Neuroprotective Effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-Induced Alzheimer’s Disease Mouse Model
Abstract
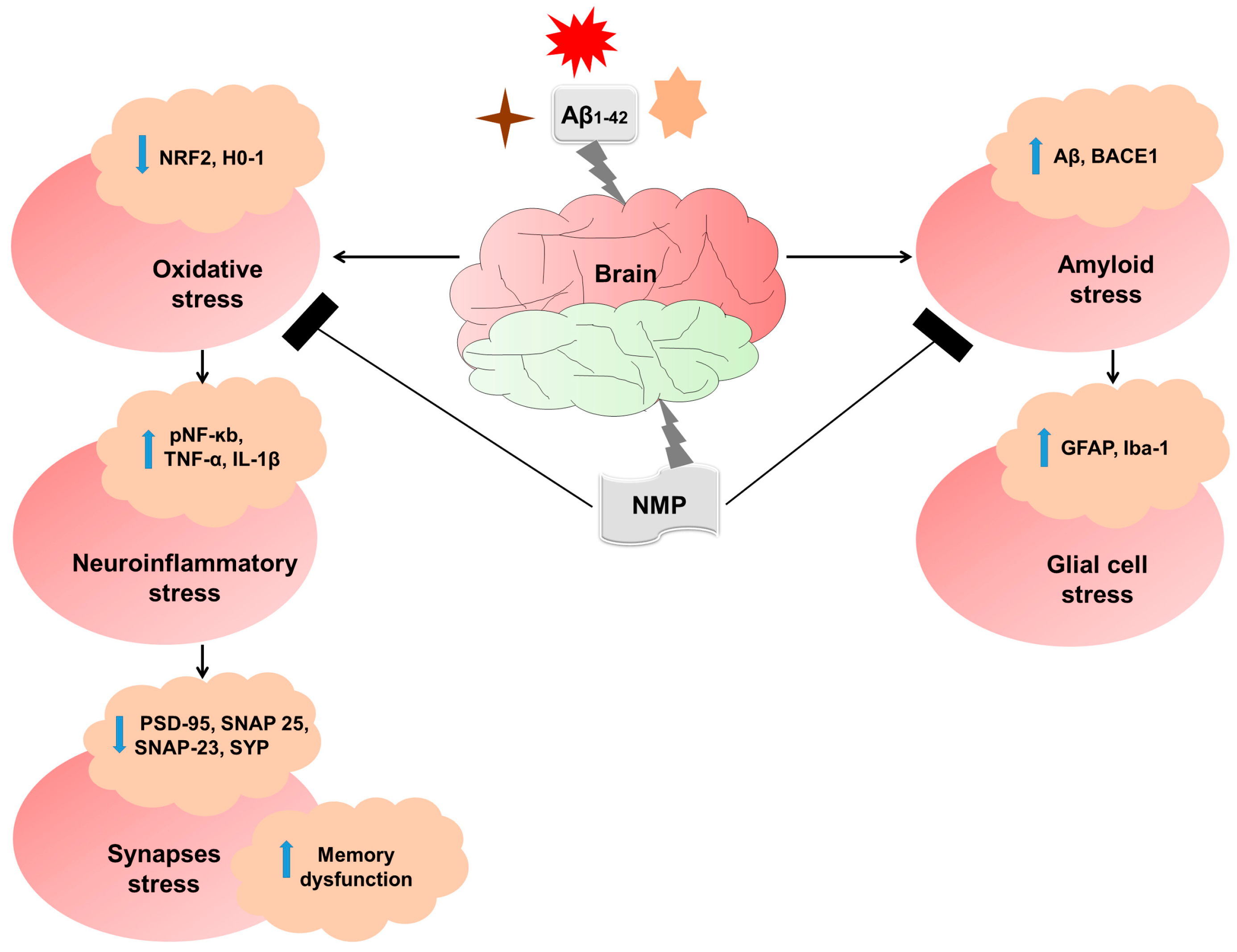
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
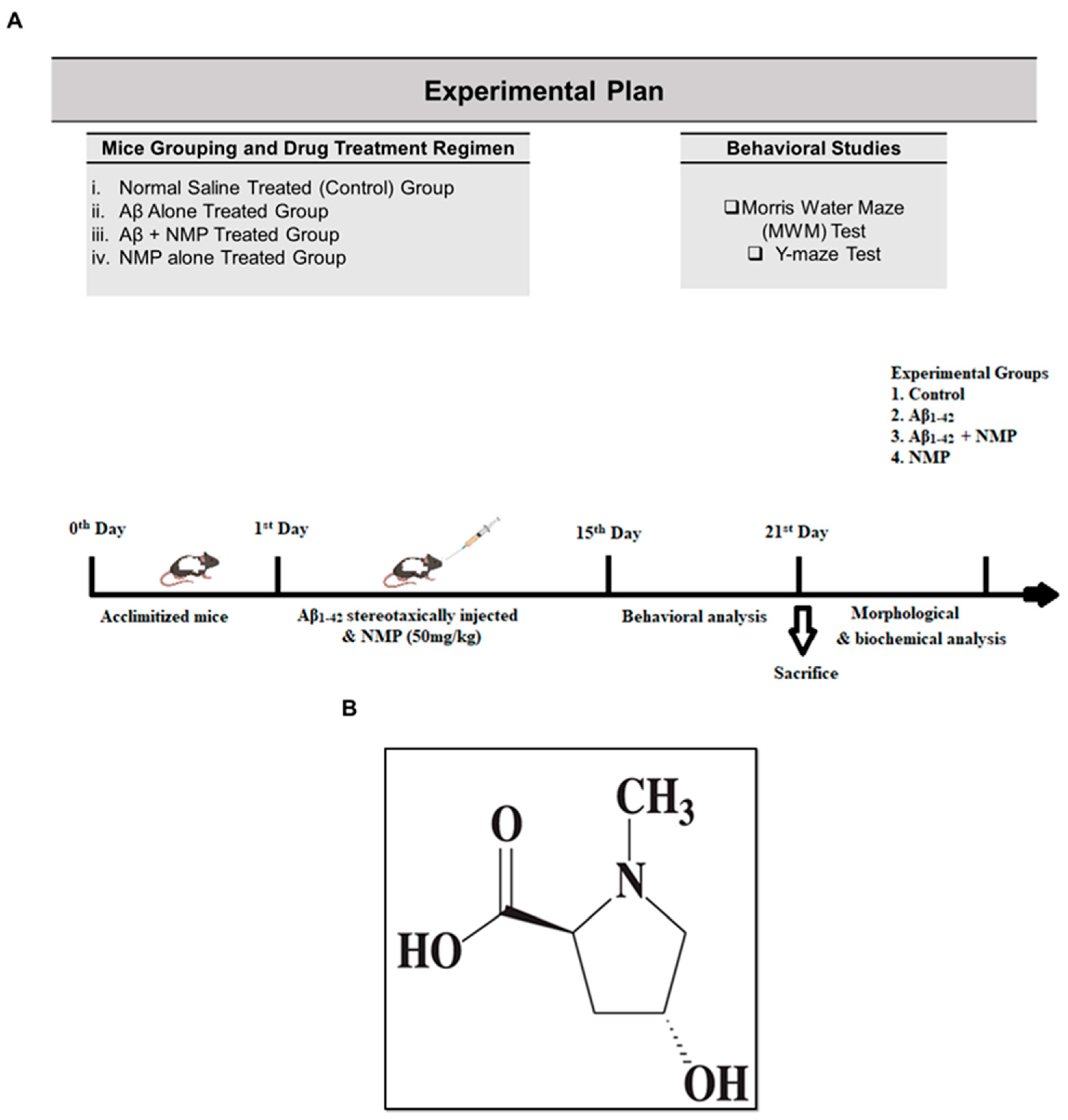
2.3. Experimental Design
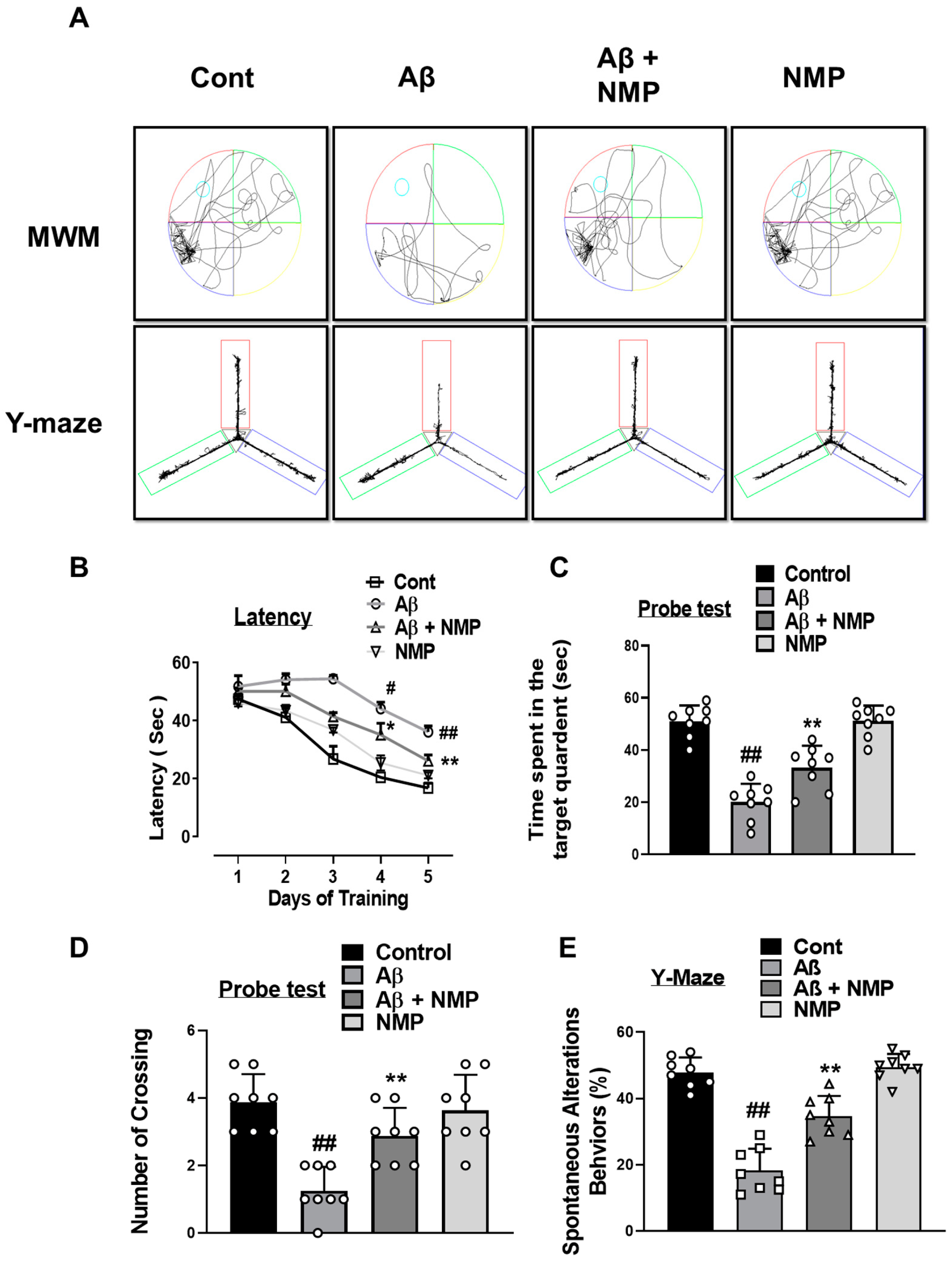
2.4. Morris Water Maze
2.5. Y-Maze Task
2.6. Extraction of Proteins from Mouse Brain
2.7. Western Blot Analysis
2.8. Immunofluorescence Staining and Confocal Imaging
2.9. Statistical Analysis
3. Results
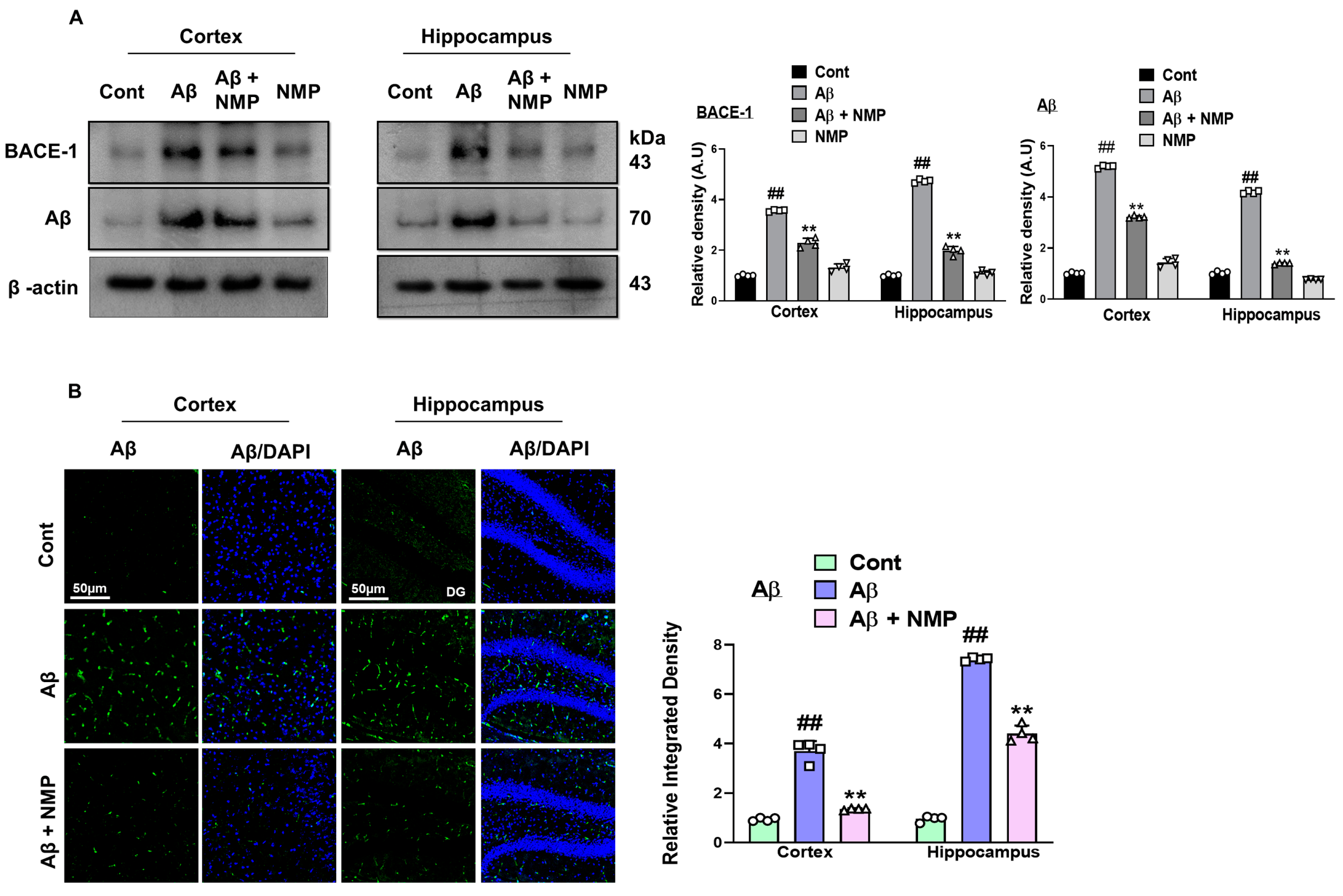
3.1. NMP Treatment Improved AD-like Pathology by Regulating the Level of BACE-1 and Aβ in Both the Cortex and Hippocampus of Aβ1–42-Induced AD Mice
3.2. NMP treatment Improved Reactive Gliosis in AD Mice Brain
3.3. NMP Treatment Improved Oxidative Stress by Regulating the Level of NRF2 and HO-1 in Both Cortex and Hippocampus of Aβ1–42-Induced AD Mice
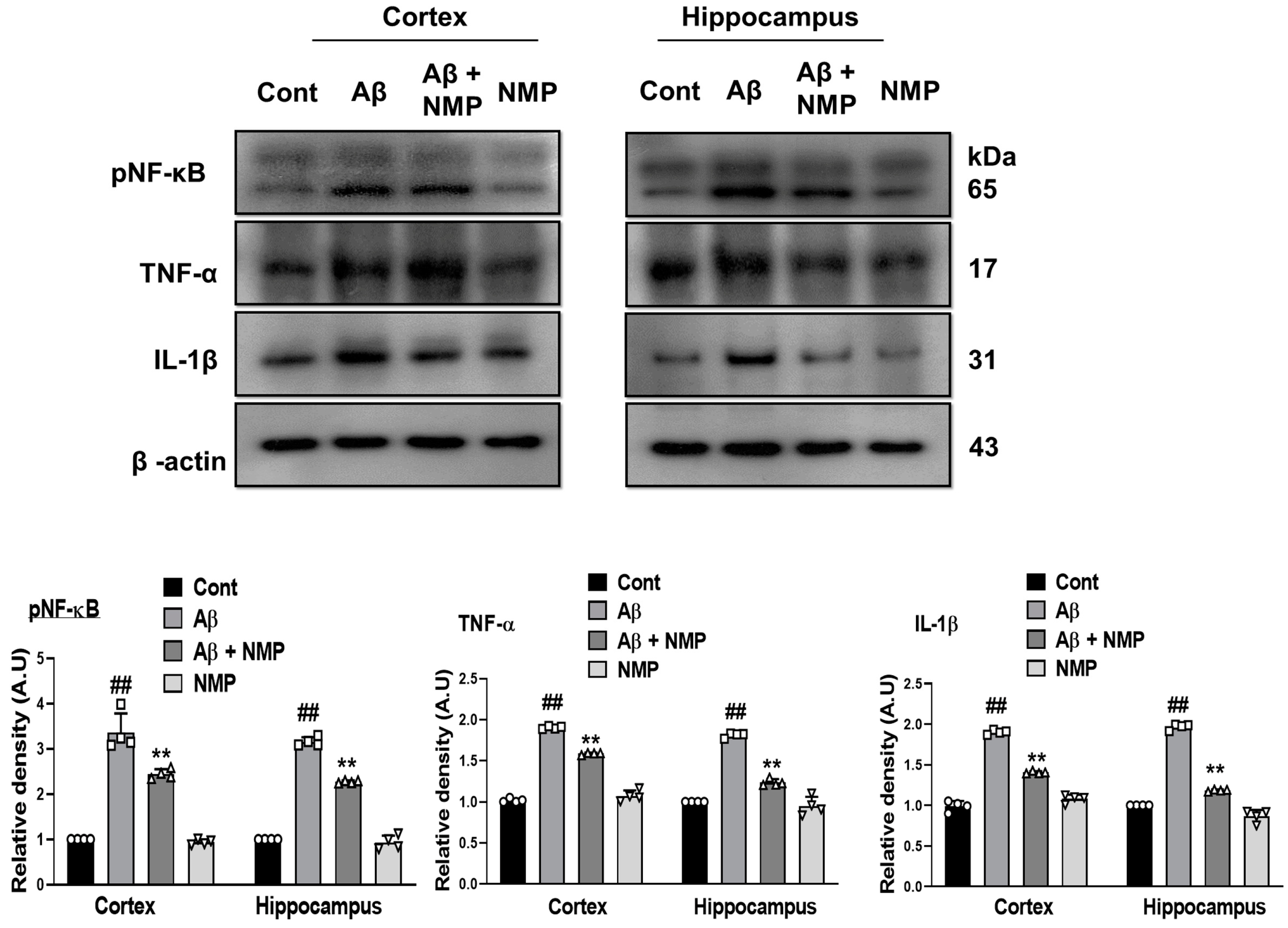
3.4. NMP Treatment Improved Neuroinflammation by Regulating the Level of pNF-ĸB and Its Downstream Targets in Aβ1–42-Induced AD Mice
3.5. NMP Treatment Improved Synaptic Dysfunction by Regulating Synaptic Markers in Both Cortex and Hippocampus of Aβ1–42-Induced AD Mice
3.6. NMP Treatment Improved Aβ1–42-Induced Behavioral and Cognitive Deficits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine natural products, multitarget therapy and repurposed agents in Alzheimer’s disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Powell, W.R.; Buckingham, W.R.; Larson, J.L.; Vilen, L.; Yu, M.; Salamat, M.S.; Bendlin, B.B.; Rissman, R.A.; Kind, A.J. Association of neighborhood-level disadvantage with Alzheimer disease neuropathology. JAMA Netw. Open 2020, 3, e207559. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Das, B.; Zhou, J.; Hu, X.; Yan, R. Targeted BACE-1 inhibition in microglia enhances amyloid clearance and improved cognitive performance. Sci. Adv. 2022, 8, eabo3610. [Google Scholar] [CrossRef] [PubMed]
- Association, A.S. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Iyaswamy, A.; Xu, D.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Yang, Y.; Li, Y.; Chen, C.; Li, M.; Li, H.-W. Real-time detection and visualization of amyloid-β aggregates induced by hydrogen peroxide in cell and mouse models of Alzheimer’s disease. ACS Appl. Mater. Interfaces 2022, 15, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Arun, S.; Liu, L.; Donmez, G. Mitochondrial biology and neurological diseases. Curr. Neuropharmacol. 2016, 14, 143–154. [Google Scholar] [CrossRef]
- Gameiro, I.; Michalska, P.; Tenti, G.; Cores, Á.; Buendia, I.; Rojo, A.I.; Georgakopoulos, N.D.; Hernández-Guijo, J.M.; Teresa Ramos, M.; Wells, G. Discovery of the first dual GSK3β inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer’s disease. Sci. Rep. 2017, 7, 45701. [Google Scholar] [CrossRef]
- Rather, M.A.; Khan, A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Rashid, S.; Alsaffar, R.M.; Kamal, M.A.; Rehman, M.U. Inflammation and Alzheimer’s disease: Mechanisms and therapeutic implications by natural products. Mediat. Inflamm. 2021, 2021, 9982954. [Google Scholar] [CrossRef]
