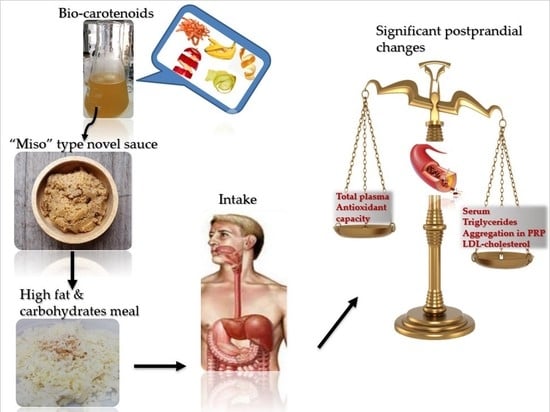
The Acute Effect of a Novel Miso-Type Sauce, Enhanced with a Carotenoid-Rich Extract from Fruit By-Products, on Postprandial Biomarkers of Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Treatments
2.4. In Vitro Functional Sauce Preliminary Determinations
2.5. Study Design
2.6. Blood Sampling and Analyses
2.7. Data Analysis
3. Results
3.1. Functional Sauce In Vitro Measurements
3.2. Baseline Characteristics
3.3. Postprandial Plasma Total Antioxidant Capacity (TAC)
3.4. Postprandial Serum Lipids, Glucose and Uric Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Rizou, M.; Aldawoud, T.M.S.; Ucak, I.; Rowan, N.J. Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci. Technol. 2021, 110, 193–200. [Google Scholar] [CrossRef]
- Helkar, P.B.; Sahoo, A. Review: Food Industry By-Products used as a Functional Food Ingredients. Int. J. Waste Resour. 2016, 6, 1000248. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour. Bioprocess. 2022, 9, 14. [Google Scholar] [CrossRef]
- Koutelidakis, A.; Dimou, C. The Effects of Functional Food and Bioactive Compounds on Biomarkers of Cardiovascular Diseases. In Functional Foods Text Book, 1st ed.; Martirosyan, D., Ed.; Functional Food Center: Dallas, TX, USA, 2017; pp. 89–117. [Google Scholar]
- Thilavech, T.; Adisakwattana, S.; Channuwong, P.; Radarit, K.; Jantarapat, K.; Ngewlai, K.; Sonprasan, N.; Chusak, C. Clitoria ternatea Flower Extract Attenuates Postprandial Lipemia and Increases Plasma Antioxidant Status Responses to a High-Fat Meal Challenge in Overweight and Obese Participants. Biology 2021, 10, 975. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, X.; Chen, K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2020, 117, 3–14. [Google Scholar] [CrossRef]
- Akter, B.; Rabeta, M.S. Synbiotic and antioxidant activity of fruit by-products and their effect on human health. Food Res. 2020, 5, 24–35. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity & rsquo’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Grasso, S.; Purewal, S.S.; Kaur, M.; Siroha, A.K.; Kumar, K.; Kumar, V.; Kumar, M. Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods 2020, 10, 70. [Google Scholar] [CrossRef]
- Santos, R.; Mansidão, A.; Mota, M.; Raymundo, A.; Prista, C. Development and physicochemical characterization of a new grass pea (Lathyrus sativus L.) miso. J. Sci. Food Agric. 2021, 101, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Pedrol, N.; Ramos, P. Protein Content Quantification by Bradford Method. In Handbook of Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2001; pp. 283–295. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Argyri, E.; Piromalis, S.; Koutelidakis, A.; Kafetzopoulos, D.; Petsas, A.S.; Skalkos, D.; Nasopoulou, C.; Dimou, C.; Karantonis, H.C. Applied Sciences Olive Paste-Enriched Cookies Exert Increased Antioxidant Activities. Appl. Sci. 2021, 11, 5515. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulou, S.; Fragopoulou, E.; Karantonis, H.C.; Mitsou, E.; Sitara, M.; Rementzis, J.; Mourelatos, A.; Ginis, A.; Phenekos, C. Effect of Traditional Greek Mediterranean Meals on Platelet Aggregation in Normal Subjects and in Patients with Type 2 Diabetes Mellitus. J. Med. Food 2006, 9, 356–362. [Google Scholar] [CrossRef]
