Improvement of Cognitive Function by Fermented Panax ginseng C.A. Meyer Berries Extracts in an AF64A-Induced Memory Deficit Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
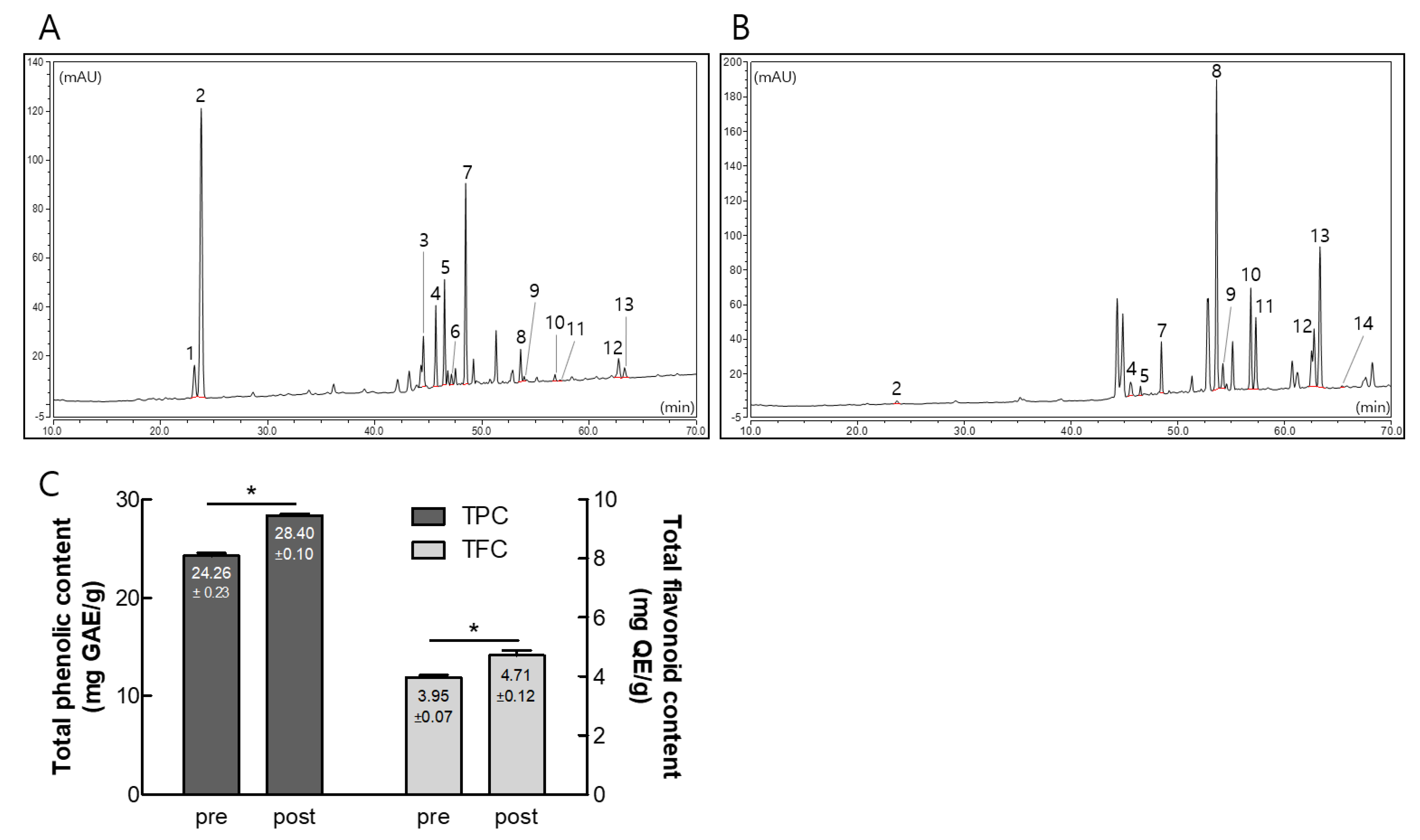
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Determination of Polyphenol and Flavonoid Contents
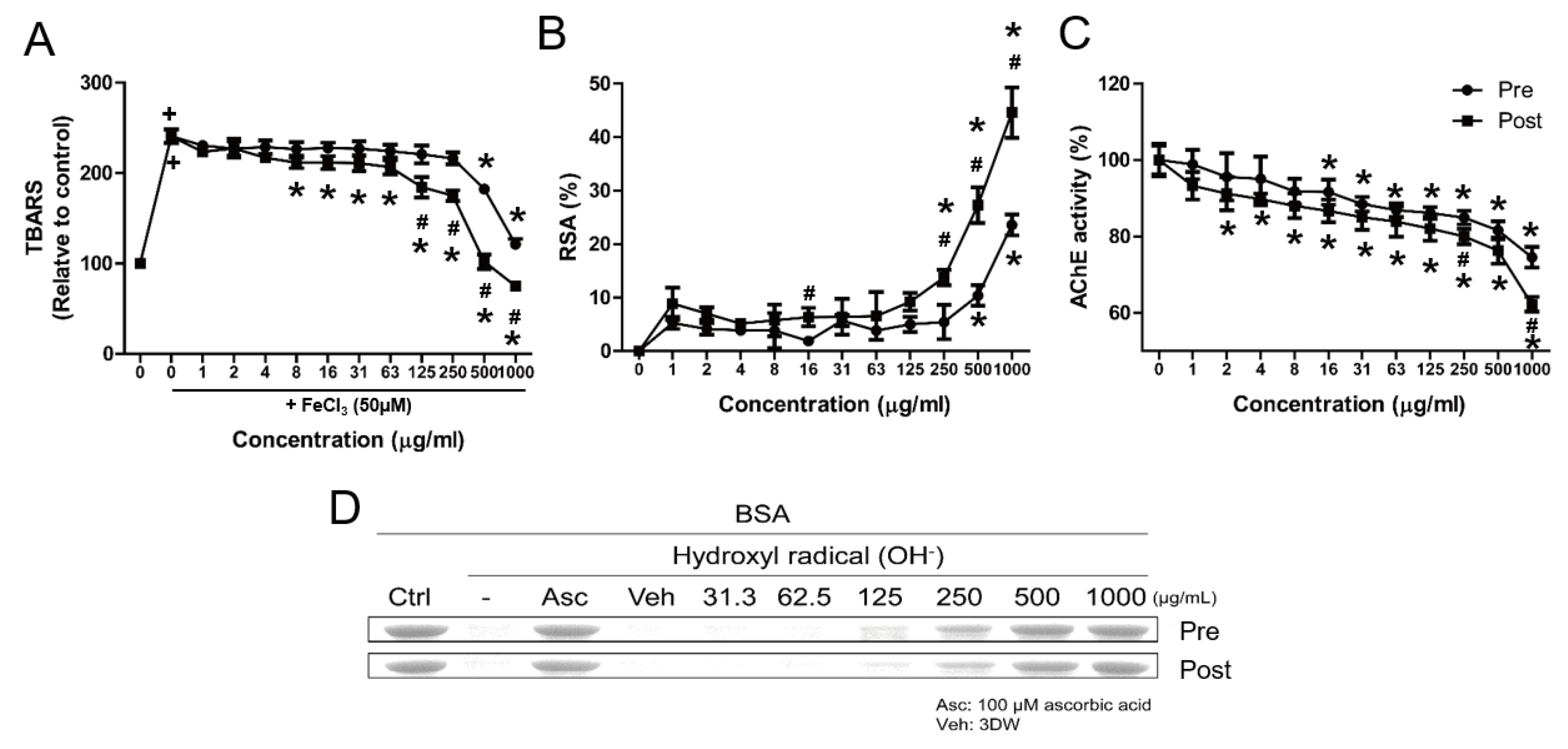
2.4. Assessment of Lipid Peroxidation Levels
2.5. Evaluation of Antioxidant Scavenging Potential
2.6. Assessment of Hydroxyl Radical-Induced Oxidative Damage
2.7. Determination of AChE Activity
2.8. Cell Culture and Maintenance
2.9. Preparation and pH Adjustment of AF64A
2.10. Assessment of Cell Viability and Protective Effects of Pre- and Post-Fermented GBE
2.11. Induction of Cognitive Deficits and Treatment in Mice
2.12. Assessment of Passive Avoidance Memory Performance
2.13. Assessment of Spatial Memory Using Morris Water Maze
2.14. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.15. Western Blot Analysis
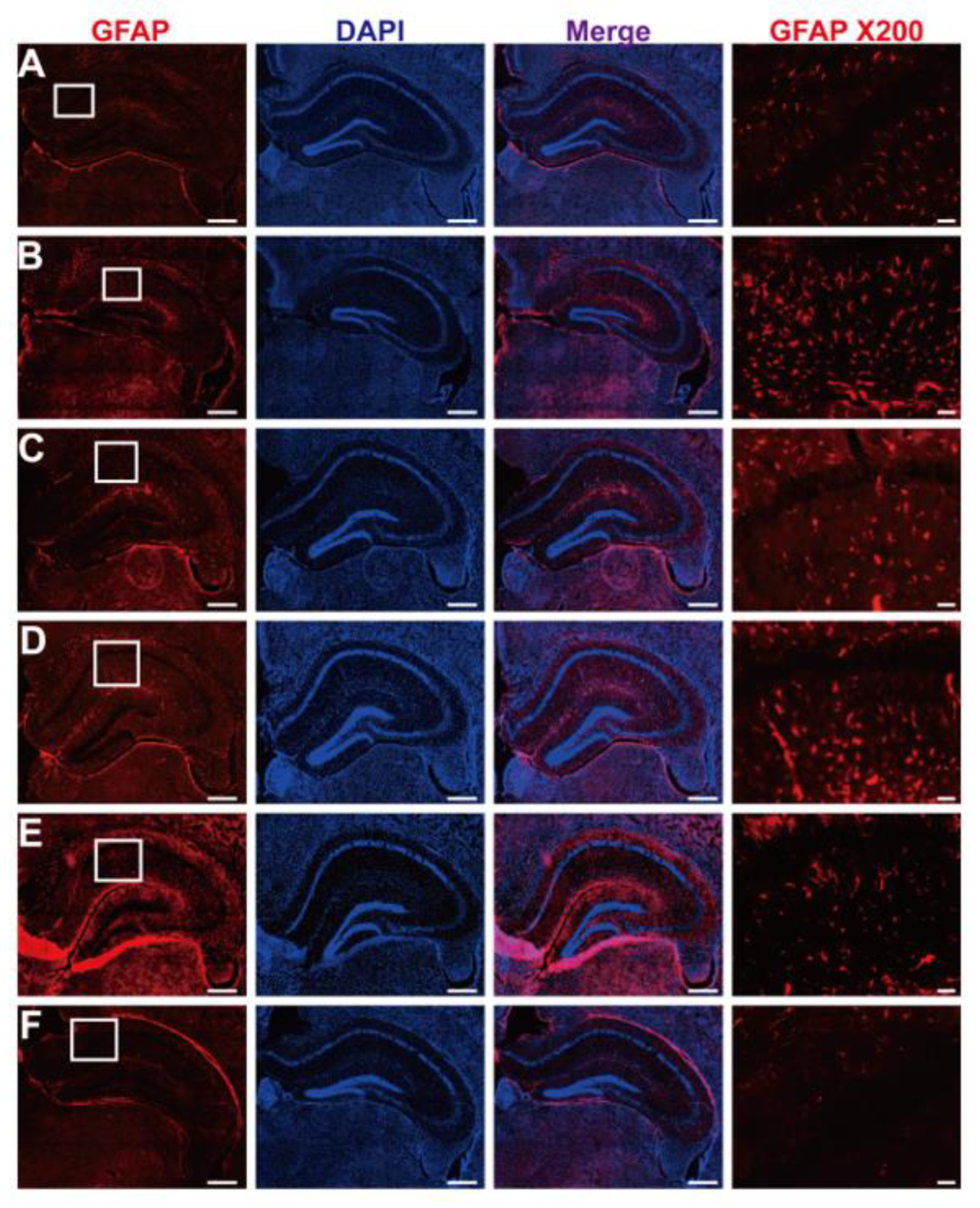
2.16. Glial Fibrillary Acidic Protein (GFAP) Immunostaining of Brain Tissue
2.17. Brain Acetylcholine Concentration Analysis
2.18. Statistical Analyses
3. Results
3.1. Composition of Ginsenosides, Polyphenols, and Flavonoids in Pre- and Post-Fermented GBE
3.2. Comparative Physiological Properties of Pre- and Post-Fermented GBE
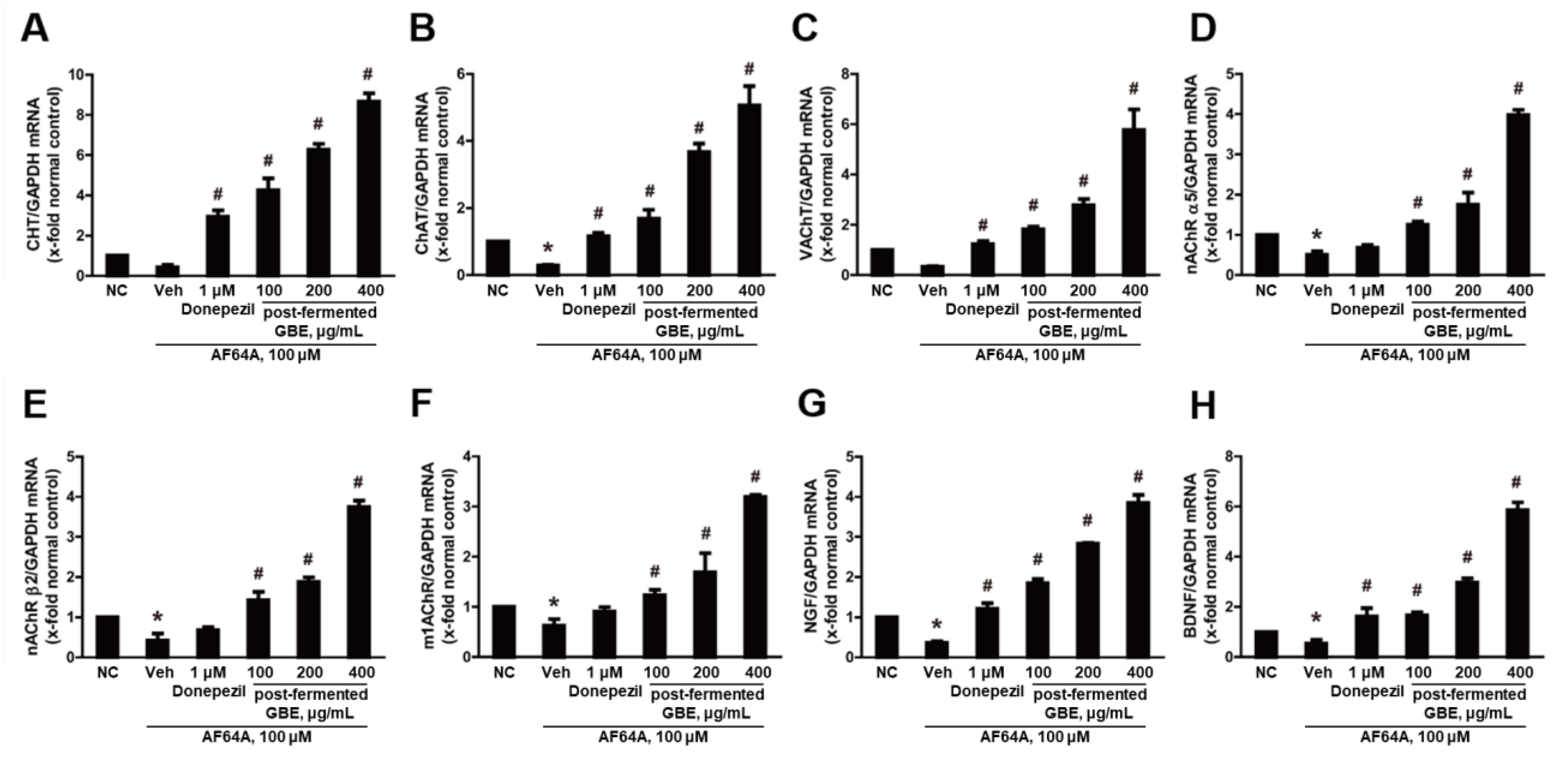
3.3. Protective Effects of Post-Fermented GBE on AF64A-Induced Damage in F3 and F3.ChAT Cells
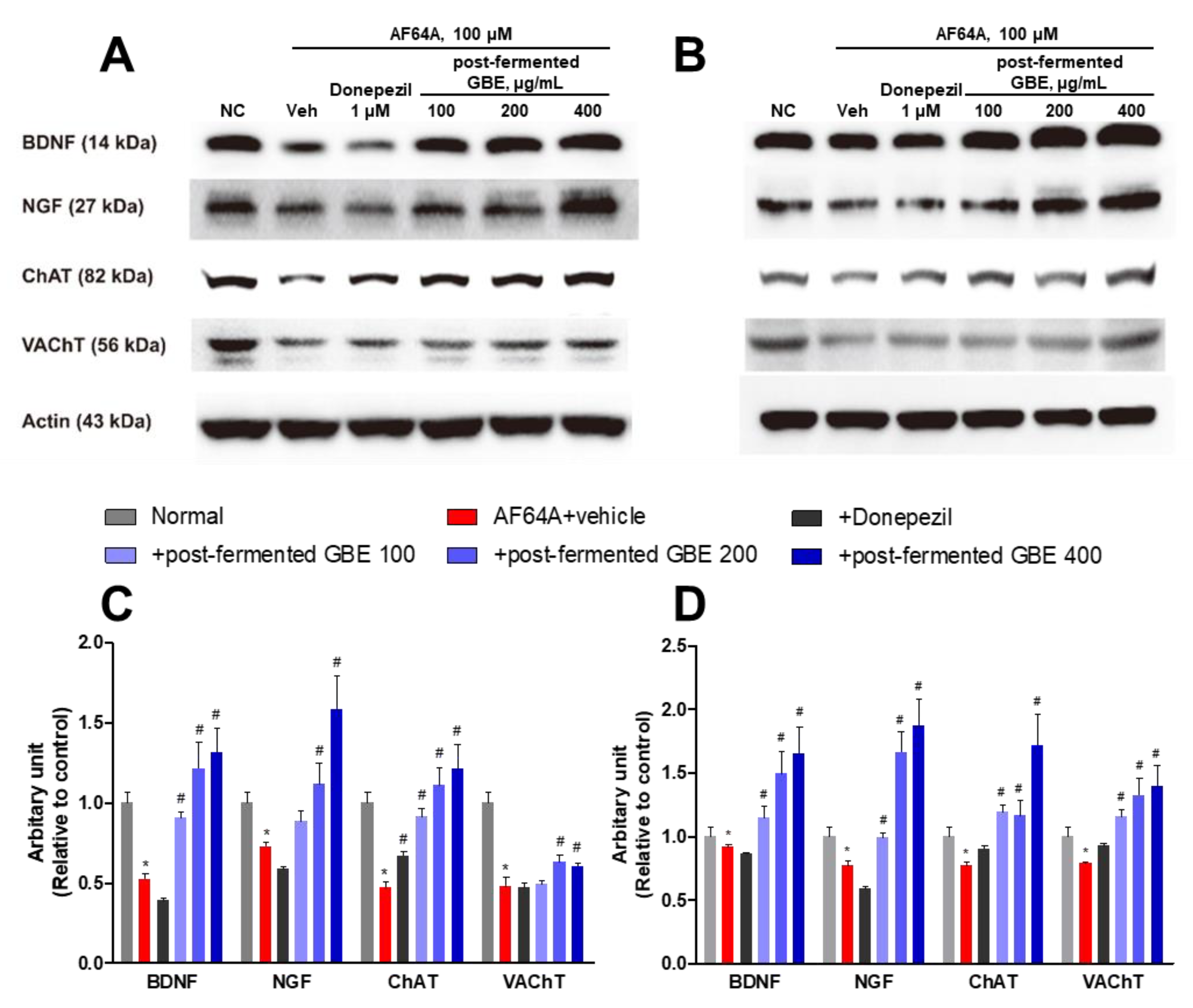
3.4. Post-Fermented GBE’s Effects on Cholinergic Pathway in AF64A-Damaged F3 and F3.ChAT Cells
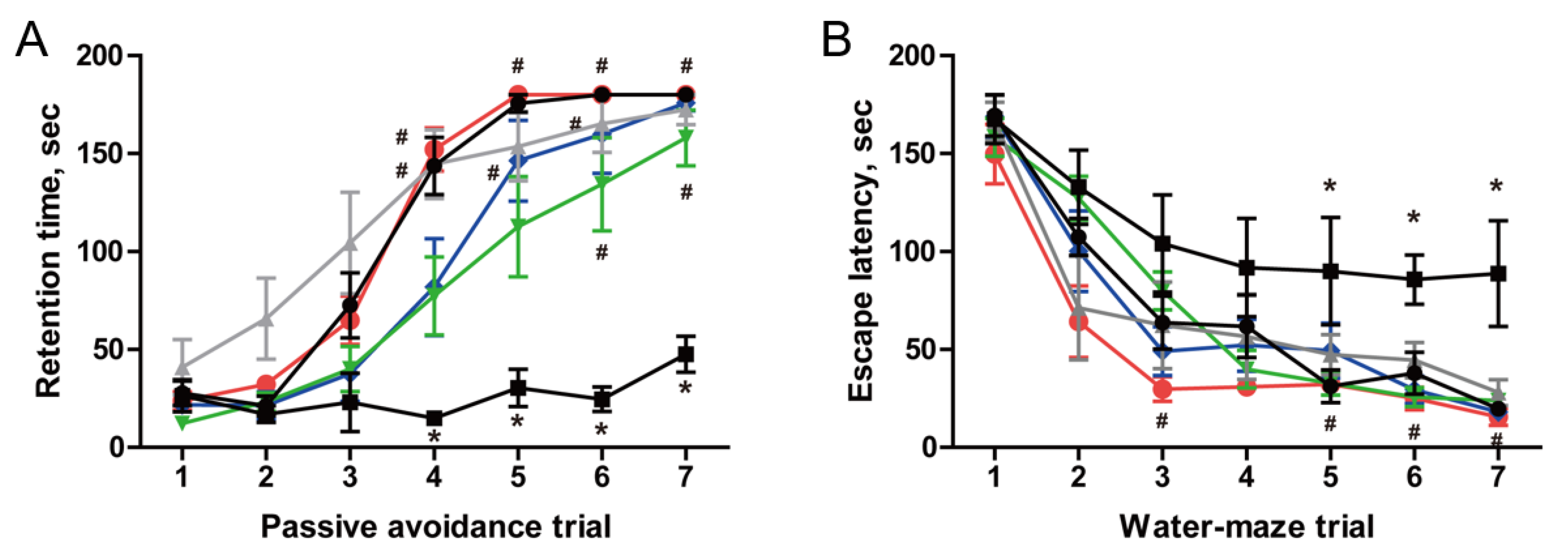
3.5. Restoration of Cognitive Function in AF64A-Induced Memory Deficit Animals
