Plant-Based Diets and Peritoneal Dialysis: A Review
Abstract
:1. Introduction
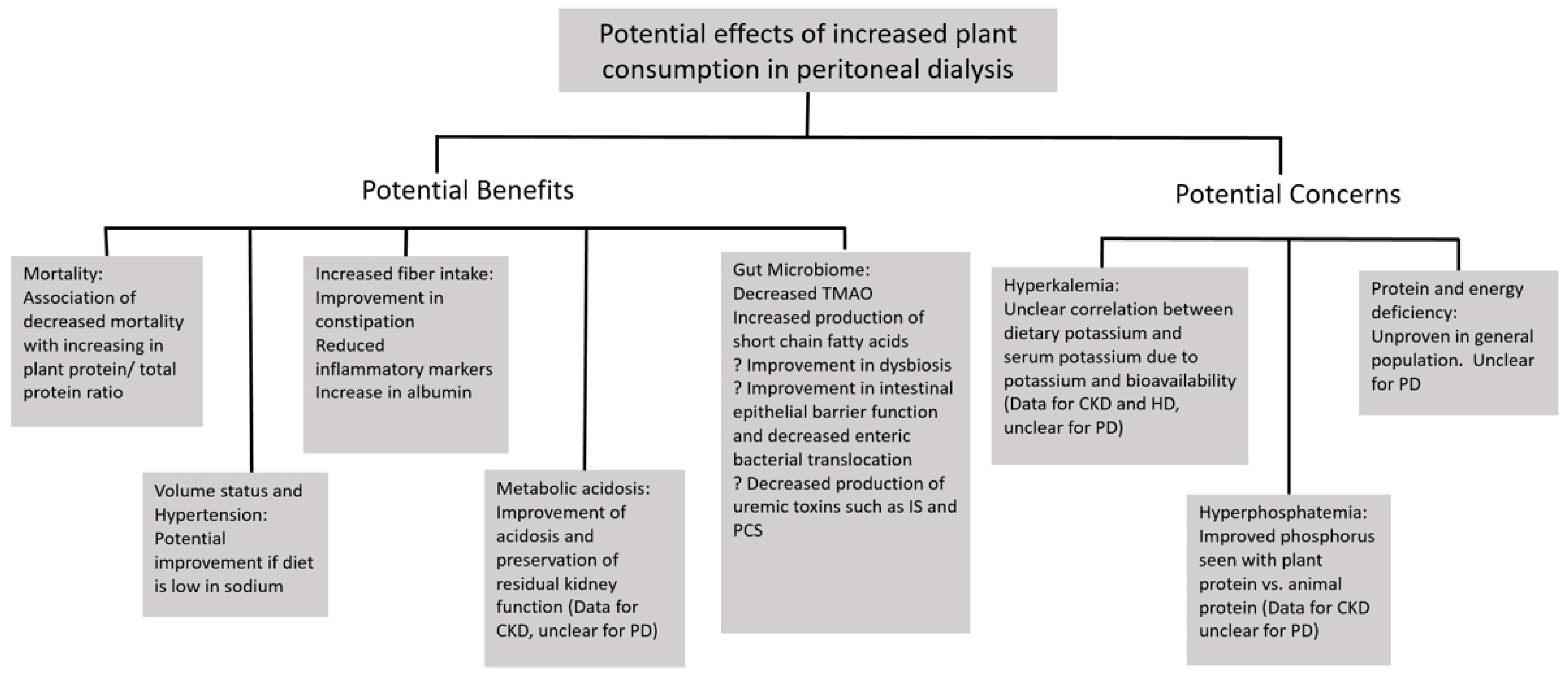
2. Potential Benefits of Plant-Based Diets in the Peritoneal Dialysis Population
2.1. Mortality
2.2. Volume Overload and Sodium Intake
2.3. Constipation/Fiber
2.4. Gut Microbiome
2.5. Hypertension
2.6. Metabolic Acidosis
3. Potential Disadvantages of Plant-Based Diets in the Peritoneal Dialysis Population
3.1. Potassium/Hyperkalemia
3.2. Phosphorus
3.3. Energy and Protein Intake
3.4. Other Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. JGC 2017, 14, 369–374. [Google Scholar] [PubMed]
- Joshi, S.; Ettinger, L.; Liebman, S.E. Plant-Based Diets and Hypertension. Am. J. Lifestyle Med. 2019, 14, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.K.; Fidahusain, M.; Campbell Ii, T.M. Evaluation of an Eight-Week Whole-Food Plant-Based Lifestyle Modification Program. Nutrients 2019, 11, 2068. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588–1596. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. JGC 2017, 14, 342–354. [Google Scholar]
- Patel, H.; Chandra, S.; Alexander, S.; Soble, J.; Williams, K.A. Plant-Based Nutrition: An Essential Component of Cardiovascular Disease Prevention and Management. Curr. Cardiol. Rep. 2017, 19, 104. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Ornish, D.; Scherwitz, L.W.; Billings, J.H.; Gould, K.L.; Merritt, T.A.; Sparler, S.; Armstrong, W.T.; Ports, T.A.; Kirkeeide, R.L.; Hogeboom, C.; et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998, 280, 2001–2007. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-Based Diets and Incident CKD and Kidney Function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682. [Google Scholar] [CrossRef] [Green Version]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Hashmi, S.; Shah, S.; Kalantar-Zadeh, K. Plant-based diets for prevention and management of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 16–21. [Google Scholar] [CrossRef]
- United States Renal Data System: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Cocchi, R.; Degli Esposti, E.; Fabbri, A.; Lucatello, A.; Sturani, A.; Quarello, F.; Boero, R.; Bruno, M.; Dadone, C.; Favazza, A.; et al. Prevalence of hypertension in patients on peritoneal dialysis: Results of an Italian multicentre study. Nephrol. Dial. Transplant. 1999, 14, 1536–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.C.; Johnson, D.W.; Hawley, C.; Boudville, N.; Lim, W.H. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci. Rep. 2018, 8, 3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Htay, H.; Cho, Y.; Pascoe, E.M.; Darssan, D.; Nadeau-Fredette, A.C.; Hawley, C.; Clayton, P.A.; Borlace, M.; Badve, S.V.; Sud, K.; et al. Center Effects and Peritoneal Dialysis Peritonitis Outcomes: Analysis of a National Registry. Am. J. Kidney Dis. 2018, 71, 814–821. [Google Scholar] [CrossRef]
- IPSOS. Retail Performance, Vegan Trends in the U.S. Available online: https://www.ipsos-retailperformance.com/en/vegan-trends/ (accessed on 3 March 2022).
- Wunsch, N.-G. Percentage of U.S. Consumers Interested in Alternative Diets 2018, by Generation. Available online: https://www.statista.com/statistics/875526/share-alternative-diet-us-generation/ (accessed on 3 March 2022).
- Saglimbene, V.M.; Wong, G.; Ruospo, M.; Palmer, S.C.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Campbell, K.L.; Carrero, J.-J.; Stenvinkel, P.; et al. Fruit and Vegetable Intake and Mortality in Adults undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hu, Z.; Xu, X.; Li, Z.; Chen, Y.; Dong, J. The associations of plant-based protein intake with all-cause and cardiovascular mortality in patients on peritoneal dialysis. Nutr. Metab. Cardiovasc. Dis. 2020, 6, 967–976. [Google Scholar] [CrossRef]
