-B.; Takeuchi, M.; Kitamura, N.; Ogawa, J. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J. Lipid Res. 2015, 56, 1340–1350. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Yoo, J.A.; Lee, C.-J.; Kim, Y.-G.; Lee, B.-E.; Yoon, M.-H. Antifungal Effect of Phenyllactic Acid Produced by Lactobacillus casei Isolated from Button Mushroom. J. Mushroom 2016, 14, 162–167. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. Genomic features of Lactobacillus species. Front. Biosci. -Landmark 2009, 14, 1362–1386. [Google Scholar] [CrossRef]
- Hirai, S.; Horii, S.; Matsuzaki, Y.; Ono, S.; Shimmura, Y.; Sato, K.; Egashira, Y. Anti-inflammatory effect of pyroglutamyl-leucine on lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2014, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shirako, S.; Kojima, Y.; Tomari, N.; Nakamura, Y.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Sato, K. Pyroglutamyl leucine, a peptide in fermented foods, attenuates dysbiosis by increasing host antimicrobial peptide. npj Sci. Food 2019, 3, 18–27. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Gavrish, G.E.; Goriainov, S.V.; Chistyakov, V.V.; Astakhova, A.A.; Azbukina, N.V.; Sergeeva, M.G. Oxylipin profiles as functional characteristics of acute inflammatory responses in astrocytes pre-treated with IL-4, IL-10, or LPS. Int. J. Mol. Sci. 2020, 21950, 1780. [Google Scholar] [CrossRef] [PubMed]
- Gosset, V.; Goëbel, C.; Laine, G.; Delaplace, P.; Du Jardin, P.; Feussner, I.; Fauconnier, M.-L. The role of oxylipins and antioxidants on off-flavor precursor formation during potato flake processing. J. Agric. Food Chem. 2008, 56, 11285–11292. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jun, H.Y.; Kim, J.H. Antiadipogenic effect of Korean glasswort (Salicornia herbacea L.) water extract on 3T3-L1 adipocytes. J. Korean Soc. Food Sci. Nutr. 2014, 43, 814–821. [Google Scholar] [CrossRef]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: Size and optical density distribution. Eur. J. Histochem. EJH 2013, 57, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Prusty, D.; Park, B.-H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Poeggeler, B.; Schulz, C.; Pappolla, M.A.; Bodó, E.; Tiede, S.; Lehnert, H.; Paus, R. Leptin and the skin: A new frontier. Exp. Dermatol. 2010, 19, 12–18. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, J.H.; Kim, M.-J. Ethanol extract of Hippophae rhamnoides L. leaves inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Korean J. Plant Resour. 2015, 28, 582–590. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-J.; Jang, S.-H.; Kim, T.H.; Kim, H.-D.; Kim, S.-W.; Won, C.-K.; Cho, J.-H. Annual wormwood leaf inhibits the adipogenesis of 3T3-L1 and obesity in high-fat diet-induced obese rats. Nutrients 2017, 9, 554. [Google Scholar] [CrossRef]
