Drastic Synergy of Lovastatin and Antrodia camphorata Extract Combination against PC3 Androgen-Refractory Prostate Cancer Cells, Accompanied by AXL and Stemness Molecules Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Lovastatin and AC Extract
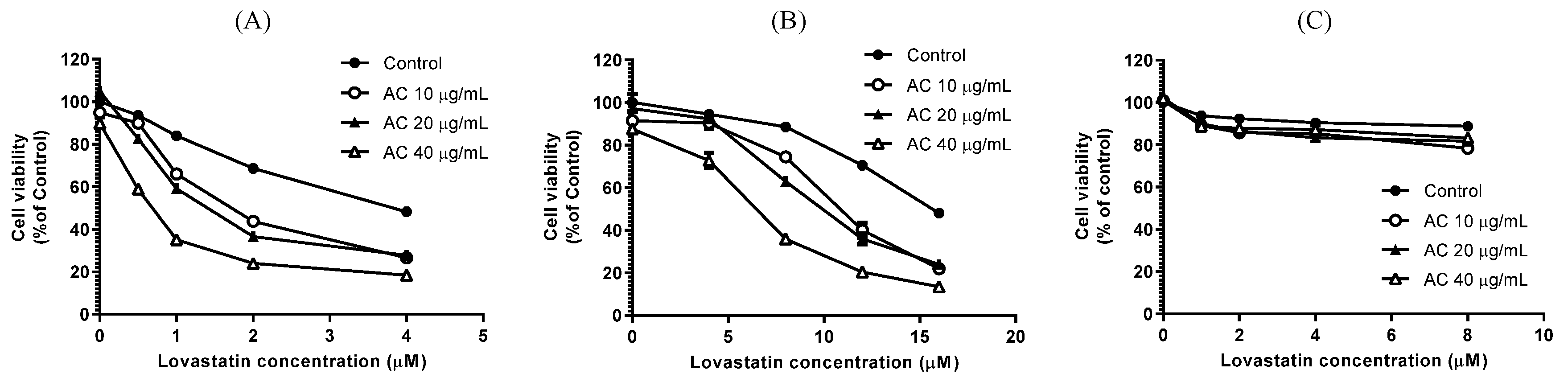
2.3. Sulforhodamine B (SRB) Assay for Cell Viability
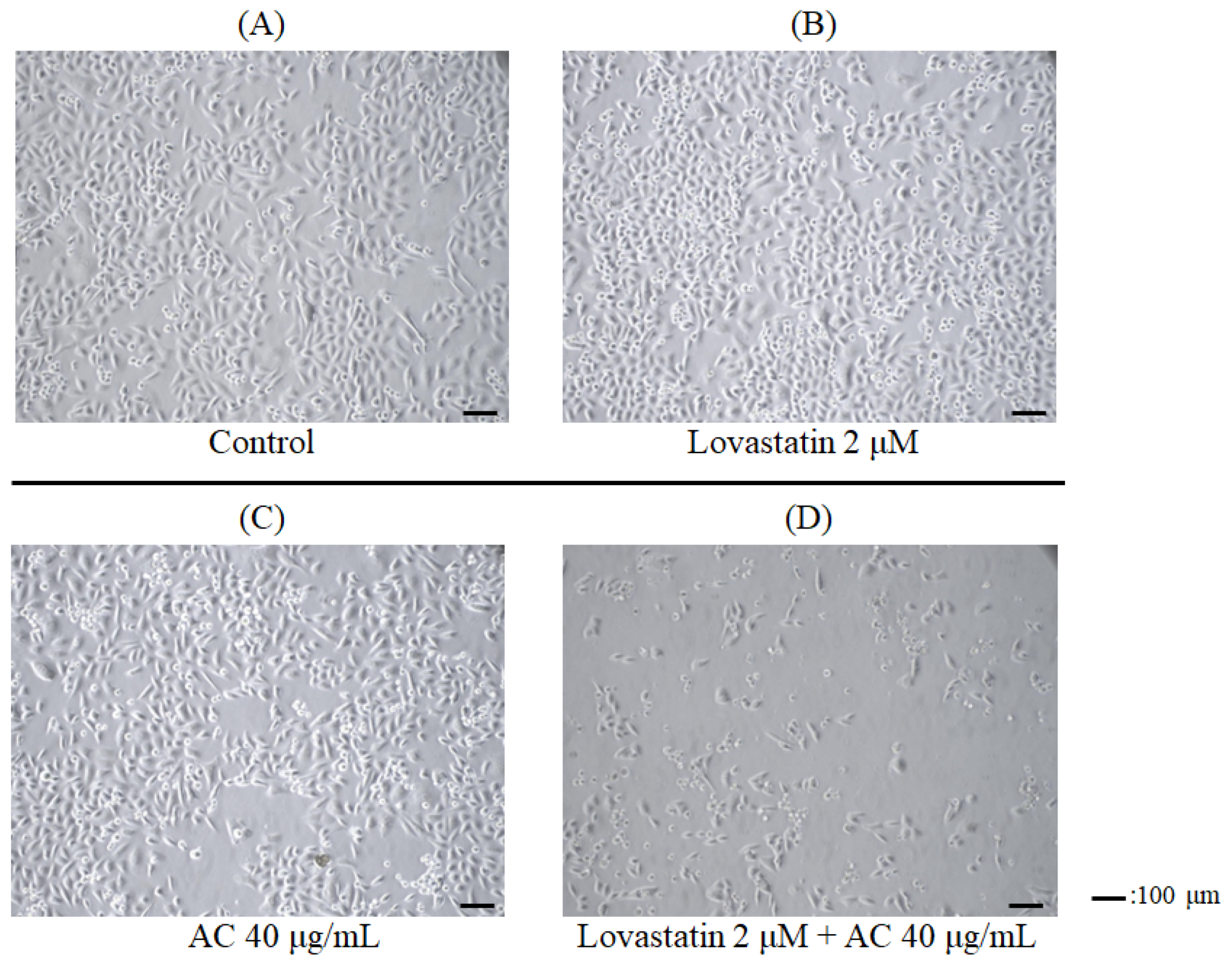
2.4. Photograph of the Cells
2.5. Cell Cycle Analysis
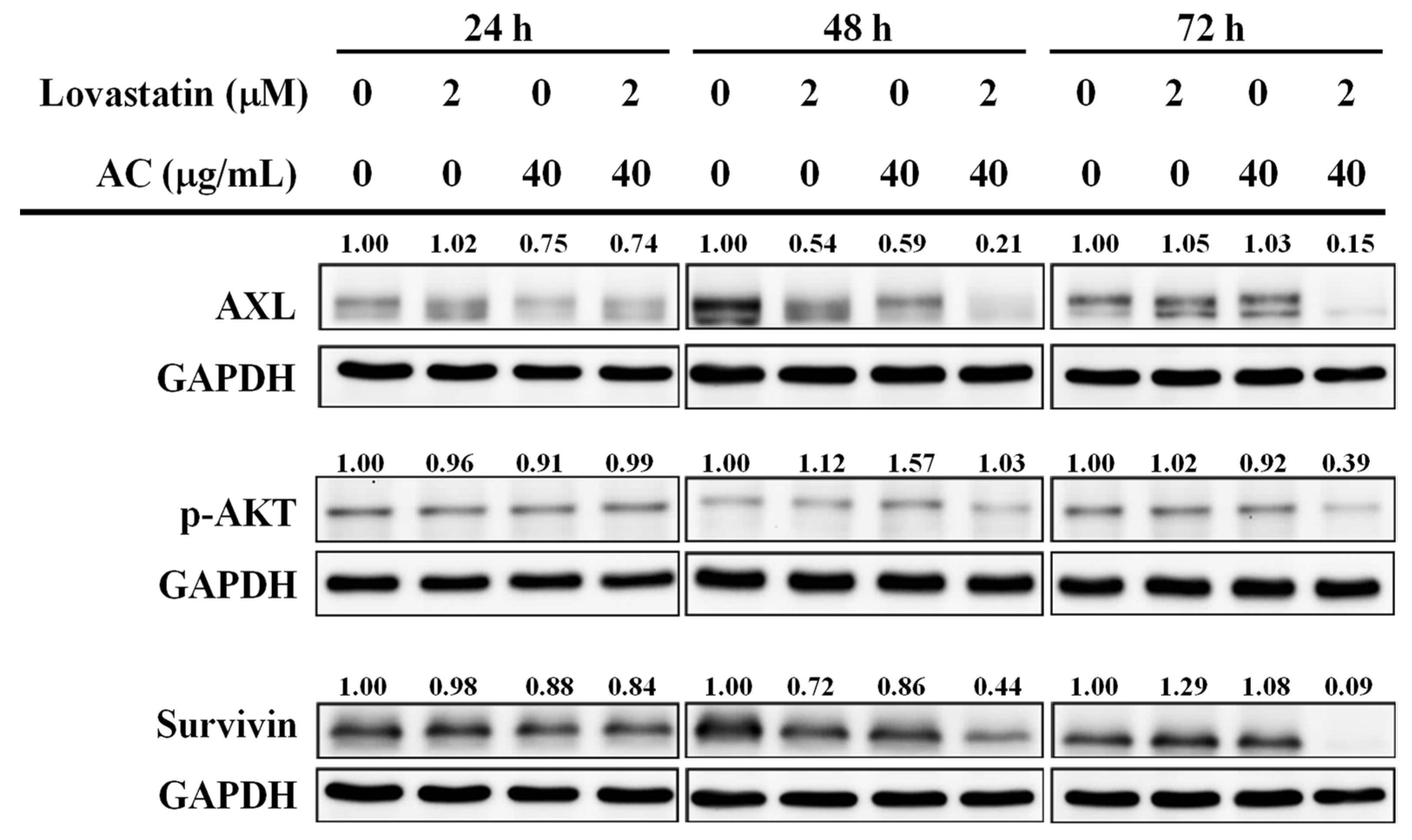
2.6. Western Blotting
3. Results
3.1. Lovastatin and AC Extract Synergistically Suppress Proliferation and Induce Apoptosis in PC3 Cells
3.2. Lovastatin and AC Extract Synergistically Reduce the Proteins Crucial for Cell Cycle Progression in PC3 Cells
3.3. Lovastatin and AC Extract Synergistically Diminish AXL and Survivin in PC3 Cells
3.4. Lovastatin and AC Extract Synergistically Suppress Stemness Molecules and Markers in PC3 Cells
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Hara, T.; Miyazaki, H.; Lee, A.; Tran, C.P.; Reiter, R.E. Androgen receptor and invasion in prostate cancer. Cancer Res. 2008, 68, 1128–1135. [Google Scholar] [CrossRef]
- Dong, L.; Zieren, R.C.; Xue, W.; de Reijke, T.M.; Pienta, K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Goto, T.; Akamatsu, S.; Yamasaki, T.; Inoue, T.; Ogawa, O.; Kobayashi, T. Castration-Resistant Prostate Cancer Refractory to Second-Generation Androgen Receptor Axis-Targeted Agents: Opportunities and Challenges. Cancers 2018, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- De Pinieux, G.; Chariot, P.; Ammi-Said, M.; Louarn, F.; Lejonc, J.L.; Astier, A.; Jacotot, B.; Gherardi, R. Lipid-lowering drugs and mitochondrial function: Effects of HMG-CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br. J. Clin. Pharmacol. 1996, 42, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies, C.; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Taylor, F.C.; Huffman, M.; Ebrahim, S. Statin therapy for primary prevention of cardiovascular disease. JAMA 2013, 310, 2451–2452. [Google Scholar] [CrossRef]