- Wang, A.Y.; Brimble, K.S.; Brunier, G.; Holt, S.G.; Jha, V.; Johnson, D.W.; Kang, S.W.; Kooman, J.P.; Lambie, M.; McIntyre, C.; et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part I—Assessment and Management of Various Cardiovascular Risk Factors. Perit. Dial. Int. 2015, 35, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Van Biesen, W.; Verger, C.; Heaf, J.; Vrtovsnik, F.; Britto, Z.M.L.; Do, J.Y.; Prieto-Velasco, M.; Martínez, J.P.; Crepaldi, C.; De Los Ríos, T.; et al. Evolution Over Time of Volume Status and PD-Related Practice Patterns in an Incident Peritoneal Dialysis Cohort. Clin. J. Am. Soc. Nephrol. 2019, 14, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Günal, A.I.; Duman, S.; Özkahya, M.; Töz, H.; Asçi, G.; Akçiçek, F.; Basçi, A. Strict volume control normalizes hypertension in peritoneal dialysis patients. Am. J. Kidney Dis. 2001, 37, 588–593. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gøbel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Li, Y.; Yang, Z.; Luo, J. Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 2010, 5, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmadakis, G.; Albaret, J.; Da Costa Correia, E.; Somda, F.; Aguilera, D. Constipation in Peritoneal Dialysis Patients. Perit. Dial. Int. 2019, 39, 399–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erthal Leinig, C.; Pecoits-Filho, R.; Kunii, L.; Claro, L.M.; Merlin, J.; Almeida, N.R.; Carvalho, C.R.S.; Moraes, T.P. Low-Fiber Intake Is Associated with High Production of Intraperitoneal Inflammation Biomarkers. J. Ren. Nutr. 2019, 29, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Z.; Chen, Y.; Liu, X.; Dong, J. Dietary fibre and mortality risk in patients on peritoneal dialysis. Br. J. Nutr. 2019, 122, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Meksawan, K.; Chaotrakul, C.; Leeaphorn, N.; Gonlchanvit, S.; Eiam-Ong, S.; Kanjanabuch, T. Effects of Fructo-Oligosaccharide Supplementation on Constipation in Elderly Continuous Ambulatory Peritoneal Dialysis Patients. Perit. Dial. Int. 2016, 36, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Sutton, D.; Dumbleton, S.; Allaway, C. Can Increased Dietary Fibre Reduce Laxative Requirement in Peritoneal Dialysis Patients? J. Ren. Care 2007, 33, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.; Ovington, S.; Engel, B. A Multi-Centre, Randomised Trial to Assess Whether Increased Dietary Fibre Intake (Using a Fibre Supplement or High-Fibre Foods) Produces Healthy Bowel Performance and Reduces Laxative Requirement in Free Living Patients on Peritoneal Dialysis. J. Ren. Care 2014, 40, 157–163. [Google Scholar] [CrossRef]
- Allès, B.; Baudry, J.; Méjean, C.; Touvier, M.; Péneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the NutriNet-Santé Study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC-Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33,883 meat-eaters and 31,546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Armani, R.G.; Ramezani, A.; Yasir, A.; Sharama, S.; Canziani, M.E.F.; Raj, D.S. Gut Microbiome in Chronic Kidney Disease. Curr. Hypertens. Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo-Salgado, J.; Vehaskari, V.M.; Stewart, T.; Ferris, M.; Zhang, Q.; Wang, G.; Blanchard, E.E.; Taylor, C.M.; Kallash, M.; Greenbaum, L.A.; et al. Intestinal microbiota in pediatric patients with end stage renal disease: A Midwest Pediatric Nephrology Consortium study. Microbiome 2016, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Kwok, J.S.; Lai, K.B.; Cheng, P.M.; Pang, W.F.; Ng, J.K.; Chan, M.H.; Lit, L.C.; et al. Circulating bacterial-derived DNA fragment level is a strong predictor of cardiovascular disease in peritoneal dialysis patients. PLoS ONE 2015, 10, e0125162. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.; Harrison, L.; Hoad, C.; Marciani, L.; Cox, E.; Buchanan, C.; Costigan, C.; Francis, S.; Lai, K.B.; Szeto, C.C.; et al. Endotoxemia in Peritoneal Dialysis Patients: A Pilot Study to Examine the Role of Intestinal Perfusion and Congestion. Perit. Dial. Int. 2017, 37, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Lai, K.B.; Chung, K.Y.; Leung, C.B.; Li, P.K. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 431–436. [Google Scholar] [CrossRef]
- Kwan, B.C.; Chow, K.M.; Leung, C.B.; Law, M.C.; Cheng, P.M.; Yu, V.; Li, P.K.; Szeto, C.C. Circulating bacterial-derived DNA fragments as a marker of systemic inflammation in peritoneal dialysis. Nephrol. Dial. Transpl. 2013, 28, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transpl. 2011, 26, 938–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viaene, L.; Meijers, B.K.; Bammens, B.; Vanrenterghem, Y.; Evenepoel, P. Serum concentrations of p-cresyl sulfate and indoxyl sulfate, but not inflammatory markers, increase in incident peritoneal dialysis patients in parallel with loss of residual renal function. Perit. Dial. Int. 2014, 34, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.J.; Pan, C.F.; Chuang, C.K.; Liu, H.L.; Sun, F.J.; Wang, T.J.; Chen, H.H.; Wu, C.J. Gastrointestinal-related uremic toxins in peritoneal dialysis: A pilot study with a 5-year follow-up. Arch. Med. Res. 2013, 44, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Glick-Bauer, M.; Yeh, M.C. The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef] [Green Version]
- Martínez, I.; Muller, C.E.; Walter, J. Long-term temporal analysis of the human fecal microbiota revealed a stable core of dominant bacterial species. PLoS ONE 2013, 8, e69621. [Google Scholar] [CrossRef]
- Kim, M.S.; Hwang, S.S.; Park, E.J.; Bae, J.W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Shen, J.; Li, W.; Wang, Y.; Zhong, Z.; Ye, H.; Huang, N.; Fan, L.; Yang, X.; Yu, X.; et al. Elevated Serum Trimethylamine N-Oxide Levels Are Associated with Mortality in Male Patients on Peritoneal Dialysis. Blood Purif. 2021, 50, 837–847. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R. Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am. J. Nephrol. 2011, 34, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Nissenson, A.R.; Batlle, D.; Coyne, D.W.; Trout, J.R.; Warnock, D.G. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am. J. Med. 2003, 115, 291–297. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.-J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of Onset of CKD-Related Metabolic Complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Wesson, D.E.; Buysse, J.M.; Bushinsky, D.A. Mechanisms of Metabolic Acidosis–Induced Kidney Injury in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 469–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Ríos-Burrows, N.; Williams, D.E.; Powe, N.R. High Dietary Acid Load Predicts ESRD among Adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef] [Green Version]
