The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Measurement of Blood Lead
2.3. Examination of BMD
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. GAMs Analysis
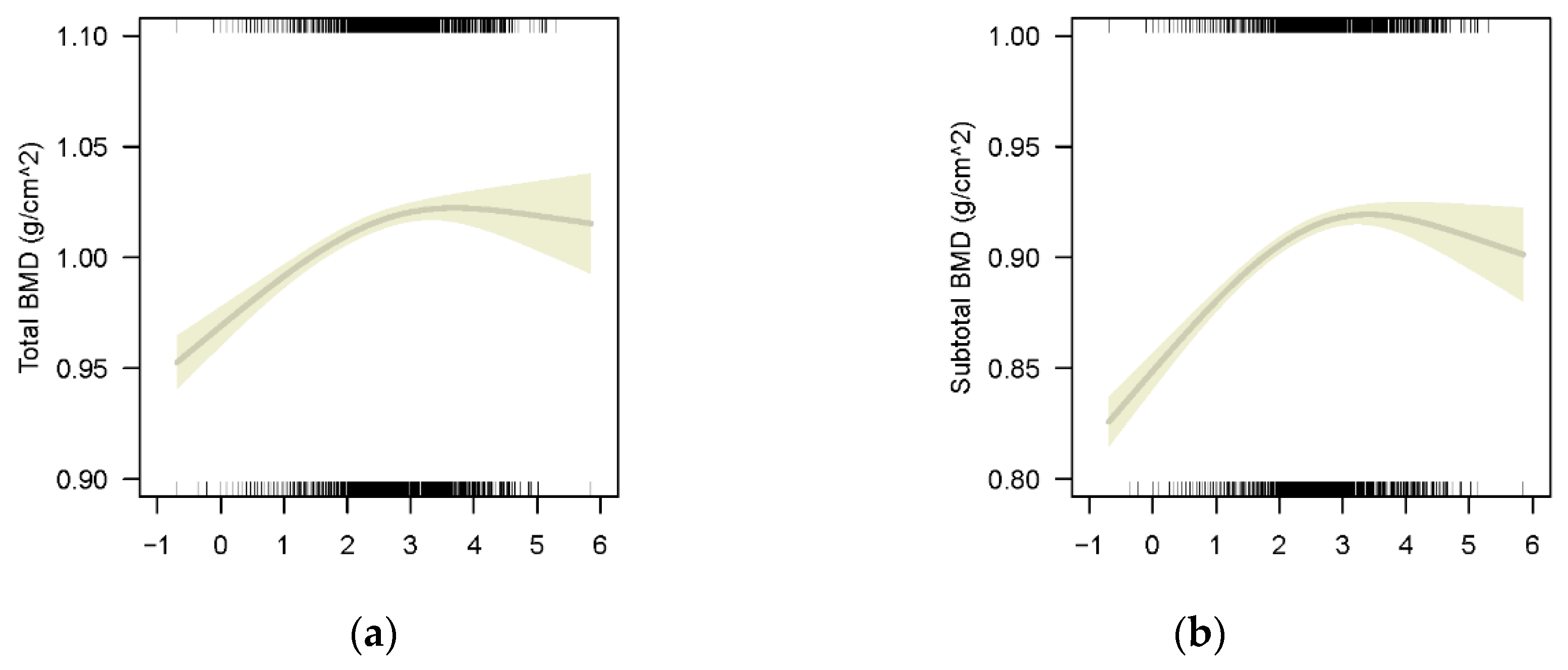
3.2.1. The Association between BLLs and BMD in Whole Participants Population
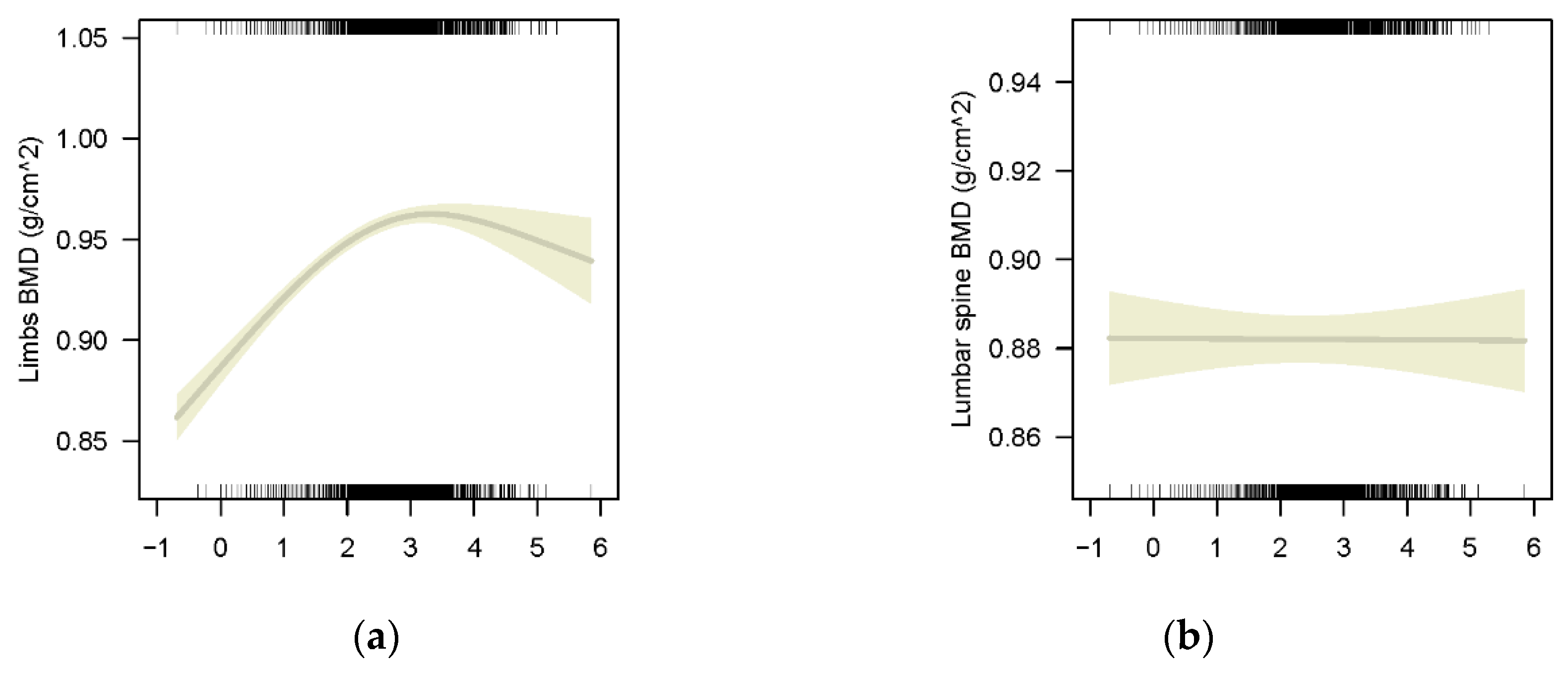
3.2.2. The Association between BLLs and BMD of Different Bony Sites
3.2.3. The Association between BLLs and BMD of Different Sex
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sioen, I.; Michels, N.; Polfliet, C.; De Smet, S.; D’Haese, S.; Roggen, I.; Deschepper, J.; Goemaere, S.; Valtuena, J.; De Henauw, S. The influence of dairy consumption, sedentary behaviour and physical activity on bone mass in Flemish children: A cross-sectional study. BMC Public Health 2015, 15, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappe, J.M.; Watson, P.; Gilsanz, V.; Hangartner, T.; Kalkwarf, H.J.; Oberfield, S.; Shepherd, J.; Winer, K.K.; Zemel, B. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J. Bone Miner. Res. 2015, 30, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masztalerz-Kozubek, D.; Zielinska-Pukos, M.A.; Hamulka, J. Maternal Diet, Nutritional Status, and Birth-Related Factors Influencing Offspring’s Bone Mineral Density: A Narrative Review of Observational, Cohort, and Randomized Controlled Trials. Nutrients 