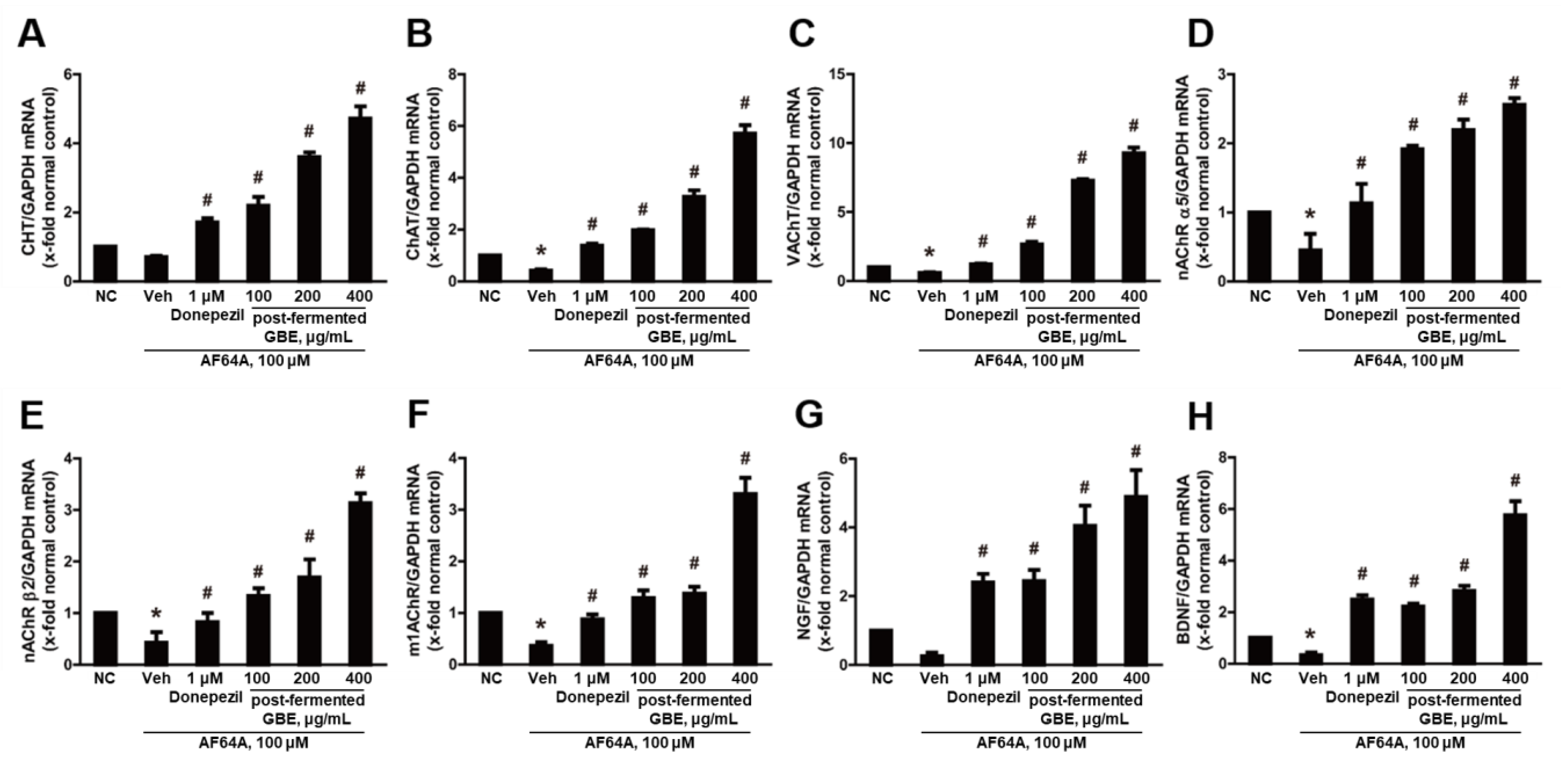
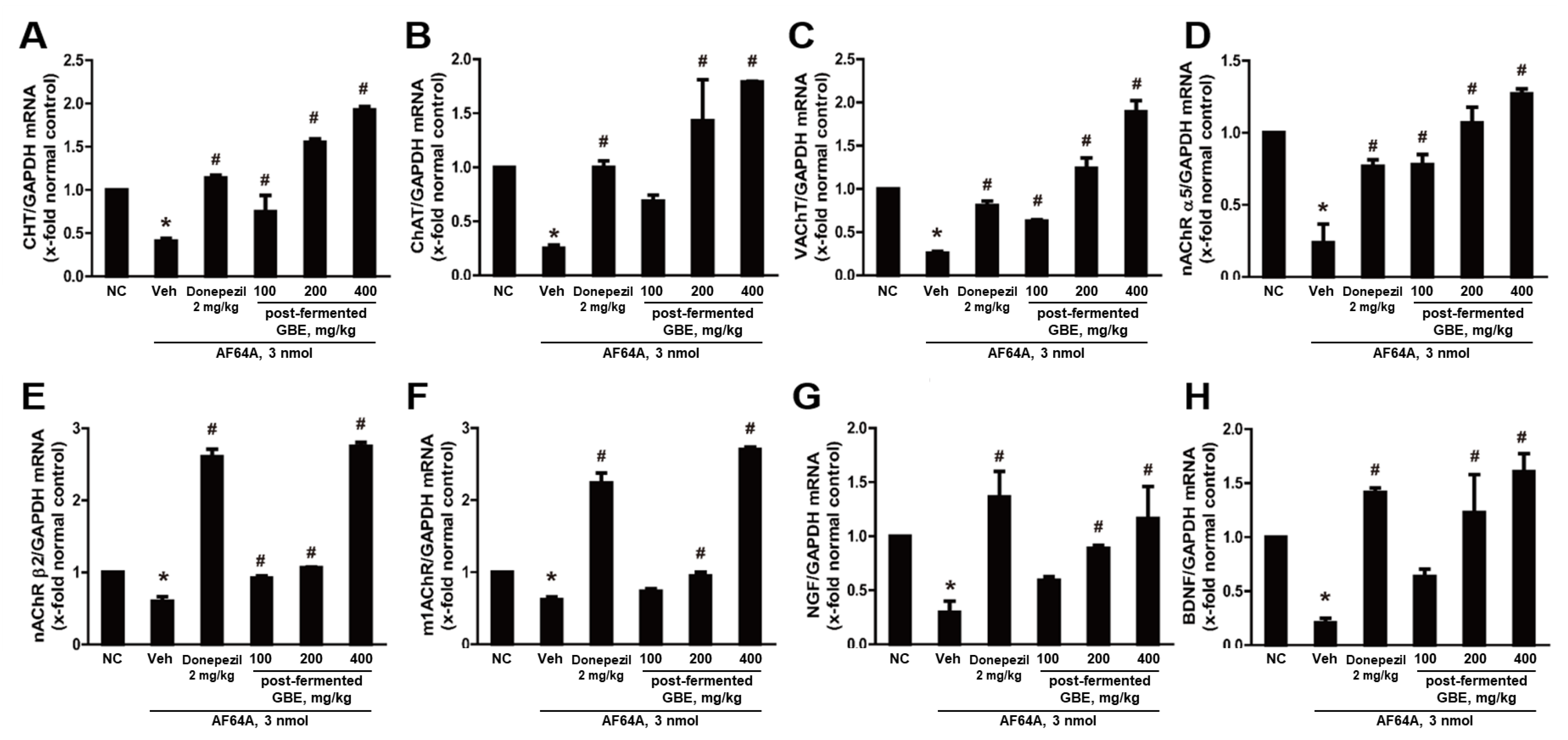
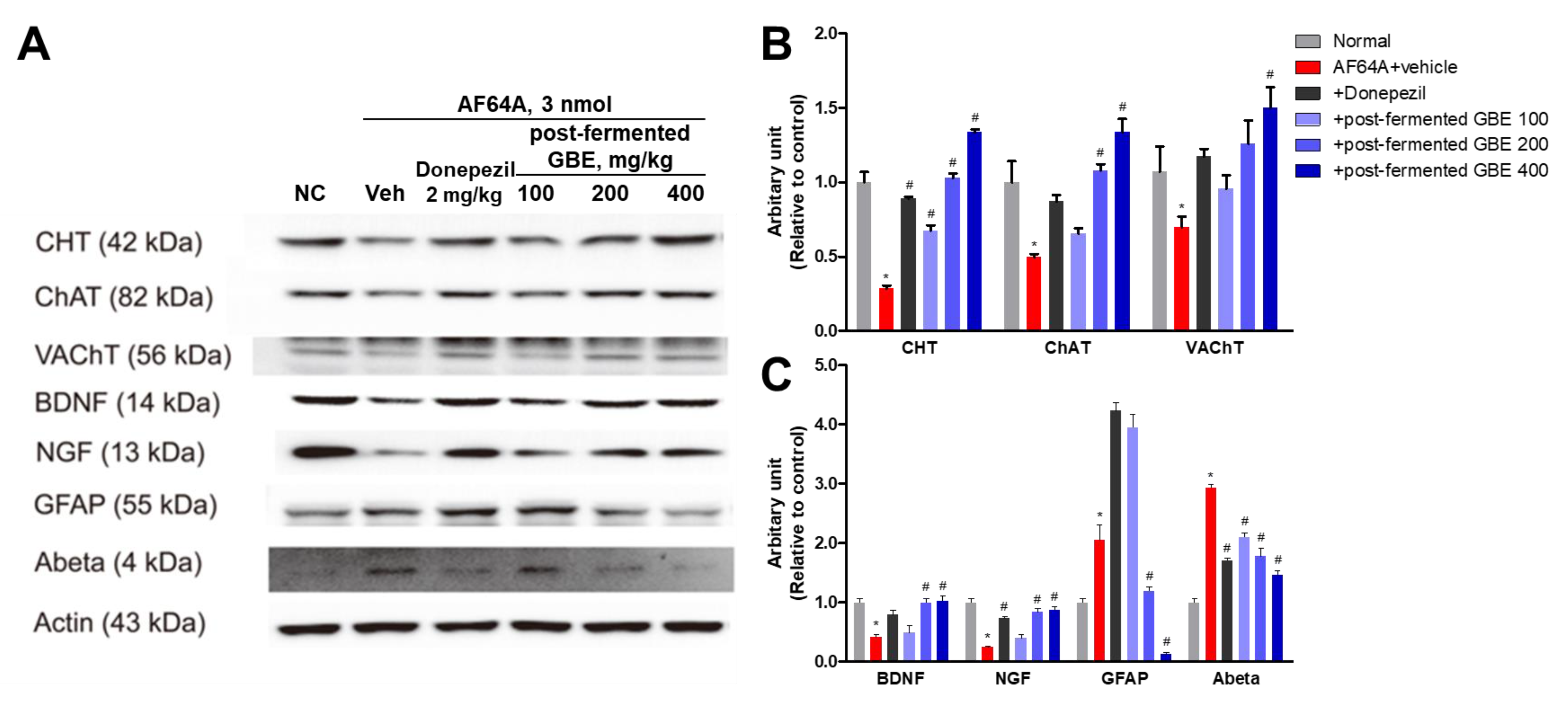
3.6. Cholinergic Pathway Modulation by Post-Fermented GBE in AF64A-Induced Brain Damage in Mice
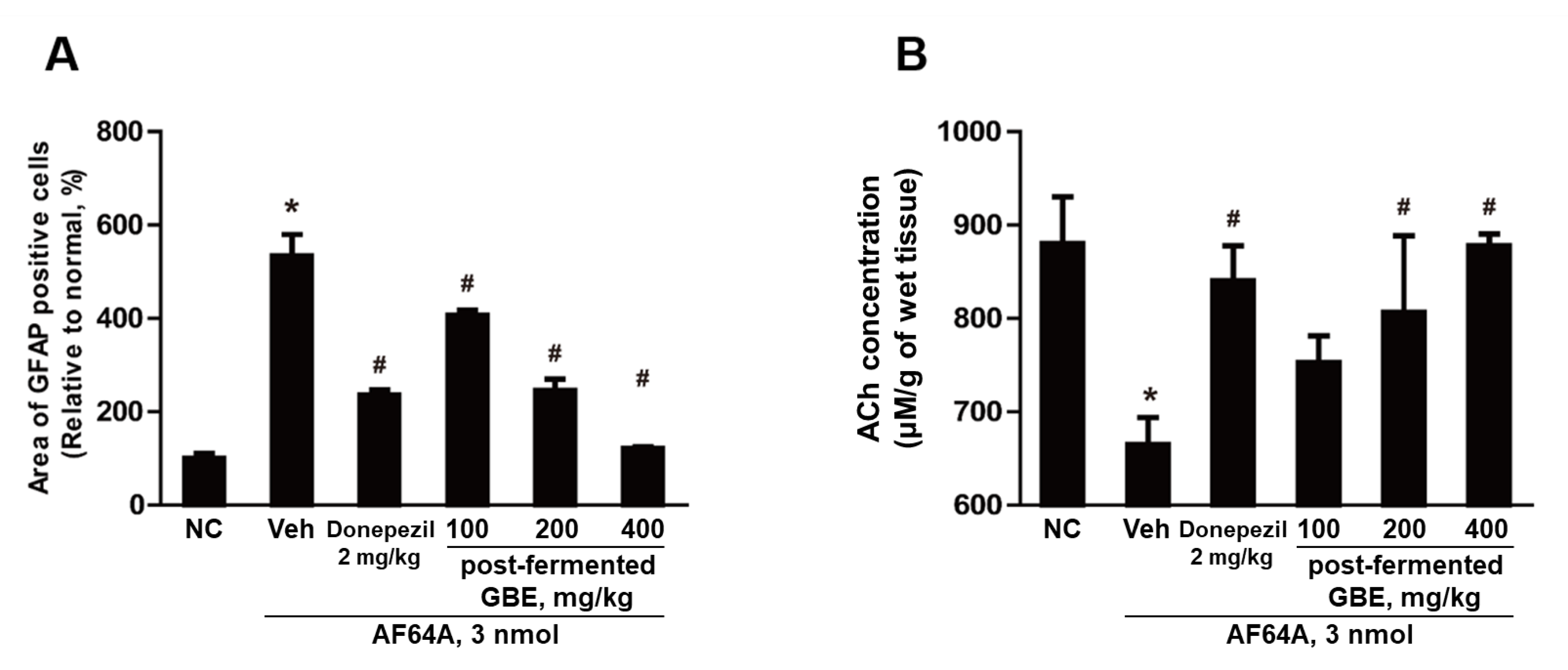
3.7. GFAP Inactivation by Post-Fermented GBE in the Context of AF64A-Induced Brain Damage in Mice
3.8. ACh Concentration Improvement in AF64A-Induced Brain Damage through Post-Fermented GBE Administration
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardo-Moreno, T.; Gonzalez-Acedo, A.; Rivas-Dominguez, A.; Garcia-Morales, V.; Garcia-Cozar, F.J.; Ramos-Rodriguez, J.J.; Melguizo-Rodriguez, L. Therapeutic approach to Alzheimer’s disease: Current treatments and new perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An english translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s disease: Mechanisms and therapeutic strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef]
- Pinheiro, L.; Faustino, C. Therapeutic strategies targeting amyloid-beta in Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 418–452. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement. 2021, 7, e12179. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.K.; Chao, S.P.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Morales, V.; Gonzalez-Acedo, A.; Melguizo-Rodriguez, L.; Pardo-Moreno, T.; Costela-Ruiz, V.J.; Montiel-Troya, M.; Ramos-Rodriguez, J.J. Current understanding of the physiopathology, diagnosis and therapeutic approach to Alzheimer’s disease. Biomedicines 2021, 9, 1910. [Google Scholar] [CrossRef]