- Prakash, D.; Gopinath, K.; Sudhandiran, G. Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. NeuroMolecular Med. 2013, 15, 192–208. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; De Teresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Spampinato, D. Alzheimer disease and neuroplasticity. Handb. Clin. Neurol. 2022, 184, 473–479. [Google Scholar] [PubMed]
- Lorenzi, H.; Matos, F. Plantas Medicinais no Brasil, Nativas e Exóticas; Instituto Plantarum: Nova Odessa, Brazil, 2002. [Google Scholar]
- Oliveira, A.P.; Raith, M.; Kuster, R.M.; Rocha, L.M.; Hamburger, M.; Potterat, O. Metabolite profiling of the leaves of the Brazilian folk medicine Sideroxylon obtusifolium. Planta Medica 2012, 78, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Neto, V.; Bomfim, R.R.; Oliveira, V.O.; Passos, A.M.; Oliveira, J.P.; Lima, C.A.; Mendes, S.S.; Estevam, C.S.; Thomazzi, S.M. Therapeutic benefits of Sideroxylon obtusifolium (Humb. ex Roem. & Schult.) TD Penn., Sapotaceae, in experimental models of pain and inflammation. Rev. Bras. Farmacogn. 2010, 20, 933–938. [Google Scholar]
- de Aquino, P.E.A.; Lustosa, Í.R.; de Sousa, C.N.S.; Chaves-Filho, A.J.M.; Lima, F.A.V.; da Conceição Santos, A.D.; Gramosa, N.V.; Silveira, E.R.; de Barros Viana, G.S. The N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline-enriched methanol fraction from Sideroxylon obtusifolium shows an anticonvulsant activity associated with its anti-inflammatory/antioxidant actions. Planta Medica Int. Open 2020, 7, e158–e169. [Google Scholar] [CrossRef]