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.J.; Le, C.T.; Beerthuijzen, J.M.; Murphy, W.J. Obesity as an immune-modifying factor in cancer immunotherapy. J. Leukoc. Biol. 2018, 104, 487–497. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.-J.; Kim, Y.-H.; Kim, D.-B.; Shin, G.-H.; Cho, J.-H.; Kim, B.K.; Lee, B.-Y.; Lee, O.-H. Codonopsis lanceolata extract prevents diet-induced obesity in C57BL/6 mice. Nutrients 2014, 6, 4663–4677. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Choi, J.-H.; Kim, Y.-G.; Lee, C.-H. Effect of dietary intake of Salicornia herbacea L. hot water extract on anti-obesity in diet-induced obese rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 950–956. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, S.; Kim, J.; Kim, B.; Wu, C.; Lee, J.H.; Jun, H.j.; Kim, N.; Lee, D.; Lee, S.J. Ursolic acid improves lipid and glucose metabolism in high-fat-fed C57BL/6J mice by activating peroxisome proliferator-activated receptor alpha and hepatic autophagy. Mol. Nutr. Food Res. 2015, 59, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2007, 65, S57–S63. [Google Scholar] [CrossRef]
- Seo, H.-B.; Kwak, Y.-Y.; Nam, J.-O.; Song, Y.-J.; Kim, B.-O.; Ryu, S.-P. Glasswort powder diet activates lipid metabolism in rat. J. Life Sci. 2012, 22, 478–485. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, S.K.; Choi, J.G.; Chung, S.H. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR mice. Arch. Pharmacal Res. 2006, 29, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Pichiah, P.T.; Cha, Y.S. Salicornia herbacea prevents weight gain and hepatic lipid accumulation in obese ICR mice fed a high-fat diet. J. Sci. Food Agric. 2015, 95, 3150–3159. [Google Scholar] [CrossRef] [PubMed]

| Gene | Name | Primer Sequence | Tm (°C) |
|---|---|---|---|
| C/EBPα | mC/EBPα_F | CAA GAA GTC GGT GGA CAA G | 55.2 |
| mC/EBPα_R | GCT TTA TCT CGG CTC TTG C | 55.2 | |
| PPARγ | mPPARγ_F | GAC ATC CAA GAC AAC CTG CT | 55.4 |
| mPPARγ_R | TGT CAT CTT CTG GAG CAC CT | 55.4 | |
| Leptin | mLeptin_F | TGA CAC CAA AAC CCT CAT CA | 53.4 |
| mLeptin_R | AGC CCA GGA ATG AAG TCC A | 55.2 | |
| Fas | mFas_F | AGA GAT CCC GAG ACG CTT CT | 57.4 |
| mFas_R | GCT TGG TCC TTT GAA GTC GAA GA | 58.2 |
| No. | Tentative Metabolite | VIP a 1 | VIP 2 | RT (min) b | MW | Measured Mass | MS/MS Fragments | Molecular Formula | Delta ppm |
|---|---|---|---|---|---|---|---|---|---|
| Negative Mode c(m/z) | |||||||||
| Peptides | |||||||||
| 1 | Pyroglutamyl-valine | 1.47 | 1.12 | 2.18 | 228 | 227.1046 | 227 > 183 > 155, 127, 82 | C10H15O4N2 | 3.698 |
| 2 | Pyroglutamyl-leucine | 1.43 | 1.15 | 4.17 | 242 | 241.1202 | 241 > 197 > 169, 141 | C11H17O4N2 | 3.441 |
| 3 | Lactoyl-tryptophan | 1.45 | 1.13 | 4.53 | 276 | 275.1049 | 275 > 231, 127 > 109 | C14H15O4N2 | 4.325 |
| Organic acid | |||||||||
| 4 | Hydroxyglutaric acid | 0.97 | 1.46 | 1.06 | 148 | 147.0307 | 147 > 129 > 101, 85 | C5H7O5 | 5.124 |
| 5 | Succinic Acid | 1.17 | 1.35 | 1.06 | 118 | 117.0200 | 117 > 99, 73 | C4H5O4 | 6.307 |
| Fatty acid | |||||||||
| 6 | Hydroxyisocaproic acid | 0.91 | 1.50 | 4.02 | 132 | 131.0722 | 131 > 113, 85 | C6H11O3 | 6.275 |