- Barrios-Gonzalez, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 2010, 85, 869–883. [Google Scholar] [CrossRef]
- Chae, Y.K.; Yousaf, M.; Malecek, M.K.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Sassano, A.; Platanias, L.C.; Giles, F. Statins as anti-cancer therapy; Can we translate preclinical and epidemiologic data into clinical benefit? Discov. Med. 2015, 20, 413–427. [Google Scholar] [PubMed]
- Lim, T.; Lee, I.; Kim, J.; Kang, W.K. Synergistic Effect of Simvastatin Plus Radiation in Gastric Cancer and Colorectal Cancer: Implications of BIRC5 and Connective Tissue Growth Factor. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 316–325. [Google Scholar] [CrossRef]
- Keskivali, T.; Kujala, P.; Visakorpi, T.; Tammela, T.L.; Murtola, T.J. Statin use and risk of disease recurrence and death after radical prostatectomy. Prostate 2016, 76, 469–478. [Google Scholar] [CrossRef]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.; Richel, D.J.; Guchelaar, H.J. The risk of cancer in users of statins. J. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef]
- Mondul, A.M.; Han, M.; Humphreys, E.B.; Meinhold, C.L.; Walsh, P.C.; Platz, E.A. Association of statin use with pathological tumor characteristics and prostate cancer recurrence after surgery. J. Urol. 2011, 185, 1268–1273. [Google Scholar] [CrossRef]
- Klimek, M.; Wang, S.; Ogunkanmi, A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm. Ther. 2009, 34, 313–327. [Google Scholar]