- Chan, E.; Wong, S.; Kezuka, M.; Oshiro, N.; Chan, H. Natto and miso: An overview on their preparation, bioactive components and health-promoting effects. Food Res. 2021, 5, 446–452. [Google Scholar] [CrossRef]
- Kasote, D.; Tiozon, R.N.J.; Sartagoda, K.J.D.; Itagi, H.; Roy, P.; Kohli, A.; Regina, A.; Sreenivasulu, N. Food Processing Technologies to Develop Functional Foods with Enriched Bioactive Phenolic Compounds in Cereals. Front. Plant Sci. 2021, 12, 771276. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Tlais, A.; Fiorino, M.; Polo, A. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, O.; Argyri, K.; Loukas, T.; Magkoutis, A.; Biagki, T.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Postprandial Bioactivity of a Spread Cheese Enriched with Mountain Tea and Orange Peel Extract in Plasma Oxidative Stress Status, Serum Lipids and Glucose Levels: An Interventional Study in Healthy Adults. Biomolecules 2021, 11, 1241. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Thilavech, T.; Adisakwattana, S. Postprandial Effect of Yogurt Enriched with Anthocyanins from Riceberry Rice on Glycemic Response and Antioxidant Capacity in Healthy Adults. Nutrients 2020, 12, 2930. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Selby-Pham, S.; Colton, B.-L.; Bennett, L.; Williamson, G.; Dordevic, A.L. Does timing of phytonutrient intake influence the suppression of postprandial oxidative stress? A systematic literature review. Redox Biol. 2021, 46, 102123. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Ávila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxidative Med. Cell. Longev. 2017, 2017, 8361493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laus, M.N.; Soccio, M.; Alfarano, M.; Pasqualone, A.; Lenucci, M.S.; Di Miceli, G.; Pastore, D. Different effectiveness of two pastas supplemented with either lipophilic or hydrophilic/phenolic antioxidants in affecting serum as evaluated by the novel Antioxidant/Oxidant Balance approach. Food Chem. 2017, 221, 278–288. [Google Scholar] [CrossRef]
- Dimina, L.; Mariotti, F. The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances. Nutrients 2019, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Chakraborti, S.; Nirmal, D.; Dikshit, M. Oxidative Stress in Heart Diseases “Role of INOS in Insulin Resistance and Endothelial Dysfunction. In Oxidative Stress in Heart Diseases; Springer: Singapore, 2019. [Google Scholar]
- Pietro, D.; Tomo, D.D.; Pandolfi, P. Carotenoids in Cardiovascular Disease Prevention. JSM Atheroscler 2016, 1, 1002. [Google Scholar]