2021, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.W.; Xu, F.H.; Davies, K.M.; Heaney, R.; Recker, R.R. Differences in bone mineral density, bone mineral content, and bone areal size in fracturing and non-fracturing women, and their interrelationships at the spine and hip. J. Bone Miner. Metab. 2002, 20, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.; Yeter, D.; Aschner, M.; Wheeler, D.C. Estimated IQ points and lifetime earnings lost to early childhood blood lead levels in the United States. Sci. Total Environ. 2021, 778, 146307. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Kasanuma, Y.; Iwai-Shimada, M.; Sakamoto, M.; Murata, K.; Satoh, H. Prenatal and postnatal lead exposures and intellectual development among 12-year-old Japanese children. Environ. Res. 2020, 189, 109844. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of Childhood Blood Lead Levels With Cognitive Function and Socioeconomic Status at Age 38 Years and With IQ Change and Socioeconomic Mobility Between Childhood and Adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef]
- Wang, W.J.; Wu, C.C.; Jung, W.T.; Lin, C.Y. The associations among lead exposure, bone mineral density, and FRAX score: NHANES, 2013 to 2014. Bone 2019, 128, 115045. [Google Scholar] [CrossRef]
- Lu, J.; Lan, J.; Li, X.; Zhu, Z. Blood lead and cadmium levels are negatively associated with bone mineral density in young female adults. Arch Public Health 2021, 79, 116. [Google Scholar] [CrossRef]
- Wei, M.H.; Cui, Y.; Zhou, H.L.; Song, W.J.; Di, D.S.; Zhang, R.Y.; Huang, Q.; Liu, J.A.; Wang, Q. Associations of multiple metals with bone mineral density: A population-based study in US adults. Chemosphere 2021, 282, 131150. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R.L.; Huang, Y.L.; Chen, W.J.; Chen, H.H.; Shiue, H.S.; Lin, Y.C.; Hsueh, Y.M. Associations between Plasma Folate and Vitamin B12, Blood Lead, and Bone Mineral Density among Adults and Elderly Who Received a Health Examination. Nutrients 2022, 14, 911. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.R.; Rosier, R.N.; Novotny, L.; Puzas, J.E. The association between environmental lead exposure and bone density in children. Environ. Health Perspect. 2004, 112, 1200–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. National Health and Nutrition Examination Survey. 2017. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 2 January 2022).
- CDC. Cadmium, Lead, Manganese, Mercury, and Selenium Lab Procedure Manual. 2018. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/PBCD-J-PBY-J-R-MET-508.pdf (accessed on 2 January 2022).
- CDC. Body Composition Procedures Manual. 2013. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Body_Composition_DXA.pdf (accessed on 2 January 2022).
