Effect of Malaria on Blood Levels of Vitamin E: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Registration, Guidelines for Reporting, and Review Questions
2.2. Eligibility Criteria
2.3. Search Strategy
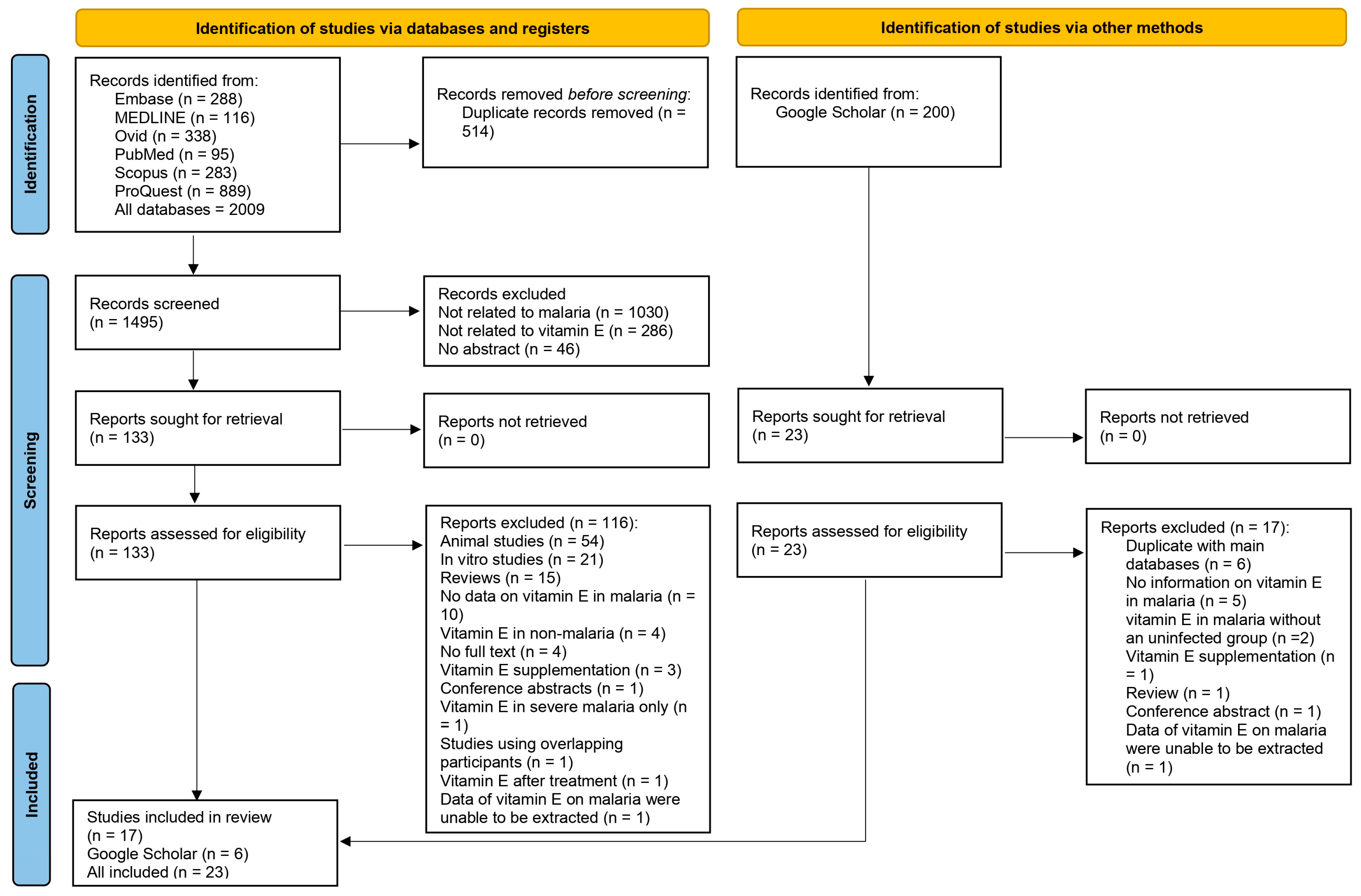