- Dou, K.X.; Tan, M.S.; Tan, C.C.; Cao, X.P.; Hou, X.H.; Guo, Q.H.; Tan, L.; Mok, V.; Yu, J.T. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: A network meta-analysis of 41 randomized controlled trials. Alzheimers Res. Ther. 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Choi, E.K.; Cho, T.H.; Joo, S.S.; Kim, Y.B. Human neural stem cells encoding ChAT gene restore cognitive function via acetylcholine synthesis, Aβ elimination, and neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the treatment of Alzheimer’s disease: Challenges and opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef]
- Thapa, A.; Carroll, N.J. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1583. [Google Scholar] [CrossRef] [Green Version]
- Carregosa, D.; Carecho, R.; Figueira, I.; Claudia, N.S. Low-molecular-weight metabolites from polyphenols as effectors for attenuating neuroinflammation. J. Agric. Food Chem. 2020, 68, 1790–1807. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.T.T.; Samarat, K.; Takamura, Y.; Azo-Oussou, A.F.; Nakazono, Y.; Vestergaard, M.C. Polyphenols modulate Alzheimer’s amyloid beta aggregation in a structure-dependent manner. Nutrients 2019, 11, 756. [Google Scholar] [CrossRef] [Green Version]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Cha, Y.; Lee, S.H.; Jang, S.K.; Guo, H.; Ban, Y.H.; Park, D.; Jang, G.Y.; Yeon, S.; Lee, J.Y.; Choi, E.K.; et al. A silk peptide fraction restores cognitive function in AF64A-induced Alzheimer disease model rats by increasing expression of choline acetyltransferase gene. Toxicol. Appl. Pharmacol. 2017, 314, 48–54. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V.; Rodriguez-Arellano, J.J.; Zorec, R. Astroglia in Alzheimer’s disease. Adv. Exp. Med. Biol. 2019, 1175, 273–324. [Google Scholar] [PubMed]