- Rangel, L.d.S.; Passos de Oliveira, A.; Falcão, D.Q.; Santos, M.G.; Von Ranke, N.L.; Rodrigues, C.R.; Santos, J.A.A.d.; Rocha, L.; Faria, R.X. Nanoemulsion of Sideroxylon obtusifolium as an Alternative to Combat Schistosomiasis. Front. Plant Sci. 2022, 13, 853002. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef]
- D’Hooge, R.; De Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- King, D.L.; Arendash, G.W. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol. Behav. 2002, 75, 627–642. [Google Scholar] [CrossRef]
- Naseer, M.; Ullah, I.; Narasimhan, M.; Lee, H.; Bressan, R.; Yoon, G.; Yun, D.; Kim, M. Neuroprotective effect of osmotin against ethanol-induced apoptotic neurodegeneration in the developing rat brain. Cell Death Dis. 2014, 5, e1150. [Google Scholar] [CrossRef]
- Alam, S.I.; Ur Rehman, S.; Ok Kim, M. Nicotinamide improves functional recovery via regulation of the RAGE/JNK/NF-κB signaling pathway after brain injury. J. Clin. Med. 2019, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Schenk, D. Alzheimer’s disease: Molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 545–584. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ1–42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef]
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.-A.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 2015, 85, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Fao, L.; Mota, S.I.; Rego, A.C. Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2019, 54, 100942. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guo, L.; Overholser, J.; Wang, X. Mitochondrial VDAC1: A potential therapeutic target of inflammation-related diseases and clinical opportunities. Cells 2022, 11, 3174. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Qin, Y.; Gao, H.; Wang, G.; Song, H.; Wang, Y.; Cai, B. Chrysophanol exerts neuroprotective effects via interfering with endoplasmic reticulum stress apoptotic pathways in cell and animal models of Alzheimer’s disease. J. Pharm. Pharmacol. 2022, 74, 32–40. [Google Scholar] [CrossRef]
- Penke, B.; Szűcs, M.; Bogár, F. New Pathways Identify Novel Drug Targets for the Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 5383. [Google Scholar] [CrossRef]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666. [Google Scholar]
- Guzmán-Vélez, E.; Diez, I.; Schoemaker, D.; Pardilla-Delgado, E.; Vila-Castelar, C.; Fox-Fuller, J.T.; Baena, A.; Sperling, R.A.; Johnson, K.A.; Lopera, F. Amyloid-β and tau pathologies relate to distinctive brain dysconnectomics in preclinical autosomal-dominant Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2113641119. [Google Scholar] [CrossRef] [PubMed]
- Dislich, B.; Lichtenthaler, S.F. The membrane-bound aspartyl protease BACE1: Molecular and functional properties in Alzheimer’s disease and beyond. Front. Physiol. 2012, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Duan, B.-Y.; Zhou, X.-P.; Gong, J.-X.; Luo, Z.-G. Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2010, 285, 27737–27744. [Google Scholar] [CrossRef] [PubMed]
- Shanker, G.; Walsh, D. Alzheimer’s disease: Synaptic dysfunction and Aβ. Mol. Neurodegener 2009, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Van Hoesen, G.W.; Hyman, B.T. Hippocampal formation: Anatomy and the patterns of pathology in Alzheimer’s disease. Prog. Brain Res. 1990, 83, 445–457. [Google Scholar]