- Zuscik, M.J.; Pateder, D.B.; Edward Puzas, J.; Schwarz, E.M.; Rosier, R.N.; O’Keefe, R.J. Lead alters parathyroid hormone-related peptide and transforming growth factor-β1 effects and AP-1 and NF-κKB signaling in chondrocytes. J. Orthop. Res. 2002, 20, 811–818. [Google Scholar] [CrossRef]
- Zanchetta, J.R.; Bogado, C.E.; Ferretti, J.L.; Wang, O.; Wilson, M.G.; Sato, M.; Gaich, G.A.; Dalsky, G.P.; Myers, S.L. Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J. Bone Miner. Res. 2003, 18, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Inskip, M.J.; Franklin, C.A.; Subramanian, K.S.; Blenkinsop, J.; Wandelmaier, F. Sampling of cortical and trabecular bone for lead analysis: Method development in a study of lead mobilization during pregnancy. Neurotoxicology 1992, 13, 825–834. [Google Scholar] [PubMed]
- Beier, E.E.; Holz, J.D.; Sheu, T.J.; Puzas, J.E. Elevated Lifetime Lead Exposure Impedes Osteoclast Activity and Produces an Increase in Bone Mass in Adolescent Mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 149, 277–288. [Google Scholar] [CrossRef]
- Potula, V.; Kleinbaum, D.; Kaye, W. Lead exposure and spine bone mineral density. J. Occup. Environ. Med. 2006, 48, 556–564. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, H.H.; Kim, T.H.; Lee, B.R. Relationship between Heavy Metal Exposure and Bone Mineral Density in Korean Adult. J. Bone Metab. 2016, 23, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Theppeang, K.; Glass, T.A.; Bandeen-Roche, K.; Todd, A.C.; Rohde, C.A.; Links, J.M.; Schwartz, B.S. Associations of bone mineral density and lead levels in blood, tibia, and patella in urban-dwelling women. Environ. Health Perspect. 2008, 116, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Junaid, M.; Chowdhuri, D.K.; Narayan, R.; Shanker, R.; Saxena, D.K. Lead-induced changes in ovarian follicular development and maturation in mice. J. Toxicol. Environ. Health 1997, 50, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | Total (n = 13,951) | Male (n = 7647) | Female (n= 6304) | p |
|---|---|---|---|---|
| Age (years, mean ± SD) | 13.53 ± 3.42 | 13.56 ± 3.40 | 13.49 ± 3.45 | 0.229 |
| Race (%) | ||||
| Mexican American | 29.0 | 29.0 | 29.0 | 0.214 |
| Other Hispanic | 6.2 | 6.0 | 6.4 | |
| Non-Hispanic White | 26.4 | 26.2 | 26.7 | |
| Non-Hispanic Black | 29.5 | 30.2 | 28.6 | |
| Other Race | 8.9 | 8.6 | 9.3 | |
| Height (cm, mean ± SD) | 157.6 ± 15.1 | 160.5 ± 16.7 | 154.0 ± 12.1 | <0.001 |
| Weight (kg, mean ± SD) | 57.1 ± 20.5 | 58.8 ± 21.7 | 55.1 ± 18.7 | <0.001 |
| Blood Lab test (μg/L, GM 95% CI) | ||||
| Lead | 8.49 (8.39, 8.59) | 9.97 (9.81, 10.13) | 6.99 (6.87, 7.10) | <0.001 |
| Cadmium | 0.18 (0.18, 0.19) | 0.19 (0.19, 0.19) | 0.18 (0.18, 0.18) | <0.001 |
| BMD (g/cm2, mean ± SD) | ||||
| Total | 0.991 ± 0.161 | 1.002 ± 0.170 | 0.977 ± 0.148 | <0.001 |
| Subtotal | 0.886 ± 0.162 | 0.904 ± 0.178 | 0.863 ± 0.137 | <0.001 |
| Lumbar Spine | 0.889 ± 0.192 | 0.865 ± 0.195 | 0.917 ± 0.183 | <0.001 |
| Limbs # | 0.924 ± 0.164 | 0.948 ± 0.180 | 0.894± 0.136 | <0.001 |
| Ratio of family income to poverty (mean ± SD) | 2.0 ± 1.5 | 2.0 ± 1.5 | 2.0 ± 1.5 | 0.484 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Xie, Y.; Wang, L.; Huang, G.; Cheng, Y.; Hou, D.; Liu, W.; Zhang, T.; Liu, J. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients 2022, 14, 1523. https://doi.org/10.3390/nu14071523
Li T, Xie Y, Wang L, Huang G, Cheng Y, Hou D, Liu W, Zhang T, Liu J. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients. 2022; 14(7):1523. https://doi.org/10.3390/nu14071523
Chicago/Turabian StyleLi, Tao, Yixuan Xie, Liang Wang, Guimin Huang, Yijing Cheng, Dongqing Hou, Wenqian Liu, Tong Zhang, and Junting Liu. 2022. "The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018" Nutrients 14, no. 7: 1523. https://doi.org/10.3390/nu14071523