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
3.1. Search Results
3.2. Characteristics and Critical Appraisal of the Studies
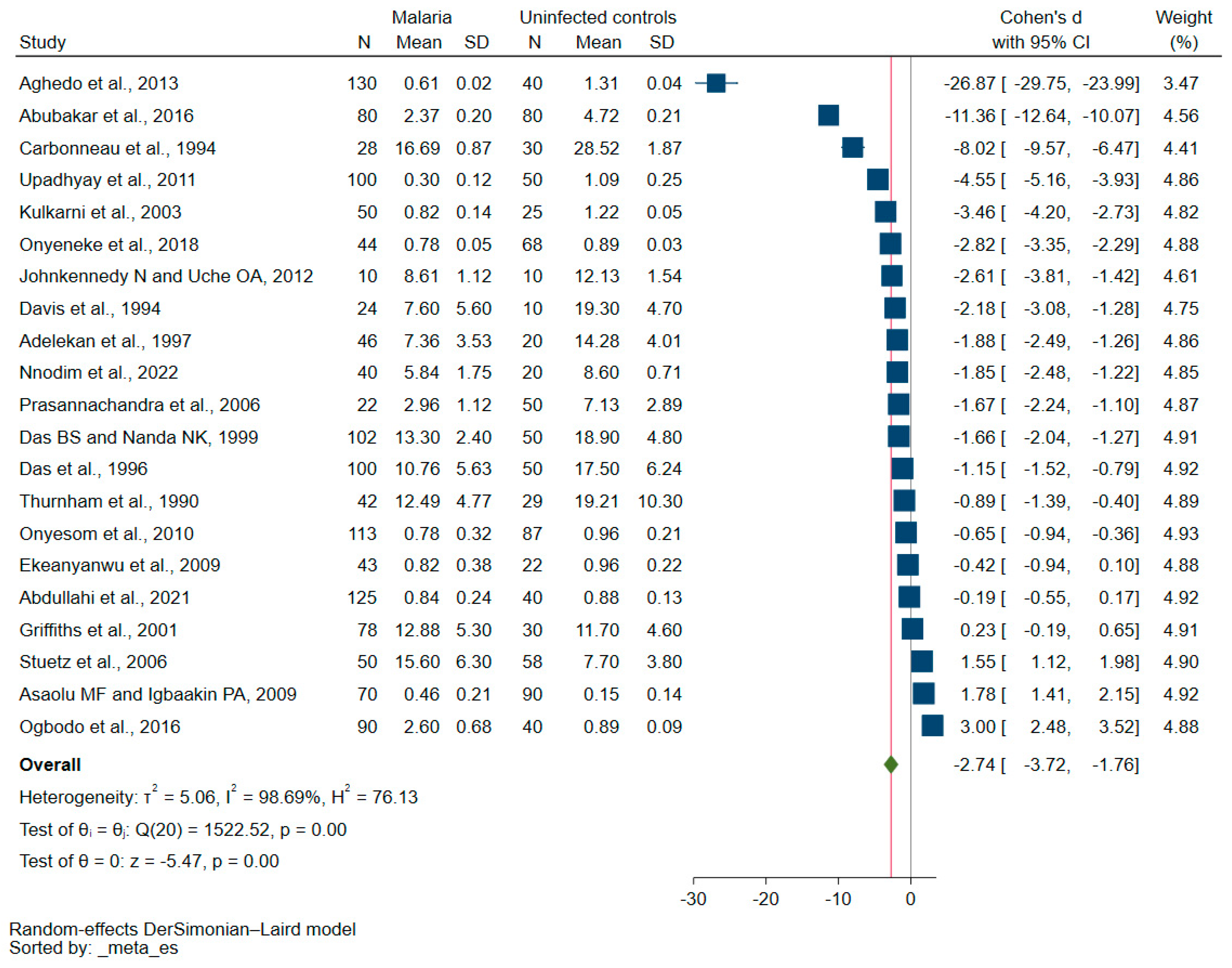
3.3. Vitamin E Levels in Malaria Patients Compared with Those Who Were Not Infected
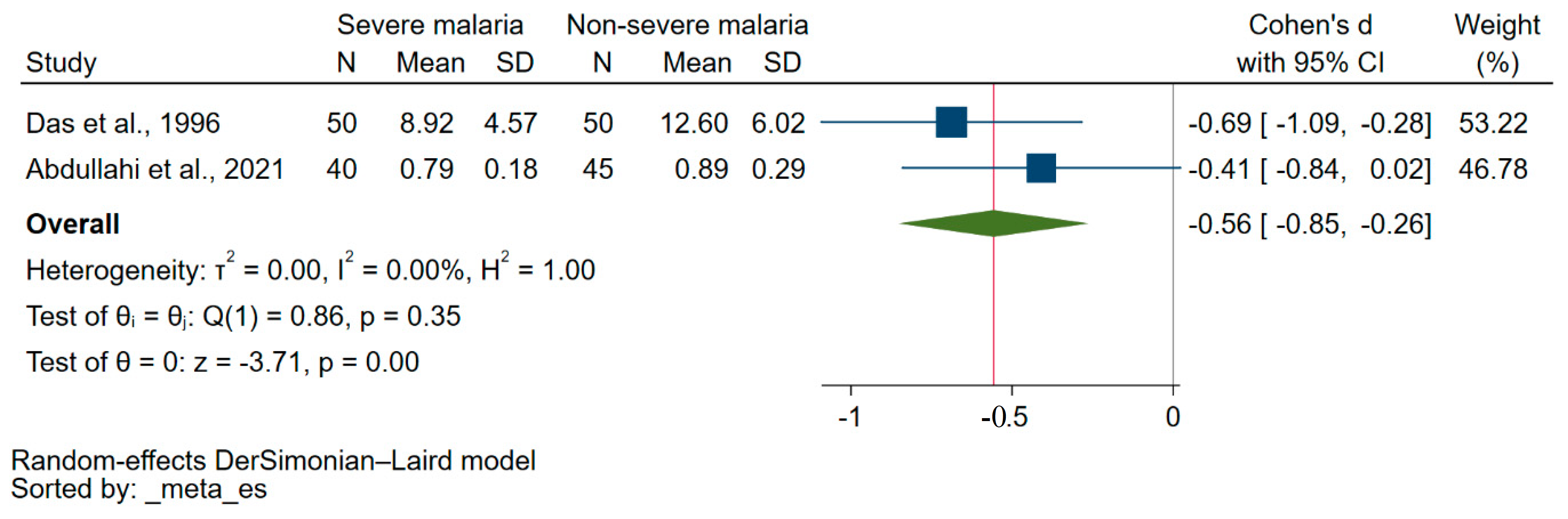