- Fan, Q.I.; Hanin, I. Effects of AF64A on gene expression of choline acetyltransferase (ChAT) in the septo-hippocampal pathway and striatum in vivo. Neurochem. Res. 1999, 24, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rapaka, D.; Adiukwu, P.C.; Bitra, V.R. Experimentally induced animal models for cognitive dysfunction and Alzheimer’s disease. MethodsX 2022, 9, 101933. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Kumar, H.; Cho, D.Y.; Yun, Y.S.; Choi, D.K. Toxin-induced experimental models of learning and memory impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.Y.; Bae, M.G.; Choi, Y.H. Stereoselective and simultaneous analysis of ginsenosides from ginseng berry extract in rat plasma by UPLC-MS/MS: Application to a pharmacokinetic study of ginseng berry extract. Molecules 2018, 23, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar] [CrossRef]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Cho, S.Y.; Kim, S.H.; Cho, D.; Kim, S.; Park, C.W.; Shimizu, T.; Cho, J.Y.; Seo, D.B.; Shin, S.S. Effects of Korean ginseng berry on skin antipigmentation and antiaging via FoxO3a activation. J. Ginseng Res. 2017, 41, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng berry aqueous extract prevents scopolamine-induced memory impairment in mice. Exp. Ther. Med. 2019, 18, 4388–4396. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, C.W.; Lee, S.J.; Park, H.R.; Kim, S.H.; Son, S.U.; Park, J.; Shin, K.S. Anti-cancer effects of Panax ginseng berry polysaccharides via activation of immune-related cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanlier, N.; Gokcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Juraskova, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Jang, S.K.; Ahn, J.W.; Jo, B.R.; Kim, H.S.; Kim, S.J.; Sung, E.A.; Lee, D.I.; Park, H.Y.; Jin, D.H.; Joo, S.S. Double-processed ginseng berry extracts enhance learning and memory in an Aβ42-induced Alzheimer’s mouse model. Korean J. Food Sci. Technol. 2019, 51, 160–168. [Google Scholar]