- Takwi, A.A.; Li, Y.; Becker Buscaglia, L.E.; Zhang, J.; Choudhury, S.; Park, A.K.; Liu, M.; Young, K.H.; Park, W.Y.; Martin, R.C.; et al. A statin-regulated microRNA represses human c-Myc expression and function. EMBO Mol. Med. 2012, 4, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, I.; Kang, W.K. Lovastatin-induced E2F-1 modulation and its effect on prostate cancer cell death. Carcinogenesis 2001, 22, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Chen, H.; Xu, X.C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.J.; Siu, L.L.; Chen, E.; Dimitroulakos, J.; Kamel-Reid, S.; Moore, M.J.; Chin, S.; Irish, J.; LaFramboise, S.; Oza, A.M. A Phase I trial of prolonged administration of lovastatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or of the cervix. Eur. J. Cancer 2005, 41, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea-An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Huang, C.C.; Huang, C.J.; Cheng, J.S.; Chen, I.S.; Tsai, J.Y.; Jiann, B.P.; Tseng, P.L.; Kuo, S.J.; Jan, C.R. Effects of antrodia camphorata on viability, apoptosis, and [Ca2+]i in PC3 human prostate cancer cells. Chin. J. Physiol. 2008, 51, 78–84. [Google Scholar] [PubMed]
- Chen, K.C.; Peng, C.C.; Peng, R.Y.; Su, C.H.; Chiang, H.S.; Yan, J.H.; Hsieh-Li, H.M. Unique formosan mushroom Antrodia camphorata differentially inhibits androgen-responsive LNCaP and -independent PC-3 prostate cancer cells. Nutr. Cancer 2007, 57, 111–121. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, F.; Lucchetti, C.; Caligiuri, I.; Marchesi, I.; Caputo, M.; Klein-Szanto, A.J.; Bagella, L.; Castronovo, M.; Giordano, A. Retinoblastoma tumor-suppressor protein phosphorylation and inactivation depend on direct interaction with Pin1. Cell Death Differ. 2012, 19, 1152–1161. [Google Scholar] [CrossRef]