3.4. Difference in Vitamin E Levels between Patients with Severe Malaria and Those Experiencing Less Severe Forms of the Disease
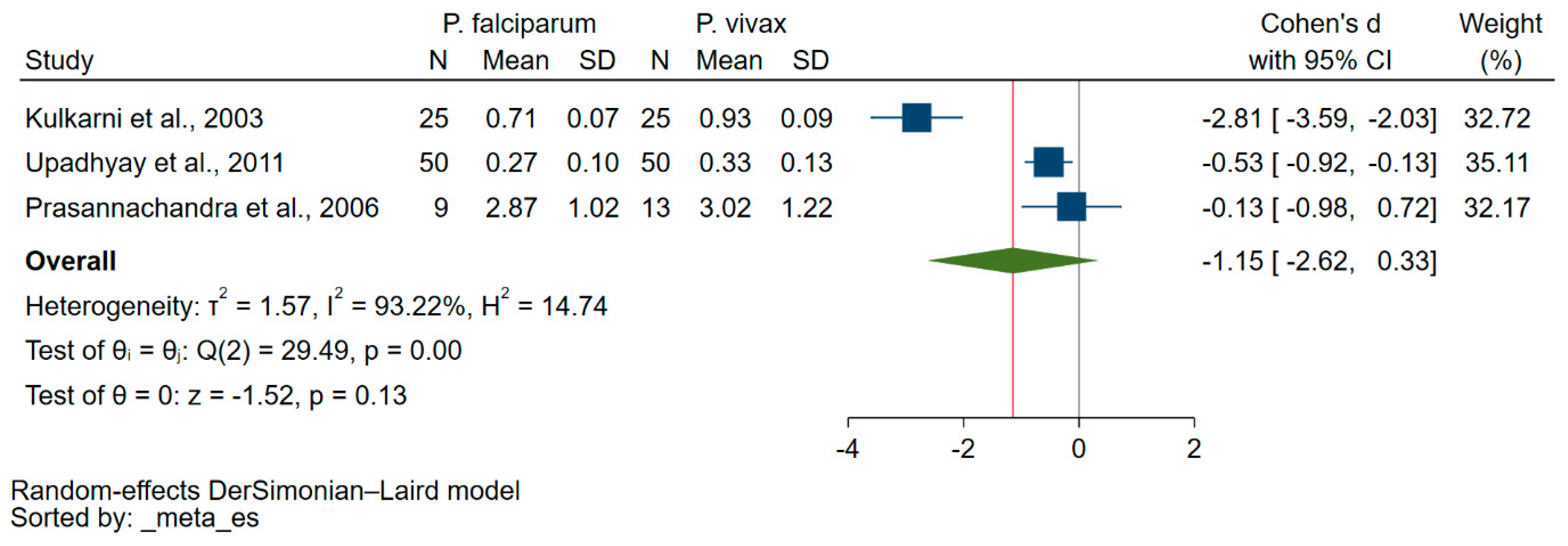
3.5. Difference in Vitamin E Levels among Patients Suffering from either P. falciparum or P. vivax Malaria