- Lee, D.I.; Kim, S.T.; Lee, D.H.; Yu, J.M.; Jang, S.K.; Joo, S.S. Ginsenoside-free molecules from steam-dried ginseng berry promote ethanol metabolism: An alternative choice for an alcohol hangover. J. Food Sci. 2014, 79, C1323–C1330. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kim, H.B.; Lee, K.H.; Choi, Y.R.; Kim, H.J.; Shin, I.S.; Gyoung, Y.S.; Joo, S.S. Steam-dried ginseng berry fermented with Lactobacillus plantarum controls the increase of blood glucose and body weight in type 2 obese diabetic db/db mice. J. Agric. Food Chem. 2012, 60, 5438–5445. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.W.; Jang, S.K.; Jo, B.R.; Kim, H.S.; Park, J.Y.; Park, H.Y.; Yoo, Y.M.; Joo, S.S. A therapeutic intervention for Alzheimer’s disease using ginsenoside Rg3: Its role in M2 microglial activation and non-amyloidogenesis. J. Physiol. Pharmacol. 2021, 72, 185–193. [Google Scholar]
- Choi, J.K.; Lee, Y.B.; Lee, K.H.; Im, H.C.; Kim, Y.B.; Choi, E.K.; Joo, S.S.; Jang, S.K.; Han, N.S.; Kim, C.H. Extraction conditions for phenolic compounds with antioxidant activities from white rose petals. J. Appl. Biol. Chem. 2015, 58, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Yoon, E.J.; Lee, M.Y.; Choi, B.I.; Lim, K.J.; Hong, S.Y.; Park, D. Pharmaceutical advantages of genotx-407, a combination of extracts from Scutellaria baicalensis root and Magnolia officinalis bark. Antioxidants 2020, 9, 1111. [Google Scholar] [CrossRef]
- Park, D.; Jeon, J.H.; Kwon, S.C.; Shin, S.; Jang, J.Y.; Jeong, H.S.; Lee, D.I.; Kim, Y.B.; Joo, S.S. Antioxidative activities of white rose flower extract and pharmaceutical advantages of its hexane fraction via free radical scavenging effects. Biochem.Cell Biol. 2009, 87, 943–952. [Google Scholar] [CrossRef]