- De Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; De La Rosa, L.A.; Geraldi, M.V.; Maróstica, M.R.; Shahidi, F.; Schwember, A.R. Is Chickpea a Potential Substitute for Soybean? Phenolic Bioactives and Potential Health Benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef] [Green Version]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Bozzetto, L.; Costabile, G.; Cipriano, P.; Mangione, A.; Patti, L.; Riccardi, G.; Rivellese, A.A.; et al. Effects of a diet naturally rich in polyphenols on lipid composition of postprandial lipoproteins in high cardiometabolic risk individuals: An ancillary analysis of a randomized controlled trial. Eur. J. Clin. Nutr. 2019, 74, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, X.; Xia, Y.; Xu, J.; Li, Q.; Zhang, C.; Li, M. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol. 2020, 103, 304–320. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salmeán, G.; Ortiz-Vilchis, P.; Vacaseydel, C.M.; Rubio-Gayosso, I.; Meaney, E.; Villarreal, F.; Ramírez-Sánchez, I.; Ceballos, G. Acute effects of an oral supplement of (−)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct. 2014, 5, 521–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzetto, L.; Della Pepa, G.; Vetrani, C.; Rivellese, A.A. Dietary Impact on Postprandial Lipemia. Front. Endocrinol. 2020, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hou, T.; Guo, D.; He, H. Evaluation of hypolipidemic peptide (Val-Phe-Val-Arg-Asn) virtual screened from chickpea peptides by pharmacophore model in high-fat diet-induced obese rat. J. Funct. Foods 2019, 54, 136–145. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jung, E.-S.; Choi, E.-K.; Jeong, D.-Y.; Jo, S.-W.; Jin, J.-H.; Lee, J.-M.; Park, B.-H.; Chae, S.-W. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef]
- Burton-Freeman, B. Postprandial metabolic events and fruit-derived phenolics: A review of the science. Br. J. Nutr. 2010, 104 (Suppl. 3), S1–S14. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Zhang, L.-L.; Li, W.; Zhang, Y.; Zhang, Y.; Liu, F.; Zou, L. Application of metabolomics for revealing the interventional effects of functional foods on metabolic diseases. Food Chem. 2021, 367, 130697. [Google Scholar] [CrossRef]




| Meals’ Nutritional Value | ||
|---|---|---|
| Control | Functional | |
| Energy (kcal) | 932.155 | 932.155 |
| Carbohydrates (g) | 140.945 | 140.945 |
| Fat, total (g) | 29.972 | 29.972 |
| Protein (g) | 29.295 | 29.298 |
| Saturated fat (g) | 17.6935 | 17.6935 |
| Unsaturated fat (g) | 8.6685 | 8.6685 |
| Cholesterol (mg) | 73.6 | 73.6 |
| Dietary fiber, total (g) | 4.6 | 4.6 |
| Sugar, total (g) | 0.845 | 0.845 |
| Total phenolics (mg/g) | 0 | 57.6 |
| Total carotenoids (mg/g) | 0 | 48.16 |
| Total antioxidants (mmol/g) | 0 | 165.18 |
| Variable | |
|---|---|
| General Characteristics | N |
| Volunteers | 14 |
| Men | 6 |
| Women | 8 |
| Smoking | 4 |
| Physical Activity | |
| Low | 2 |
| Medium | 6 |
| High | 6 |
| Mean ± SD | |
| Age (years) | 23.5 ± 2.7 a |
| Anthropometry and Body Composition | |
| Weight (kg) | 71.5 ± 15.3 b |
| Height (cm) | 170.1 ± 8.1 c |
| BMI | 24.5 ± 3.5 d |
| Fat mass (kg) | 20.9 ± 7 |
| Muscle mass (kg) | 53.4 ± 11.1 |
| Body water (kg) | 55 ± 6.8 |
| Bone mass (kg) | 2.8 ± 0.3 |
| Waist/hip circumference ratio | 0.7 ± 0.17 |
| Control | Functional | |
|---|---|---|