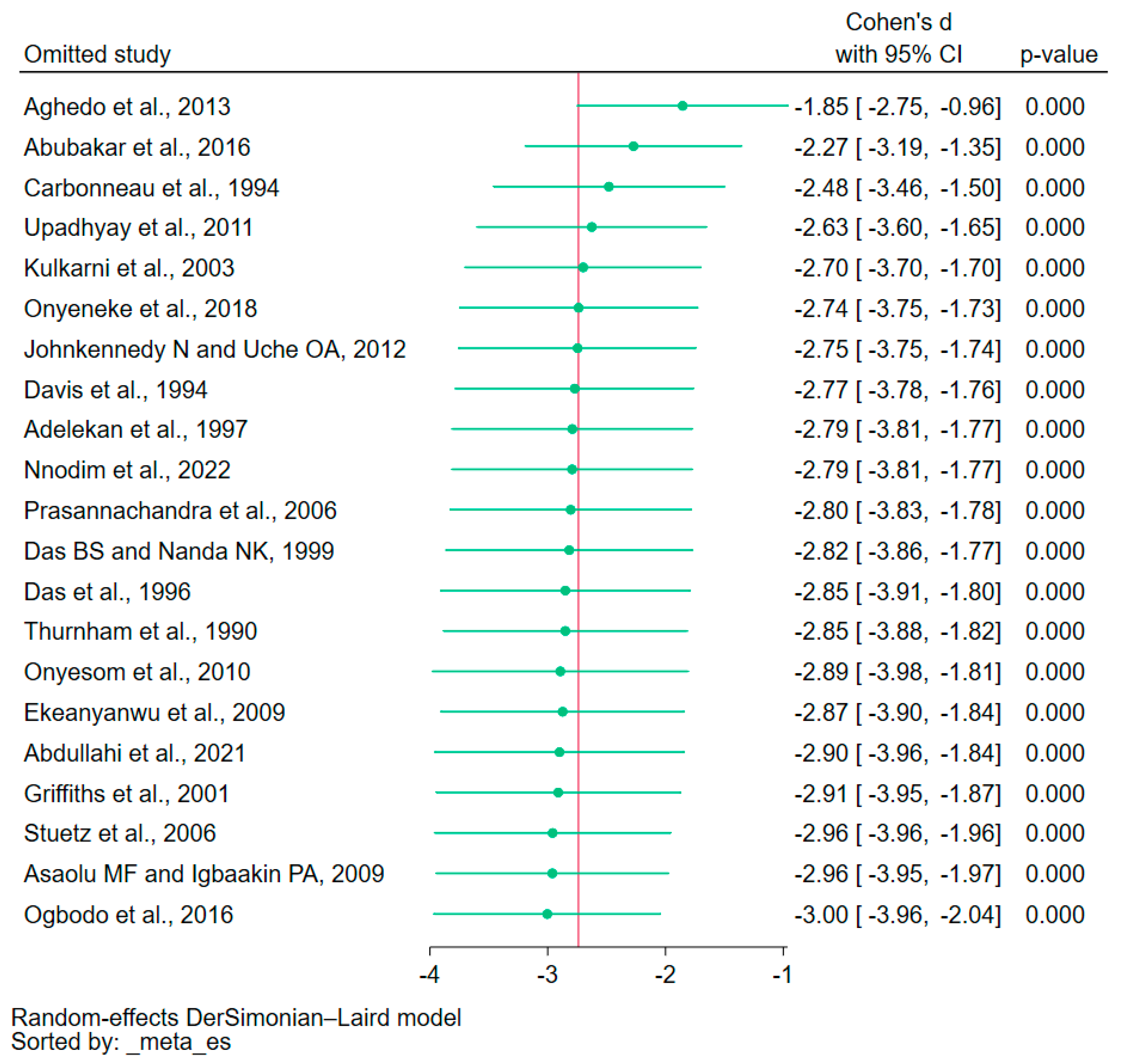
3.6. Sensitivity Analysis
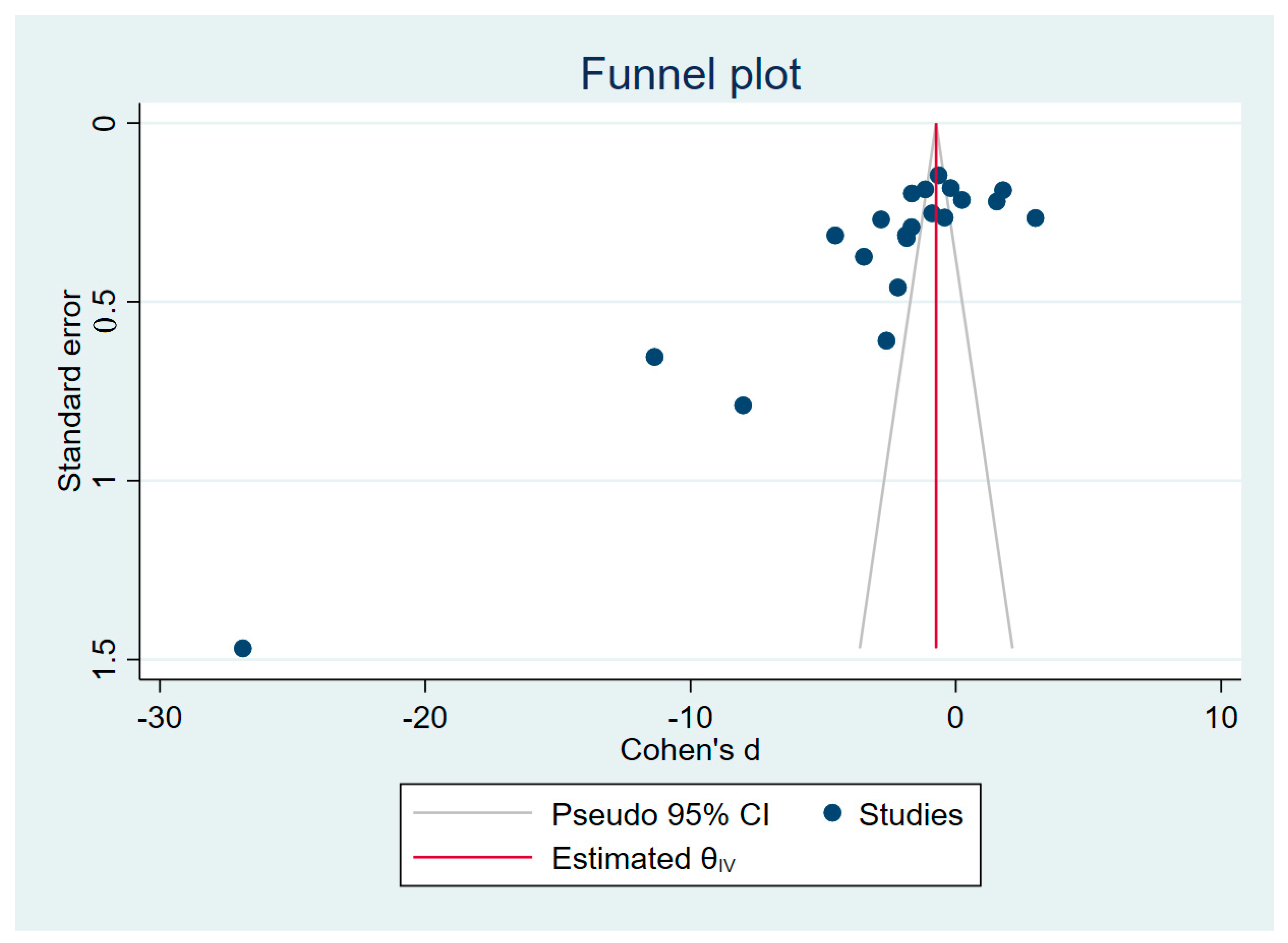
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crutcher, J.M.; Hoffman, S.L. Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Millar, S.B.; Cox-Singh, J. Human infections with Plasmodium knowlesi—Zoonotic malaria. Clin. Microbiol. Infect. 2015, 21, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Malaria Report 2022. 2022. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 23 June 2023).