- Yoon, E.J.; Choi, Y.; Park, D. Effects of AF64A induction on the cholinergic and amyloidogenic pathways in human neural stem cells and rat brain for Alzheimer’s disease models. Brain Digit. Learn. 2022, 12, 607–620. [Google Scholar]
- Yon, J.M.; Kim, Y.B.; Park, D. The ethanol fraction of white rose petal extract abrogates excitotoxicity-induced neuronal damage in vivo and in vitro through inhibition of oxidative stress and proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, Y.J.; Kim, W.; Kim, D.O.; Kim, B.Y.; Lee, H.; Baik, M.Y. Change of ginsenoside profiles in processed ginseng by drying, steaming, and puffing. J. Microbiol. Biotechnol. 2019, 28, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Hyun, S.H.; Bhilare, K.D.; In, G.; Park, C.K.; Kim, J.H. Effects of Panax ginseng and ginsenosides on oxidative stress and cardiovascular diseases: Pharmacological and therapeutic roles. J. Ginseng Res. 2022, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kim, H.J.; Jang, S.K.; Lee, D.I.; Joo, S.S. Establishment of optimal fermentation conditions for steam-dried ginseng berry via friendly bacteria and its antioxidant activities. Korean J. Food Sci. Technol. 2013, 45, 77–83. [Google Scholar] [CrossRef]
- Kim, M.S.; Yu, J.M.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Jang, S.K.; Choi, Y.W.; Lee, D.I.; Joo, S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol. Pharm. Bull. 2014, 37, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A potential neuroprotective agent. Biomed. Res. Int. 2018, 