- Dobre, M.; Yang, W.; Chen, J.; Drawz, P.; Hamm, L.L.; Horwitz, E.; Hostetter, T.; Jaar, B.; Lora, C.M.; Nessel, L.; et al. Association of Serum Bicarbonate with Risk of Renal and Cardiovascular Outcomes in CKD: A Report from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2013, 62, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Driver, T.H.; Shlipak, M.G.; Katz, R.; Goldenstein, L.; Sarnak, M.J.; Hoofnagle, A.N.; Siscovick, D.S.; Kestenbaum, B.; De Boer, I.H.; Ix, J.H. Low Serum Bicarbonate and Kidney Function Decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 2014, 64, 534–541. [Google Scholar] [CrossRef] [Green Version]
- De Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate Supplementation Slows Progression of CKD and Improves Nutritional Status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084. [Google Scholar] [CrossRef] [Green Version]
- Passey, C. Reducing the Dietary Acid Load: How a More Alkaline Diet Benefits Patients with Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.I.; Kang, E.W.; Kim, H.W.; Ryu, G.W.; Park, C.H.; Park, J.T.; Yoo, T.-H.; Shin, S.K.; Kang, S.-W.; Choi, K.H.; et al. Low Serum Bicarbonate Predicts Residual Renal Function Loss in Peritoneal Dialysis Patients. Medicine 2015, 94, e1276. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, X.M.; Zhang, N.; Chen, R.; Wu, F.; Tao, X.C.; Li, C.J.; Zhang, P.; Yu, P. Oral Bicarbonate Slows Decline of Residual Renal Function in Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2017, 42, 565–574. [Google Scholar] [CrossRef]
- Szeto, C.-C.; Wong, T.Y.-H.; Chow, K.-M.; Leung, C.-B.; Li, P.K.-T. Oral Sodium Bicarbonate for the Treatment of Metabolic Acidosis in Peritoneal Dialysis Patients: A Randomized Placebo-Control Trial. J. Am. Soc. Nephrol. 2003, 14, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Palmer, B.F.; Colbert, G.; Clegg, D.J. Potassium Homeostasis, Chronic Kidney Disease, and the Plant-Enriched Diets. Kidney360 2020, 1, 65–71. [Google Scholar] [CrossRef]
- Goncalves, F.A.; De Jesus, J.S.; Cordeiro, L.; Piraciaba, M.C.T.; De Araujo, L.; Steller Wagner Martins, C.; Dalboni, M.A.; Pereira, B.J.; Silva, B.C.; Moysés, R.M.A.; et al. Hypokalemia and hyperkalemia in patients on peritoneal dialysis: Incidence and associated factors. Int. Urol. Nephrol. 2020, 52, 393–398. [Google Scholar] [CrossRef]
- Szeto, C.-C.; Chow, K.-M.; Kwan, B.C.-H.; Leung, C.-B.; Chung, K.-Y.; Law, M.-C.; Li, P.K.-T. Hypokalemia in Chinese Peritoneal Dialysis Patients: Prevalence and Prognostic Implication. Am. J. Kidney Dis. 2005, 46, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Torlén, K.; Kalantar-Zadeh, K.; Molnar, M.Z.; Vashistha, T.; Mehrotra, R. Serum Potassium and Cause-Specific Mortality in a Large Peritoneal Dialysis Cohort. Clin. J. Am. Soc. Nephrol. 2012, 7, 1272–1284. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.C.; Figueiredo, A.E.; Barretti, P.; Pecoits-Filho, R.; De Moraes, T.P. Low Serum Potassium Levels Increase the Infectious-Caused Mortality in Peritoneal Dialysis Patients: A Propensity-Matched Score Study. PLoS ONE 2015, 10, e0127453. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-W.; Shu, K.-H.; Yu, T.-M.; Cheng, C.-H.; Chen, C.-H. Hypokalaemia: An independent risk factor of enterobacteriaceae peritonitis in CAPD patients. Nephrol. Dial. Transplant. 2009, 24, 1603–1608. [Google Scholar] [CrossRef] [Green Version]