- Vasquez, M.; Zuniga, M.; Rodriguez, A. Oxidative stress and pathogenesis in malaria. Front. Cell. Infect. Microbiol. 2021, 11, 768182. [Google Scholar] [CrossRef]
- Percario, S.; Moreira, D.R.; Gomes, B.A.; Ferreira, M.E.; Goncalves, A.C.; Laurindo, P.S.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ty, M.C.; Zuniga, M.; Gotz, A.; Kayal, S.; Sahu, P.K.; Mohanty, A.; Mohanty, S.; Wassmer, S.C.; Rodriguez, A. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol. Med. 2019, 11, e9903. [Google Scholar] [CrossRef] [PubMed]
- Narsaria, N.; Mohanty, C.; Das, B.K.; Mishra, S.P.; Prasad, R. Oxidative stress in children with severe malaria. J. Trop. Pediatr. 2012, 58, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. Vitam. Horm. 2007, 76, 1–21. [Google Scholar] [CrossRef]
- George, B.O.; Atalor, B.; Okpoghono, J. Role of Vitamin E, Vitamin A and aqueous extract of Aframomum sceptrum in mice, infected with malaria parasites. Niger. J. Nutr. Sci. 2019, 40, 113–117. [Google Scholar]
- Ibrahim, M.A.; Isah, M.B.; Okafor, A.I.; Bashir, M.; Bisalla, M.; Umar, I.A. Effects of combined administration of vitamins C and E on some Plasmodium berghei-induced pathological changes and oxidative stress in mice. Comp. Clin. Pathol. 2012, 21, 1677–1682. [Google Scholar] [CrossRef]
- Suzuki, H.; Kume, A.; Herbas, M.S. Potential of vitamin E deficiency, induced by inhibition of alpha-tocopherol efflux, in murine malaria infection. Int. J. Mol. Sci. 2018, 20, 64. [Google Scholar] [CrossRef]
- Sussmann, R.A.C.; Fotoran, W.L.; Kimura, E.A.; Katzin, A.M. Plasmodium falciparum uses vitamin E to avoid oxidative stress. Parasites Vectors 2017, 10, 461. [Google Scholar] [CrossRef] [Green Version]
- Olofin, I.O.; Spiegelman, D.; Aboud, S.; Duggan, C.; Danaei, G.; Fawzi, W.W. Supplementation with multivitamins and vitamin A and incidence of malaria among HIV-infected tanzanian women. J. Acquir. Immune Defic. Syndr. 2014, 67, S173–S178. [Google Scholar] [CrossRef] [Green Version]
- Villamor, E.; Msamanga, G.; Saathoff, E.; Fataki, M.; Manji, K.; Fawzi, W.W. Effects of maternal vitamin supplements on malaria in children born to HIV-infected women. Am. J. Trop. Med. Hyg. 2007, 76, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Jordan, Z.; McArthur, A. Developing the review question and inclusion criteria. Am. J. Nurs. 2014, 114, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 15 May 2023).
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hoaglin, D.C. Misunderstandings about Q and ‘Cochran’s Q test’ in meta-analysis. Stat. Med. 2016, 35, 485–495. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (updated February 2022); Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 15 May 2023).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ 2007, 176, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.G.; Usman, S.M.; Dandare, S.U. Oxidant status of children infected with Plasmodium falciparum malaria in Katsina Metropolis, Northwestern Nigeria. Afr. J. Infect. Dis. 2016, 10, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Adelekan, D.A.; Adeodu, O.O.; Thurnham, D.I. Comparative effects of malaria and malnutrition on plasma concentrations of antioxidant micronutrients in children. Ann. Trop. Paediatr. 1997, 17, 223–227. [Google Scholar] [CrossRef]
- Aghedo, F.I.; Shehu, R.A.; Umar, R.A.; Jiya, M.N.; Erhabor, O. Antioxidant vitamin levels among preschool children with uncomplicated Plasmodium falciparum malaria in Sokoto, Nigeria. J. Multidiscip. Healthc. 2013, 6, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asaolu, M.F.; Igbaakin, P.A. Serum Levels of micronutrients and antioxidants during malaria in pregnant women In Ado-Ekiti, Ekiti State, Nigeria. Int. J. Med. Sci. 2009, 1, 523–526. [Google Scholar]
- Carbonneau, M.A.; Sess, D.; Peuchant, E.; Dubourg, L.; Thomas, M.J.; Perromat, A.; Clerc, M.; Receveur, M.C. Comparison of methods evaluating lipoperoxidation in plasma of malaria patients. J. Liq. Chromatogr. Relat. Technol. 1994, 17, 2247–2272. [Google Scholar] [CrossRef]