8174345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, K.S.; Choi, Y.O.; Lee, S.M.; Ryu, H.Y.; Kang, S.J.; Yeon, Y.; Kim, Y.R.; Lee, J.G.; Kim, C.J.; Lee, Y.J. Ginsenosides Rg5 and Rk1 enriched cultured wild ginseng root extract bioconversion of Pediococcus pentosaceus HLJG0702: Effect on scopolamine-induced memory dysfunction in mice. Nutrients 2019, 11, 1120. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Lee, H.J.; Joo, S.S.; Bae, D.K.; Yang, G.; Yang, Y.H.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp. Neurol. 2012, 234, 521–526. [Google Scholar] [CrossRef]
- Amalia, L. Glial fibrillary acidic protein (GFAP): Neuroinflammation biomarker in acute ischemic stroke. J. Inflamm. Res. 2021, 14, 7501. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, J.; Lier, J.; Franke, H.; Hammer, N.; Matschke, J.; Trautz, F.; Tse, R.; Ondruschka, B. GFAP positivity in neurons following traumatic brain injuries. Int. J. Legal Med. 2021, 135, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Balzamino, B.O.; Squitti, R.; Varano, M.; Calissano, P.; Micera, A. Nerve growth factor-based therapy in Alzheimer’s disease and age-related macular degeneration. Front. Neurosci. 2021, 15, 735928. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Yoon, E.J.; Choi, Y.; Park, D. Improvement of cognitive function in ovariectomized rats by human neural stem cells overexpressing choline acetyltransferase via secretion of NGF and BDNF. Int. J. Mol. Sci. 2022, 23, 5560. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, T.K.; Kim, Y.H.; Lee, J.; Moon, J.M.; Park, Y.S.; Sung, C.M. Pharmacokinetics of ginsenoside Rb1, Rg3, Rk1, Rg5, F2, and Compound K from red ginseng extract in healthy Korean volunteers. Evid. Based Complement. Alternat. Med. 2022, 2022, 8427519. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, J.; Yan, K.; Guo, J. Ginsenoside Rk1 protects human melanocytes from H2O2-induced oxidative injury via regulation of the PI3K/AKT/Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 24, 821. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Zhao, W.; Gao, S.; Cui, Z. Antidepressant-like effects of ginsenoside Rg5 in mice: Involving of hippocampus BDNF signaling pathway. Neurosci. Lett. 2017, 645, 97–105. [Google Scholar] [CrossRef]
- You, Z.; Yao, Q.; Shen, J.; Gu, Z.; Xu, H.; Wu, Z.; Chen, C.; Li, L. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J. Nat. Med. 2017, 71, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mok, H.; Yeo, W.S.; Ahn, J.H.; Choi, Y.K. Role of ginseng in the neurovascular unit of neuroinflammatory diseases focused on the blood-brain barrier. J. Ginseng Res. 2021, 45, 599–609. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, E.-J.; Ahn, J.-W.; Kim, H.-S.; Choi, Y.; Jeong, J.; Joo, S.-S.; Park, D. Improvement of Cognitive Function by Fermented Panax ginseng C.A. Meyer Berries Extracts in an AF64A-Induced Memory Deficit Model. Nutrients 2023, 15, 3389. https://doi.org/10.3390/nu15153389
Yoon E-J, Ahn J-W, Kim H-S, Choi Y, Jeong J, Joo S-S, Park D. Improvement of Cognitive Function by Fermented Panax ginseng C.A. Meyer Berries Extracts in an AF64A-Induced Memory Deficit Model. Nutrients. 2023; 15(15):3389. https://doi.org/10.3390/nu15153389
Chicago/Turabian StyleYoon, Eun-Jung, Jeong-Won Ahn, Hyun-Soo Kim, Yunseo Choi, Jiwon Jeong, Seong-Soo Joo, and Dongsun Park. 2023. "Improvement of Cognitive Function by Fermented Panax ginseng C.A. Meyer Berries Extracts in an AF64A-Induced Memory Deficit Model" Nutrients 15, no. 15: 3389. https://doi.org/10.3390/nu15153389