- Shariat, S.F.; Lotan, Y.; Saboorian, H.; Khoddami, S.M.; Roehrborn, C.G.; Slawin, K.M.; Ashfaq, R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 2004, 100, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Patel, L.R.; Ziegler, A.M.; Havens, A.M.; Jung, Y.; Wang, J.; Zalucha, S.; Loberg, R.D.; Pienta, K.J.; et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 2010, 12, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Vanli, N.; Sheng, J.; Li, S.; Xu, Z.; Hu, G.F. Ribonuclease 4 is associated with aggressiveness and progression of prostate cancer. Commun. Biol. 2022, 5, 625. [Google Scholar] [CrossRef]
- Yang, J.; Aljitawi, O.; Van Veldhuizen, P. Prostate Cancer Stem Cells: The Role of CD133. Cancers 2022, 14, 5448. [Google Scholar] [CrossRef]
- Li, W.J.; Liu, X.; Dougherty, E.M.; Tang, D.G. MicroRNA-34a, Prostate Cancer Stem Cells, and Therapeutic Development. Cancers 2022, 14, 4538. [Google Scholar] [CrossRef]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, C.; Boopathi, E.; Liu, Y.; Haber, A.; Ertel, A.; Bhardwaj, A.; Addya, S.; Williams, N.; Ciment, S.J.; Cotzia, P.; et al. RB Loss Promotes Prostate Cancer Metastasis. Cancer Res. 2017, 77, 982–995. [Google Scholar] [CrossRef]
- McNair, C.; Xu, K.; Mandigo, A.C.; Benelli, M.; Leiby, B.; Rodrigues, D.; Lindberg, J.; Gronberg, H.; Crespo, M.; De Laere, B.; et al. Differential impact of RB status on E2F1 reprogramming in human cancer. J. Clin. Investig. 2018, 128, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Mandigo, A.C.; Shafi, A.A.; McCann, J.J.; Yuan, W.; Laufer, T.S.; Bogdan, D.; Gallagher, L.; Dylgjeri, E.; Semenova, G.; Vasilevskaya, I.A.; et al. Novel Oncogenic Transcription Factor Cooperation in RB-Deficient Cancer. Cancer Res. 2022, 82, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, N.; Gorn-Hondermann, I.; Ma, L.; Zahr, S.; Johnson-Obeseki, S.; Corsten, M.; Dimitroulakos, J. Lovastatin-induced apoptosis is mediated by activating transcription factor 3 and enhanced in combination with salubrinal. Int. J. Cancer 2014, 134, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulakos, J.; Ye, L.Y.; Benzaquen, M.; Moore, M.J.; Kamel-Reid, S.; Freedman, M.H.; Yeger, H.; Penn, L.Z. Differential sensitivity of various pediatric cancers and squamous cell carcinomas to lovastatin-induced apoptosis: Therapeutic implications. Clin. Cancer Res. 2001, 7, 158–167. [Google Scholar]
- Thibault, A.; Samid, D.; Tompkins, A.C.; Figg, W.D.; Cooper, M.R.; Hohl, R.J.; Trepel, J.; Liang, B.; Patronas, N.; Venzon, D.J.; et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin. Cancer Res. 1996, 2, 483–491. [Google Scholar]
- Dulak, J.; Jozkowicz, A. Anti-angiogenic and anti-inflammatory effects of statins: Relevance to anti-cancer therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar] [CrossRef]
- Craig, E.L.; Stopsack, K.H.; Evergren, E.; Penn, L.Z.; Freedland, S.J.; Hamilton, R.J.; Allott, E.H. Statins and prostate cancer-hype or hope? The epidemiological perspective. Prostate Cancer Prostatic Dis. 2022, 25, 641–649. [Google Scholar] [CrossRef]
- Longo, J.; Freedland, S.J.; Penn, L.Z.; Hamilton, R.J. Statins and prostate cancer-hype or hope? The biological perspective. Prostate Cancer Prostatic Dis. 2022, 25, 650–656. [Google Scholar] [CrossRef]
- Mandigo, A.C.; Yuan, W.; Xu, K.; Gallagher, P.; Pang, A.; Guan, Y.F.; Shafi, A.A.; Thangavel, C.; Sheehan, B.; Bogdan, D.; et al. RB/E2F1 as a Master Regulator of Cancer Cell Metabolism in Advanced Disease. Cancer Discov. 2021, 11, 2334–2353. [Google Scholar] [CrossRef] [PubMed]