- Das, B.S.; Nanda, N.K. Evidence for erythrocyte lipid pepoxidation in acute falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 58–62. [Google Scholar] [CrossRef]
- Das, B.S.; Thurnham, D.I.; Das, D.B. Plasma α-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am. J. Clin. Nutr. 1996, 64, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.M.; Binh, T.Q.; Danh, P.T.; Dyer, J.R.; St John, A.; Garcia-Webb, P.; Anh, T.K. Serum vitamin A and E concentrations in acute falciparum malaria: Modulators or markers of severity? Clin. Sci. 1994, 87, 505–511. [Google Scholar] [CrossRef]
- Delmas-Beauvieux, M.C.; Peuchant, E.; Dumon, M.F.; Receveur, M.C.; Le Bras, M.; Clerc, M. Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin. Biochem. 1995, 28, 163–169. [Google Scholar] [CrossRef]
- Ekeanyanwu, R.C.; Achuka, N.; Akpoilih, B.U. Serum level of antioxidant vitamins (vitamin A, C and E) in Plasmodium falciparum malaria infected children in Owerri, Eastern Nigeria. Biokemistri 2009, 21, 53–58. [Google Scholar] [CrossRef]
- Johnkennedy, N.; Uche, O.A. The plasma vitamin C and E status in type II diabetes with malaria in Owerri, Nigeria. Pak. J. Nutr. 2012, 11, 62–63. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.G.; Suryakar, A.N.; Sardeshmukh, A.S.; Rathi, D.B. Studies on biochemical changes with special reference to oxidant and antioxidants in malaria patients. Indian J. Clin. Biochem. 2003, 18, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Nnodim, J.; Israe, O.K.; Ukamaka, E. Pattern of antioxidant status and lipid peroxidation product in Plasmodium falciparum patients. IJMPT 2022, 2, 16–22. [Google Scholar]
- Onyeneke, E.C.; Ofoha, P.C.; Anyanwu, G.O.; Onovughakpo-Sakpa, E.O.; Anionye, J.C.; Anekwe, A.I. Evaluation of nitric oxide and antioxidant status of Plasmodium falciparum infected pregnant Nigerian women with malaria. Int. Sci. Res. Organ. J. 2018, 3, 56–68. [Google Scholar]
- Onyesom, I.; Ekeanyanwu, R.C.; Achuka, N. Correlation between moderate Plasmodium falciparum malarial parasitaemia and antioxidant vitamins in serum of infected children in South Eastern Nigeria. Afr. J. Biochem. Res. 2010, 4, 261–264. [Google Scholar]
- Prasannachandra; D’Souza, V.; D’Souza, B. Comparative study on lipid peroxidation and antioxidant vitamins E and C in falciparum and vivax malaria. Indian J. Clin. Biochem. 2006, 21, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Thurnham, D.I.; Singkamani, R.; Kaewichit, R.; WongwoRapat, K. Influence of malaria infection on peroxyl-radical trapping capacity in plasma from rural and urban Thai adults. Br. J. Nutr. 1990, 64, 257–271. [Google Scholar] [CrossRef]
- Upadhyay, D.N.; Vyas, R.K.; Sharma, M.L.; Soni, Y.; Rajnee. Comparison in serum profile of peroxidants (MDA) and non enzymatic anti oxidants (Vitamins E and C) among patients suffering from Plasmodium falciparum and vivax malaria. J. Postgrad. Med. Inst. 2011, 25, 96–100. [Google Scholar]
- Abdullahi, I.N.; Musa, S.; Emeribe, A.U.; Muhammed, M.; Mustapha, J.O.; Shuwa, H.A.; Haruna, S.; Abubakar, S.D.; Billyrose, O.M.A.; Bakare, M. Immunological and anti-oxidant profiles of malarial children in Abuja, Nigeria. BioMedicine 2021, 11, 41–50. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Ndungu, F.; Baird, K.L.; Muller, D.P.; Marsh, K.; Newton, C.R. Oxidative stress and erythrocyte damage in Kenyan children with severe Plasmodium falciparum malaria. Br. J. Haematol. 2001, 113, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Nwagha, U.I.; Okeke, T.C.; Nwagha, T.U.; Ejezie, F.E.; Ogbodo, S.O.; Dim, C.C.; Anyaehie, B.U. Asymptomatic malaria parasitemia does not induce additional oxidative stress in pregnant women of South East Nigeria. Asian Pac. J. Trop. Med. 2011, 4, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, W.; McGready, R.; Cho, T.; Prapamontol, T.; Biesalski, H.K.; Stepniewska, K.; Nosten, F. Relation of DDT residues to plasma retinol, alpha-tocopherol, and beta-carotene during pregnancy and malaria infection: A case-control study in Karen women in northern Thailand. Sci. Total Environ. 2006, 363, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ogbodo, S.O.; Okaka, A.N.C.; Nwagha, U.I.; Chukwurah, E.F. Immune response and parasite virulence in malaria parasitemic pregnant women may depend on the antioxidant vitamins levels. J. Glob. Health 2016, 7, 78–85. [Google Scholar]