| 7 | 9,12,13-TriHOME | 1.09 | 0.79 | 6.59 | 330 | 329.2343 | 329 > 229 > 211, 125 | C18H33O5 | 2.742 |
| 8 | 9-DiHODE | 0.96 | 1.42 | 7.29 | 312 | 311.2241 | 311 > 293 > 275, 185 | C18H31O4 | 4.297 |
| 9 | 9,10-DHOME | 1.36 | 0.97 | 7.99 | 314 | 313.2393 | 313 > 295 > 277, 195 | C18H33O4 | 2.737 |
| 10 | Hydroxymyristic acid | 0.92 | 1.13 | 8.99 | 244 | 243.1974 | 243 > 225 > 207, 181 | C14H27O3 | 3.257 |
| 11 | Hydroxystearic acid | 0.18 | 1.03 | 9.64 | 300 | 299.2598 | 299 > 281, 253 > 249, 225 | C18H35O3 | 2.211 |
| Phenolic acids and deriatives | |||||||||
| 12 | Hydroxyphenyllactic acid | 0.89 | 1.51 | 2.37 | 182 | 181.0522 | 181 > 163 > 119 | C9H9O4 | 4.462 |
| 13 | Dihydrocaffeic acid | 0.90 | 1.50 | 3.54 | 182 | 181.0515 | 181 > 137 > 119, 109 | C9H9O4 | 4.849 |
| 14 | Caffeic acid | 0.60 | 1.61 | 4.02 | 180 | 179.0360 | 179 > 135 > 107, 91 | C9H7O4 | 5.574 |
| 15 | Phenyllactic acid | 0.91 | 1.50 | 4.56 | 166 | 165.0571 | 165 > 147 > 121,97 | C9H9O3 | 5.226 |
| 16 | Coumaric acid | 0.60 | 1.60 | 4.69 | 164 | 163.0409 | 163 > 119 > 91 | C9H7O3 | 5.168 |
| 17 | Scopoletin | 1.42 | 1.16 | 4.89 | 192 | 191.0358 | 191 > 177 > 104 | C10H7O4 | 4.177 |
| 18 | Ferulic acid | 1.42 | 1.01 | 4.96 | 194 | 193.0516 | 193 > 178, 149 > 134 | C10H9O4 | 5.117 |
| 19 | Phloretic acid | 1.08 | 1.41 | 4.97 | 166 | 165.0564 | 165 > 147, 121 > 106, 93 | C9H9O3 | 4.68 |
| Quinic acid and deriatives | |||||||||
| 20 | Quinic acid | 1.29 | 1.26 | 0.85 | 192 | 191.0569 | 191 > 173, 127, 111, 85 | C7 H11O6 | 4.128 |
| 21 | 3-caffeoylquinic acid | 1.50 | 1.08 | 2.29 | 354 | 353.0894 | 353 > 191 > 173, 127, 85 | C16H17O9 | 2.222 |
| 22 | 5-caffeoylquinic acid | 1.51 | 1.07 | 3.85 | 354 | 353.0894 | 353 > 191 > 173, 127, 85 | C16H17O9 | 4.573 |
| 23 | 3,4-di-O-caffeoylquinic acid | 1.50 | 1.08 | 4.39 | 516 | 515.1215 | 515 > 353 > 191, 179 | C25H23O12 | 3.923 |
| 24 | 3-feruloyl-4-caffeoylquinic acid | 1.50 | 1.09 | 4.78 | 530 | 529.1362 | 529 > 367 > 193 | C26H25O12 | 4.688 |
| 25 | 3,5-di-O-caffeoylquinic acid | 1.18 | 0.83 | 5.03 | 516 | 515.1213 | 515 > 353 > 191, 179, 135 | C25H23O12 | 2.389 |
| 26 | 3-feruloyl-5-caffeoylquinic acid | 1.44 | 1.15 | 5.48 | 530 | 529.1367 | 529 > 367 > 191, 173 | C26H25O12 | 2.855 |
| Benzoic acid and derivatives | |||||||||
| 27 | Protocatechuic acid-O-glucoside | 0.77 | 1.56 | 1.51 | 316 | 315.0735 | 315 > 153 > 123, 109 | C13H15O9 | 4.332 |
| 28 | Vanillic acid | 0.11 | 1.66 | 1.61 | 168 | 167.0359 | 167 > 152, 149 > 121 | C8H7O4 | 5.675 |
| 29 | Protocatechuic acid | 1.13 | 1.37 | 1.96 | 154 | 153.0201 | 153 > 138, 109 > 81 | C7H5O4 | 5.281 |
| 30 | Hydroxybenzoic acid | 1.42 | 1.15 | 2.83 | 138 | 137.0251 | 137 > 93 | C7H5O3 | 5.201 |
| 31 | 4-vinylcatechol | 1.14 | 1.36 | 5.29 | 136 | 135.0452 | 135 > 107, 91 | C8H7O2 | 5.163 |
| 32 | 4-ethylcatechol | 0.88 | 1.50 | 5.56 | 138 | 137.0615 | 137 > 93 | C8H9O2 | 4.721 |