- Paccez, J.D.; Vasques, G.J.; Correa, R.G.; Vasconcellos, J.F.; Duncan, K.; Gu, X.; Bhasin, M.; Libermann, T.A.; Zerbini, L.F. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene 2013, 32, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Hammes, S.R. AXL cooperates with EGFR to mediate neutrophil elastase-induced migration of prostate cancer cells. iScience 2021, 24, 103270. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef]
- Garcia-Peterson, L.M.; Li, X. Trending topics of SIRT1 in tumorigenicity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129952. [Google Scholar] [CrossRef]
- Huang, S.B.; Thapa, D.; Munoz, A.R.; Hussain, S.S.; Yang, X.; Bedolla, R.G.; Osmulski, P.; Gaczynska, M.E.; Lai, Z.; Chiu, Y.C.; et al. Androgen deprivation-induced elevated nuclear SIRT1 promotes prostate tumor cell survival by reactivation of AR signaling. Cancer Lett. 2021, 505, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Nihal, M.; Zhong, W.; Ahmad, N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J. Biol. Chem. 2009, 284, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Ohhashi, R.; Fujita, Y.; Hamada, N.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem. Biophys. Res. Commun. 2008, 373, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, X. The SIRT1-c-Myc axis in regulation of stem cells. Front. Cell Dev. Biol. 2023, 11, 1236968. [Google Scholar] [CrossRef]
- Shin, S.W. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019, 381, e19. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, T.; Riedinger, M.; Lin, S.; Faltermeier, C.M.; Smith, B.A.; Zhang, K.X.; Going, C.C.; Goldstein, A.S.; Lee, J.K.; Drake, J.M.; et al. Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6457–E6466. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Fletcher, S.; Chauhan, J.; Chalfant, V.; Riveros, C.; Mackeyev, Y.; Singh, P.K.; Krishnan, S.; Osumi, T.; Balaji, K.C. 3JC48-3 (methyl 4′-methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1′-biphenyl]-3-carboxylate): A novel MYC/MAX dimerization inhibitor reduces prostate cancer growth. Cancer Gene Ther. 2022, 29, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
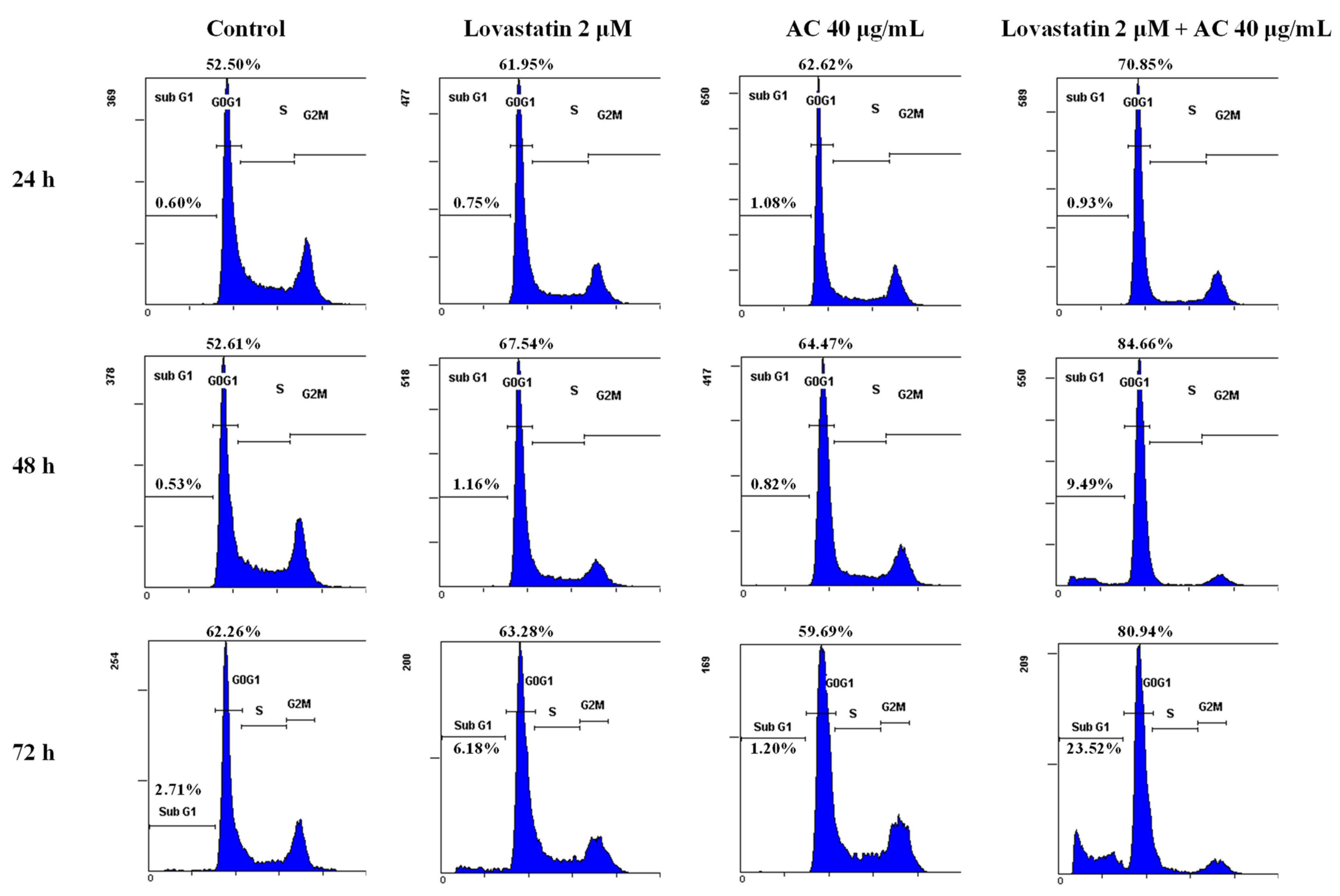


| Lovastatin (μM) | 0 | 2 | 0 | 2 |
| AC (μg/mL) | 0 | 0 | 40 | 40 |
| Sub-G1 (%) | 2.7 | 6.2 | 1.2 | 23.5 |
| G0/G1 (%) | 62.2 | 63.3 | 59.7 | 81.0 |
| S (%) | 21.7 | 19.0 | 18.5 | 10.4 |
| G2/M (%) | 16.1 | 17.7 | 21.8 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.-J.; Chang, C.-L.; Hu, M.-H.; Liao, C.-H.; Lai, G.-M.; Chiou, T.-J.; Ho, H.-L.; Kuo, H.-C.; Yang, Y.-Y.; Whang-Peng, J.; et al. Drastic Synergy of Lovastatin and Antrodia camphorata Extract Combination against PC3 Androgen-Refractory Prostate Cancer Cells, Accompanied by AXL and Stemness Molecules Inhibition. Nutrients 2023, 15, 4493. https://doi.org/10.3390/nu15214493
Yao C-J, Chang C-L, Hu M-H, Liao C-H, Lai G-M, Chiou T-J, Ho H-L, Kuo H-C, Yang Y-Y, Whang-Peng J, et al. Drastic Synergy of Lovastatin and Antrodia camphorata Extract Combination against PC3 Androgen-Refractory Prostate Cancer Cells, Accompanied by AXL and Stemness Molecules Inhibition. Nutrients. 2023; 15(21):4493. https://doi.org/10.3390/nu15214493
Chicago/Turabian StyleYao, Chih-Jung, Chia-Lun Chang, Ming-Hung Hu, Chien-Huang Liao, Gi-Ming Lai, Tzeon-Jye Chiou, Hsien-Ling Ho, Hui-Ching Kuo, Ya-Yu Yang, Jacqueline Whang-Peng, and et al. 2023. "Drastic Synergy of Lovastatin and Antrodia camphorata Extract Combination against PC3 Androgen-Refractory Prostate Cancer Cells, Accompanied by AXL and Stemness Molecules Inhibition" Nutrients 15, no. 21: 4493. https://doi.org/10.3390/nu15214493