- Chun, H.; Marriott, I.; Lee, C.J.; Cho, H. Elucidating the interactive roles of glia in Alzheimer’s disease using established and newly developed experimental models. Front. Neurol. 2018, 9, 797. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Galvan, V.; Lange, M.B.; Tang, H.; Sowell, R.A.; Spilman, P.; Fombonne, J.; Gorostiza, O.; Zhang, J.; Sultana, R. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid β-peptide of APP. Free. Radic. Biol. Med. 2010, 48, 136–144. [Google Scholar] [CrossRef]
- Wan, L.; Nie, G.; Zhang, J.; Luo, Y.; Zhang, P.; Zhang, Z.; Zhao, B. β-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free. Radic. Biol. Med. 2011, 50, 122–129. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Aquino, P.; de Siqueira, E.; Paes, L.; Magalhães, E.; Barbosa, T.; de Carvalho, M.; Azul, F.; Lustosa, I.R.; Mottin, M.; Sampaio, T. N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline, the major bioactive compound from Sideroxylon obtusifolium, attenuates pilocarpine-induced injury in cultured astrocytes. Braz. J. Med. Biol. Res. 2022, 55, e12381. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Paris, D.; Mathura, V.; Quadros, A.N.; Crawford, F.C.; Mullan, M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflamm. 