| 33 | Syringic aldehyde | 1.05 | 1.43 | 6.09 | 182 | 181.0513 | 181 > 166 > 138 | C9H9O4 | 3.689 |
| Flavonoids | |||||||||
| 34 | Rutin | 0.84 | 1.09 | 4.81 | 610 | 609.1485 | 609 > 301 > 271, 179 | C27H29O16 | 3.73 |
| 35 | Quercetin-3-glycoside | 1.47 | 1.09 | 4.95 | 464 | 463.0905 | 463 > 301 > 271, 179, 151 | C21H19O12 | 5.012 |
| 36 | Isorhamnetin 3-O-β-d-glucoside | 0.57 | 1.61 | 5.23 | 478 | 477.1061 | 477 > 314 > 300, 285 | C22H21O12 | 4.613 |
| 37 | Casticin | 1.44 | 1.15 | 7.42 | 374 | 373.0937 | 375 > 358 > 343 > 328 | C19H17O8 | 2.142 |
| Etc | |||||||||
| 38 | Shikimic acid | 1.17 | 1.34 | 1.31 | 174 | 173.0463 | 173 > 155, 129 | C7H9O5 | 4.238 |
| 39 | Pantothenic acid | 1.37 | 1.21 | 1.46 | 219 | 218.1044 | 218 > 187 > 143, 130 | C9H16O5N | 4.604 |
| N.I | |||||||||
| 40 | N.I 1 | 1.51 | 1.08 | 6.63 | 174 | 173.1190 | 173 > 127 > 123, 97 | - | - |
| 41 | N.I 2 | 1.50 | 1.06 | 6.86 | 926 | 925.4467 | 939 > 808 > 645 | - | - |
| 42 | N.I 3 | 1.51 | 1.07 | 6.90 | 216 | 215.1298 | 215 > 173 > 127 | - | - |
| Group | Blood Chemistry | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AST (U/L) | ALT (U/L) | GLU (mg/dL) | TG (mg/dL) | T-Chol (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | |||
| ND | N | Mean | 94.00 | 29.38 * | 118.00 ** | 74.00 ** | 113.13 ** | 81.88 ** | 9.63 ** |
| 8 | SE | 8.80 | 1.05 | 10.49 | 3.56 | 5.11 | 1.14 | 0.26 | |
| HFD Control | N | Mean | 122.75 | 76.75 | 226.00 | 97.75 | 210.75 | 97.13 | 18.88 |
| 8 | SE | 5.57 | 8.07 | 4.76 | 5.31 | 8.39 | 2.73 | 1.33 | |
| AW 100 | N | Mean | 163.38 * | 122.50 * | 212.63 | 88.13 | 195.75 | 96.50 | 20.00 |
| 8 | SE | 20.60 | 31.01 | 12.40 | 2.40 | 7.57 | 2.28 | 1.46 | |
| AW 300 | N | Mean | 96.63 | 67.00 | 210.50 | 101.13 | 177.50 ** | 89.25 * | 16.13 |
| 8 | SE | 7.25 | 8.61 | 17.02 | 7.17 | 9.60 | 2.94 | 1.42 | |
| GW 100 | N | Mean | 117.63 | 58.88 | 192.25 * | 92.13 | 182.13 ** | 95.88 | 14.75 * |
| 8 | SE | 12.06 | 7.49 | 5.58 | 1.54 | 5.40 | 2.84 | 0.70 | |
| GW 300 | N | Mean | 137.88 | 46.63 | 203.13 | 80.00 ** | 163.25 ** | 91.50 | 13.63 ** |
| 8 | SE | 8.01 | 3.30 | 7.90 | 3.33 | 6.30 | 2.17 | 0.91 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
On, J.-Y.; Kim, S.-H.; Kim, J.-M.; Park, S.; Kim, K.-H.; Lee, C.-H.; Kim, S.-K. Effects of Fermented Artemisia annua L. and Salicornia herbacea L. on Inhibition of Obesity In Vitro and In Mice. Nutrients 2023, 15, 2022. https://doi.org/10.3390/nu15092022
On J-Y, Kim S-H, Kim J-M, Park S, Kim K-H, Lee C-H, Kim S-K. Effects of Fermented Artemisia annua L. and Salicornia herbacea L. on Inhibition of Obesity In Vitro and In Mice. Nutrients. 2023; 15(9):2022. https://doi.org/10.3390/nu15092022
Chicago/Turabian StyleOn, Jeong-Yeon, Su-Hyun Kim, Jeong-Mee Kim, Sungkwon Park, Ki-Hyun Kim, Choong-Hwan Lee, and Soo-Ki Kim. 2023. "Effects of Fermented Artemisia annua L. and Salicornia herbacea L. on Inhibition of Obesity In Vitro and In Mice" Nutrients 15, no. 9: 2022. https://doi.org/10.3390/nu15092022