- Sun, Y.; Ma, A.; Li, Y.; Han, X.; Wang, Q.; Liang, H. Vitamin E supplementation protects erythrocyte membranes from oxidative stress in healthy Chinese middle-aged and elderly people. Nutr. Res. 2012, 32, 328–334. [Google Scholar] [CrossRef]
- Hassan, G.I.; Gregory, U.; Maryam, H. Serum ascorbic acid concentration in patients with acute falciparum malaria infection: Possible significance. Braz. J. Infect. Dis. 2004, 8, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E.; Ortega, H.; Alvino, G.; Giovannini, N.; Amusquivar, E.; Cetin, I. Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. Eur. J. Clin. Nutr. 2004, 58, 1231–1238. [Google Scholar] [CrossRef]
| Characteristics | n. (23) | % |
|---|---|---|
| Year of publication | ||
| 2011–2022 | 9 | 39.1 |
| 2000–2010 | 7 | 30.4 |
| Before 2000 | 7 | 30.4 |
| Study designs | ||
| Case-control studies | 11 | 47.8 |
| Cross-sectional studies | 10 | 43.5 |
| Cohort studies | 2 | 8.70 |
| Study areas | ||
| Africa | 13 | 56.5 |
| Nigeria | 12 | 92.3 |
| Kenya | 1 | 7.70 |
| Asia | 8 | 34.8 |
| India | 5 | 62.5 |
| Thailand | 2 | 25.0 |
| Vietnam | 1 | 12.5 |
| Europe | 2 | 8.70 |
| Plasmodium spp. | ||
| P. falciparum | 16 | 69.6 |
| P. falciparum, P. vivax | 4 | 17.4 |
| P. falciparum, P. vivax, P. ovale | 1 | 4.34 |
| Not specified | 2 | 8.70 |
| Participants | ||
| Children | 9 | 39.1 |
| Adults | 7 | 30.4 |
| Pregnant women | 4 | 17.4 |
| All age groups | 1 | 4.34 |
| Pregnant and nonpregnant women | 1 | 4.34 |
| Not specified | 1 | 4.34 |
| Clinical status | ||
| Symptomatic malaria | 16 | 69.6 |
| Asymptomatic malaria | 1 | 4.34 |
| Status not defined | 6 | 26.1 |
| Methods for malaria detection | ||
| Microscopy | 22 | 95.7 |
| Not specified | 1 | 4.30 |
| Form of vitamin E | ||
| α-tocopherol | 9 | 39.1 |
| Total tocopherols | 2 | 8.70 |
| Form was not specified | 12 | 52.2 |
| Subgroup Analyses | p-Value | Cohen’s d (95% CI) | I2 (%) | Number of Studies |
|---|---|---|---|---|
| Continent | ||||
| Africa | <0.01 | −3.10, −4.54–(−1.66) | 98.95 | 12 |
| Asia | <0.01 | −1.74, −2.95–(−0.52) | 97.81 | 8 |
| Age group | ||||
| Children | <0.01 | −3.97, −5.35–(−2.59) | 98.76 | 9 |
| Adults | <0.01 | −3.66, −5.07–(−2.25) | 94.41 | 6 |
| Pregnant women | 0.43 | 0.88, −1.31–3.07 | 98.92 | 4 |
| Children and adults | N/A | −0.89, −1.39–(−0.40) | N/A | 1 |
| Not specified | N/A | −1.85, −2.48–(−1.22) | N/A | 1 |
| Plasmodium species | ||||
| P. falciparum | <0.01 | −2.79, −3.99–(−1.60) | 98.81 | 14 |
| P. falciparum, P. vivax, P. Ovale | N/A | −8.02, −9.57–(−6.47) | N/A | 1 |
| P. falciparum, P. vivax | <0.01 | −2.64, −4.32–(−0.95) | 96.88 | 4 |
| Not specified | 0.93 | −0.16, −3.52–3.20 | 98.75 | 2 |
| Forms of vitamin E | ||||
| Total tocopherols | 0.30 | −13.66, −39.24–11.92 | 99.68 | 2 |
| α-tocopherol | 0.08 | −0.69, −1.48–(−0.09) | 96.20 | 8 |
| Not specified | <0.01 | −2.87, −4.81–(−0.93) | 99.01 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotepui, M.; Masangkay, F.R.; Mahittikorn, A.; Kotepui, K.U. Effect of Malaria on Blood Levels of Vitamin E: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3472. https://doi.org/10.3390/nu15153472
Kotepui M, Masangkay FR, Mahittikorn A, Kotepui KU. Effect of Malaria on Blood Levels of Vitamin E: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(15):3472. https://doi.org/10.3390/nu15153472
Chicago/Turabian StyleKotepui, Manas, Frederick Ramirez Masangkay, Aongart Mahittikorn, and Kwuntida Uthaisar Kotepui. 2023. "Effect of Malaria on Blood Levels of Vitamin E: A Systematic Review and Meta-Analysis" Nutrients 15, no. 15: 3472. https://doi.org/10.3390/nu15153472