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, P.E.A.; Magalhães, T.R.; Nicolau, L.A.D.; Leal, L.K.A.M.; de Aquino, N.C.; Dos Santos, S.M.; Neves, K.R.T.; Silveira, E.R.; de Barros Viana, G.S. The anti-inflammatory effects of N-methyl-(2S, 4R)-Trans-4-hydroxy-l-proline from Syderoxylon obtusifolium are related to its inhibition of TNF-alpha and inflammatory enzymes. Phytomedicine 2017, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Klann, E. Amyloid β: Linking synaptic plasticity failure to memory disruption in Alzheimer’s disease. J. Neurochem. 2012, 120, 140–148. [Google Scholar] [CrossRef]
- Bereczki, E.; Branca, R.M.; Francis, P.T.; Pereira, J.B.; Baek, J.-H.; Hortobágyi, T.; Winblad, B.; Ballard, C.; Lehtiö, J.; Aarsland, D. Synaptic markers of cognitive decline in neurodegenerative diseases: A proteomic approach. Brain 2018, 141, 582–595. [Google Scholar] [CrossRef]
- Hsieh, H.; Boehm, J.; Sato, C.; Iwatsubo, T.; Tomita, T.; Sisodia, S.; Malinow, R. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 2006, 52, 831–843. [Google Scholar] [CrossRef]
- de Aquino, P.E.A.; Rabelo Bezerra, J.; de Souza Nascimento, T.; Tavares, J.; Rosal Lustosa, Í.; Chaves Filho, A.J.M.; Mottin, M.; Macêdo Gaspar, D.; Andrade, G.M.d.; Tavares Neves, K.R. A proline derivative-enriched fraction from Sideroxylon obtusifolium protects the hippocampus from intracerebroventricular pilocarpine-induced injury associated with status epilepticus in mice. Int. J. Mol. Sci. 2020, 21, 4188. [Google Scholar] [CrossRef]



| Protein Target | Source | Application | Dilution | Catalog Number | Manufacturer |
|---|---|---|---|---|---|
| BACE-1 | Mouse | WB | 1:1000 | sc-33711 | Santa Cruz Biotechnology |
| Aβ | Mouse | WB/IF | 1:1000/1:100 | sc-28365 | Santa Cruz Biotechnology |
| Iba-1 | Mouse | WB | 1:1000 | sc-39840 | Santa Cruz Biotechnology |