- Parpia, A.S.; L’Abbé, M.; Goldstein, M.; Arcand, J.; Magnuson, B.; Darling, P.B. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients with Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Picard, K. Potassium Additives and Bioavailability: Are We Missing Something in Hyperkalemia Management? J. Ren. Nutr. 2019, 29, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Jean, A.; Wong, G.; Saglimbene, V.; Ruospo, M.; Palmer, S.C.; Natale, P.; Garcia-Larsen, V.; Johnson, D.W.; Tonelli, M.; Hegbrant, J.; et al. Dietary Potassium Intake and All-Cause Mortality in Adults Treated with Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1851–1861. [Google Scholar] [CrossRef]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Murali, S.B.; Bross, R.; Nissenson, A.R.; Kopple, J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Naismith, D.J.; Braschi, A. An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int. J. Food Sci. Nutr. 2008, 59, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Wolf, M.; Yang, W.; Zhang, X.; Sozio, S.M.; Miller, E.R., 3rd; Bazzano, L.A.; Cuevas, M.; Glenn, M.J.; et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: The Chronic Renal Insufficiency Cohort study. J. Ren. Nutr. 2012, 22, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Tyson, C.C.; Lin, P.-H.; Corsino, L.; Batch, B.C.; Allen, J.; Sapp, S.; Barnhart, H.; Nwankwo, C.; Burroughs, J.; Svetkey, L.P. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: A pilot feeding study. Clin. Kidney J. 2016, 9, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Blumenkrantz, M.J.; Kopple, J.D.; Moran, J.K.; Coburn, J.W. Metabolic balance studies and dietary protein requirements in patients undergoing continuous ambulatory peritoneal dialysis. Kidney Int. 1982, 21, 849–861. [Google Scholar] [CrossRef] [Green Version]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, C.; McLeod, M.; Robinson, R. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans. Assoc. Am. Physicians 1967, 80, 207–216. [Google Scholar]
- Rivara, M.B.; Ravel, V.; Kalantar-Zadeh, K.; Streja, E.; Lau, W.L.; Nissenson, A.R.; Kestenbaum, B.; De Boer, I.H.; Himmelfarb, J.; Mehrotra, R. Uncorrected and Albumin-Corrected Calcium, Phosphorus, and Mortality in Patients Undergoing Maintenance Dialysis. J. Am. Soc. Nephrol. 2015, 26, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.T.; Lin, Y.C.; Lin, Y.C.; Kao, C.C.; Chen, H.H.; Hsu, C.C.; Wu, M.S. Roles of Serum Calcium, Phosphorus, PTH and ALP on Mortality in Peritoneal Dialysis Patients: A Nationwide, Population-based Longitudinal Study Using TWRDS 2005–2012. Sci. Rep. 2017, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, M. Association of Single and Serial Measures of Serum Phosphorus with Adverse Outcomes in Patients on Peritoneal Dialysis: Results from the International PDOPPS, “Protect the Memrane” Free Comincation Session. In Proceedings of the 58th ERA-EDTA Congress, Fully Virtual, 5–8 June 2021. [Google Scholar]
- KDIGO. 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.C.; Gardner, S.; Tonelli, M.; Mavridis, D.; Johnson, D.W.; Craig, J.C.; French, R.; Ruospo, M.; Strippoli, G.F. Phosphate-Binding Agents in Adults with CKD: A Network Meta-analysis of Randomized Trials. Am. J. Kidney Dis. 2016, 68, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-W.; Teitelbaum, I.; Misra, M.; De Leon, E.M.; Adzize, T.; Mehrotra, R. Pill Burden, Adherence, Hyperphosphatemia, and Quality of Life in Maintenance Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1089–1096. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Contributions to total phosphorus intake: All sources considered. Semin. Dial. 2013, 26, 54–61. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- The Food and Nutrition Board, Institute of Medicine, National Academies. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes, Total Water and Macronutrients. Available online: https://ods.od.nih.gov/HealthInformation/Dietary_Reference_Intakes.aspx (accessed on 12 January 2022).