| Total cholesterol at baseline (mg/dL) | 170.53 ± 34.5 | 145.15 ± 33.2 |
| Δ 30 min | −0.61 ± 0.43 | 10.53 ± 7.45 |
| Δ 1.5 h | −10.28 ± 7.27 | −10.3 ± 7.28 |
| Δ 3 h | 3.16 ± 2.23 | 2.07 ± 2.17 |
| iAUC | 408.9 ± 180.78 | 446.16 ± 81.15 |
| Glucose at baseline (mg/dL) | 87.9 ± 10.77 | 91.07 ± 19.2 |
| Δ 30 min | 13.18 ± 9.32 | 13.38 ± 9.46 |
| Δ 1.5 h | −18.18 ± 12.85 | −21.37 ± 15.11 |
| Δ 3 h | 0.9 ± 0.64 | −3.91 ± 2.76 |
| iAUC | 262.13 ± 37.7 | 246.7 ± 66.59 |
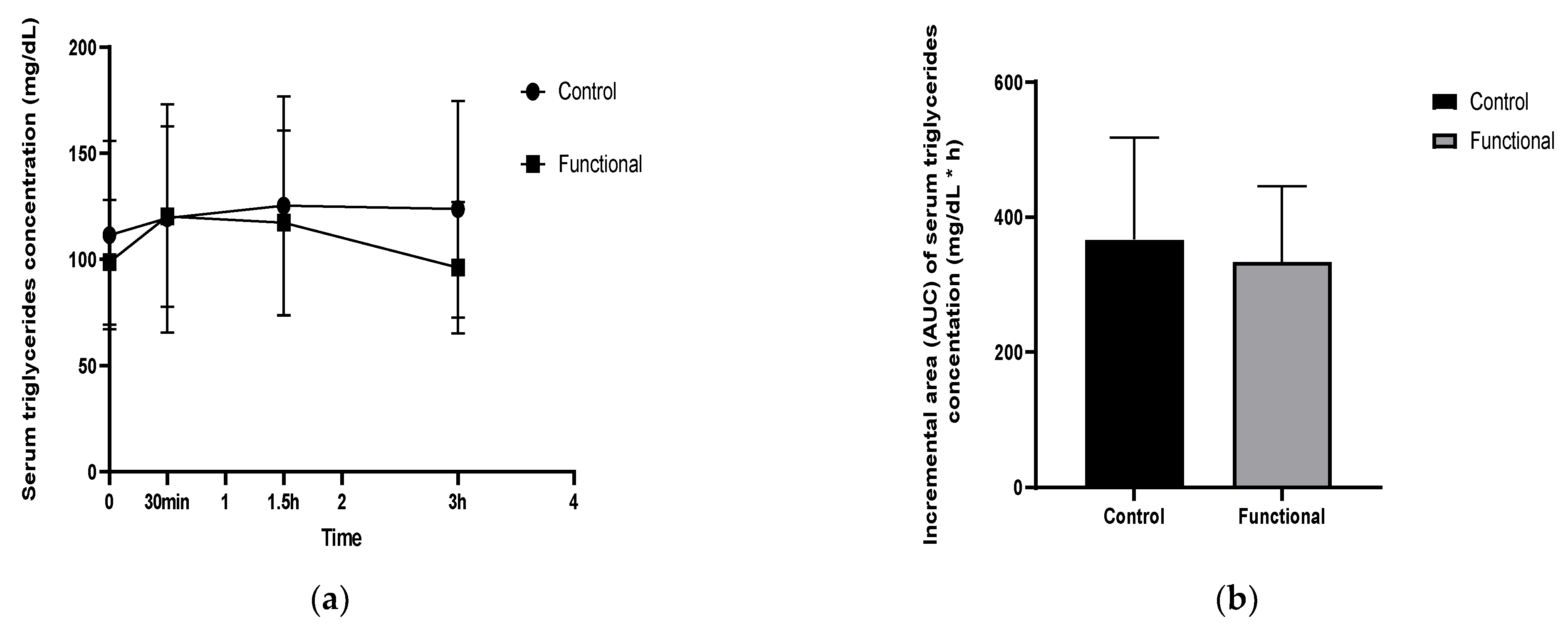
| Triglycerides at baseline (mg/dL) | 111.61 ± 44.48 | 98.76 ± 29.4 |
| Δ 30 min | 7.84 ± 5.54 | 21.61 ± 15.28 |
| Δ 1.5 h | 5.92 ± 4.18 | −3.07 ± 2.17 |
| Δ 3 h | −12.5 ± 8.86 | −20.9 ± 10.55 |
| iAUC | 412.61 ± 152.08 | 298.27 ± 112.07 |
| HDL-cholesterol at baseline (mg/dL) | 98.12 ± 28.78 | 98.57 ± 31.36 |
| Δ 30 min | −0.76 ± 0.47 | 21.61 ± 15.28 |
| Δ 1.5 h | 0.13 ± 0.09 | −3.07 ± 2.17 |
| Δ 3 h | −10.37 ± 7.33 | −17.9 ± 10.55 |
| iAUC | 270.61 ± 100,47 | 276.68 ± 97.71 |
| LDL-cholesterol at baseline (mg/dL) | 129.16 ± 24.11 | 145.36 ± 25.91 |
| Δ 30 min | 18.64 ± 13.18 | −12.72 ± 8.99 |
| Δ 1.5 h | −18.68 ± 13.21 | −0.41 ± 0.29 |
| Δ 3 h | 12.73 ± 9 | −9.89 ± 6.22 |
| iAUC | 422.18 ± 39.36 | 432.65 ± 57.76 |
| Uric acid at baseline (mg/dL) | 5.68 ± 1.37 | 5.44 ± 1.84 |
| Δ 30 min | −0.88 ± 0.62 | 0.33 ± 0.23 |
| Δ 1.5 h | 0.19 ± 0.13 | −0.35 ± 0.24 |
| Δ 3 h | −0.33 ± 0.23 | −0.9 ± 0.65 |
| iAUC | 14.74 ± 5.48 | 15.47 ± 4.54 |
| Total plasma antioxidant capacity (TAC) (mmol/L) | 0.93 ± 0.31 | 0.91 ± 0.74 |
| Δ 30 min | 0.23 ± 0.16 | 0.18 ± 0.12 |
| Δ 1.5 h | −0.55 ± 0.32 | −0.27 ± 0.19 |
| Δ 3 h | −0.09 ± 0 | 0.7 ± 0.42 |
| iAUC | 2.06 ± 0.69 | 3.46 ± 1.69 |
| Aggregation in PRP (cm) | 10.01 ± 3.3 | 0.82 ± 4.18 |
| Δ 30 min | 0.05 ± 0.03 | −2.55 ± 1.8 |
| Δ 1.5 h | −1.37 ± 0.97 | 1.82 ± 1.29 |
| Δ 3 h | −0.02 ± 0.06 | −2.7 ± 1.2 |
| iAUC | 29 ± 6.08 | 23.98 ± 7.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagianni, O.; Delli, E.; Vasila, M.-E.; Loukas, T.; Magkoutis, A.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. The Acute Effect of a Novel Miso-Type Sauce, Enhanced with a Carotenoid-Rich Extract from Fruit By-Products, on Postprandial Biomarkers of Oxidative Stress and Inflammation. Nutrients 2022, 14, 1316. https://doi.org/10.3390/nu14061316
Papagianni O, Delli E, Vasila M-E, Loukas T, Magkoutis A, Dimou C, Karantonis HC, Koutelidakis AE. The Acute Effect of a Novel Miso-Type Sauce, Enhanced with a Carotenoid-Rich Extract from Fruit By-Products, on Postprandial Biomarkers of Oxidative Stress and Inflammation. Nutrients. 2022; 14(6):1316. https://doi.org/10.3390/nu14061316
Chicago/Turabian StylePapagianni, Olga, Eleni Delli, Melina-Eleni Vasila, Thomas Loukas, Athanasios Magkoutis, Charalampia Dimou, Haralampos C. Karantonis, and Antonios E. Koutelidakis. 2022. "The Acute Effect of a Novel Miso-Type Sauce, Enhanced with a Carotenoid-Rich Extract from Fruit By-Products, on Postprandial Biomarkers of Oxidative Stress and Inflammation" Nutrients 14, no. 6: 1316. https://doi.org/10.3390/nu14061316