| GFAP | Mouse | WB/IF | 1:1000/1:100 | sc-33673 | Santa Cruz Biotechnology |
| pNF-ĸb | Mouse | WB | 1:1000 | sc-136548 | Santa Cruz Biotechnology |
| TNF-α | Mouse | WB | 1:1000 | sc-52746 | Santa Cruz Biotechnology |
| IL-1β | Mouse | WB | 1:1000 | sc-32294 | Santa Cruz Biotechnology |
| NRF2 | Mouse | WB/IF | 1:1000/1:100 | sc-365949 | Santa Cruz Biotechnology |
| HO-1 | Mouse | WB | 1:1000 | sc-136961 | Santa Cruz Biotechnology |
| PSD-95 | Mouse | WB | 1:1000 | sc-71933 | Santa Cruz Biotechnology |
| SNAP-25 | Mouse | WB | 1:1000 | sc-20038 | Santa Cruz Biotechnology |
| SNAP-23 | Mouse | WB | 1:1000 | sc-374215 | Santa Cruz Biotechnology |
| Synaptophysin | Mouse | WB | 1:1000 | sc-17750 | Santa Cruz Biotechnology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, J.; Khan, A.; Park, J.S.; Tahir, M.; Ahmad, W.; Choe, K.; Kim, M.O. Neuroprotective Effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-Induced Alzheimer’s Disease Mouse Model. Nutrients 2023, 15, 4986. https://doi.org/10.3390/nu15234986
Ali J, Khan A, Park JS, Tahir M, Ahmad W, Choe K, Kim MO. Neuroprotective Effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-Induced Alzheimer’s Disease Mouse Model. Nutrients. 2023; 15(23):4986. https://doi.org/10.3390/nu15234986
Chicago/Turabian StyleAli, Jawad, Amjad Khan, Jun Sung Park, Muhammad Tahir, Waqas Ahmad, Kyonghwan Choe, and Myeong Ok Kim. 2023. "Neuroprotective Effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-Induced Alzheimer’s Disease Mouse Model" Nutrients 15, no. 23: 4986. https://doi.org/10.3390/nu15234986