- The Food and Nutrition Board, Institute of Medicine, National Academies. Dietary Reference Intakes (DRIs): Acceptable Macronutrient Distribution Ranges. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56068/table/summarytables.t5/?report=objectonly (accessed on 12 January 2022).
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of Plant-Based Proteins in Chronic Kidney Disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Ou, S.H.; Yen, M.C.; Lee, M.S.; Chen, N.C.; Yin, C.H.; Chen, C.L. Vegetarian diet in dialysis patients: A significant gap between actual intake and current nutritional recommendations. Medicine 2021, 100, e24617. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H.; Chen, M.Y.; Huang, C.W.; Chen, N.C.; Wu, C.H.; Hsu, C.Y.; Chou, K.J.; Lee, P.T.; Fang, H.C.; Chen, C.L. Potential Role of Vegetarianism on Nutritional and Cardiovascular Status in Taiwanese Dialysis Patients: A Case-Control Study. PLoS ONE 2016, 11, e0156297. [Google Scholar] [CrossRef]
- He, Y.; Lu, Y.; Yang, S.; Li, Y.; Yang, Y.; Chen, J.; Huang, Y.; Lin, Z.; Li, Y.; Kong, Y.; et al. Dietary Plant Protein and Mortality Among Patients Receiving Maintenance Hemodialysis: A Cohort Study. Am. J. Kidney Dis. 2021, 78, 649–657. [Google Scholar] [CrossRef]
- Blumenkrantz, M.J.; Gahl, G.M.; Kopple, J.D.; Kamdar, A.V.; Jones, M.R.; Kessel, M.; Coburn, J.W. Protein losses during peritoneal dialysis. Kidney Int. 1981, 19, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaysen, G.A. Biological basis of hypoalbuminemia in ESRD. J. Am. Soc. Nephrol. 1998, 9, 2368–2376. [Google Scholar] [CrossRef]
- Ballmer, P.E.; McNurlan, M.A.; Hulter, H.N.; Anderson, S.E.; Garlick, P.J.; Krapf, R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J. Clin. Investig. 1995, 95, 39–45. [Google Scholar] [CrossRef]
- Shah, N.; Bernardini, J.; Piraino, B. Prevalence and correction of 25(OH) vitamin D deficiency in peritoneal dialysis patients. Perit. Dial. Int. 2005, 25, 362–366. [Google Scholar] [CrossRef]
- Hansen, T.H.; Madsen, M.T.B.; Jørgensen, N.R.; Cohen, A.S.; Hansen, T.; Vestergaard, H.; Pedersen, O.; Allin, K.H. Bone turnover, calcium homeostasis, and vitamin D status in Danish vegans. Eur. J. Clin. Nutr. 2018, 72, 1046–1105. [Google Scholar] [CrossRef]
- Liu, G.L.; Pi, H.C.; Hao, L.; Li, D.D.; Wu, Y.G.; Dong, J. Vitamin D Status Is an Independent Risk Factor for Global Cognitive Impairment in Peritoneal Dialysis Patients. PLoS ONE 2015, 10, e0143782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez Fontán, M.; Borràs Sans, M.; Bajo Rubio, M.A.; Rodriguez-Carmona, A.; Betriu, A.; Valdivielso, J.M.; Fernández, E. Low Serum Levels of Vitamin D are Associated with Progression of Subclinical Atherosclerotic Vascular Disease in Peritoneal Dialysis Patients: A Prospective, Multicenter Study. Nephron 2017, 136, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, J.; Xie, X.; Sun, D. Low serum vitamin D concentration is correlated with anemia, microinflammation, and oxidative stress in patients with peritoneal dialysis. J. Transl. Med. 2021, 19, 411. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.C.; Ren, Y.P.; Wang, Q.; Xu, R.; Dong, J. Serum 25-Hydroxyvitamin D Level Could Predict the Risk for Peritoneal Dialysis-Associated Peritonitis. Perit. Dial. Int. 2015, 35, 729–735. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebman, S.E.; Joshi, S. Plant-Based Diets and Peritoneal Dialysis: A Review. Nutrients 2022, 14, 1304. https://doi.org/10.3390/nu14061304
Liebman SE, Joshi S. Plant-Based Diets and Peritoneal Dialysis: A Review. Nutrients. 2022; 14(6):1304. https://doi.org/10.3390/nu14061304
Chicago/Turabian StyleLiebman, Scott E., and Shivam Joshi. 2022. "Plant-Based Diets and Peritoneal Dialysis: A Review" Nutrients 14, no. 6: 1304. https://doi.org/10.3390/nu14061304





