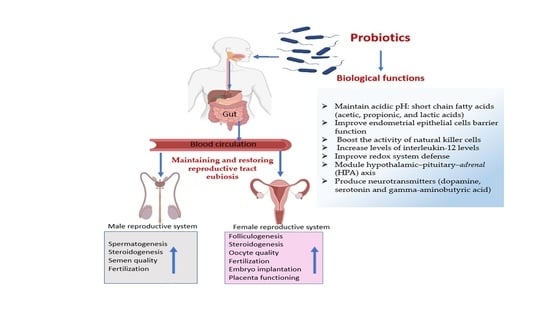
The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function
Abstract
:1. Introduction
2. Biodiversity of the Reproductive Tract Microbiota and Fertility
2.1. Human Model
2.2. Animal Model
3. Manipulation of Reproductive Tract Microbiota
4. Probiotics and Reproductive Health
4.1. Definition and Characteristics
4.2. Potential Sources
4.2.1. Human Reproductive Tract
4.2.2. Animal Reproductive Tract
4.3. Mechanisms of Action
4.4. Benefits of Probiotic for Female Reproduction
4.4.1. Women’s Fertility
4.4.2. Animal Fertility
4.5. Benefits of Probiotics for Male Reproduction
4.5.1. Men’s Fertility
4.5.2. Animal Fertility
5. Safety and Hazards of Probiotic and Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubic-Pavlovic, A.; Romero, B.; Clavero, A.; Mozas-Moreno, J.; Fontes, J.; Altmae, S. New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future. Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, N.; Hutchinson, A.P.; Lekovich, J.P.; Hobeika, E.; Elias, R.T. Antibiotic prophylaxis for gynecologic procedures prior to and during the utilization of assisted reproductive technologies: A systematic review. J. Pathog. 2016, 2016, 4698314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silla, A.J.; Keogh, L.M.; Byrne, P.G. Antibiotics and oxygen availability affect the short-term storage of spermatozoa from the critically endangered booroolong frog, Litoria booroolongensis. Reprod. Fertil. Dev. 2015, 27, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Javurek, A.B.; Johnson, S.A.; Lei, Z.; Sumner, L.W.; Hess, R.A. Seminal fluid metabolome and epididymal changes after antibiotic treatment in mice. Reproduction 2018, 156, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Simon, C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhanasekar, K.R.; Shilpa, B.; Gomathy, N.; Kundavi, S. Prenatal Probiotics: The Way Forward in Prevention of Preterm Birth. J. Clin. Gynecol. Obstet. 2019, 8, 63–69. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Tye, K.D.; Luo, H.; Tang, X.; Liao, Y.; Wang, D.; Zhou, J.; Yang, P.; Li, Y.; et al. Probiotic Supplementation During Human Pregnancy Affects the Gut Microbiota and Immune Status. Front. Cell. Infect. Microbiol. 2019, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Helli, B.; Kavianpour, M.; Ghaedi, E.; Dadfar, M.; Haghighian, H.K. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum. Fertil. 2020, 1–9. [Google Scholar] [CrossRef]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Quereda, J.J.; Garcia-Rosello, E.; Barba, M.; Moce, M.L.; Gomis, J.; Jimenez-Trigos, E.; Bataller, E.; Martinez-Bovi, R.; Garcia-Munoz, A.; Gomez-Martin, A. Use of Probiotics in Intravaginal Sponges in Sheep: A Pilot Study. Animals 2020, 10, 719. [Google Scholar] [CrossRef]
- Gu, X.L.; Li, H.; Song, Z.H.; Ding, Y.N.; He, X.; Fan, Z.Y. Effects of isomaltooligosaccharide and Bacillus supplementation on sow performance, serum metabolites, and serum and placental oxidative status. Anim. Reprod. Sci. 2019, 207, 52–60. [Google Scholar] [CrossRef]
- Mastromarino, P.; Macchia, S.; Meggiorini, L.; Trinchieri, V.; Mosca, L.; Perluigi, M.; Midulla, C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009, 15, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, C.S.; Pirotta, M.; De Guingand, D.; Hocking, J.S.; Morton, A.N.; Garland, S.M.; Fehler, G.; Morrow, A.; Walker, S.; Vodstrcil, L.A. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: Randomised placebo-controlled double-blind trial. PLoS ONE 2012, 7, e34540. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Gartner, M.A.; Michel, G.; Ibrahim, M.; Klopfleisch, R.; Lubke-Becker, A.; Jung, M.; Einspanier, R.; Gabler, C. Influence of intrauterine administration of Lactobacillus buchneri on reproductive performance and pro-inflammatory endometrial mRNA expression of cows with subclinical endometritis. Sci. Rep. 2018, 8, 5473. [Google Scholar] [CrossRef]
- Younge, N.; McCann, J.R.; Ballard, J.; Plunkett, C.; Akhtar, S.; Araújo-Pérez, F.; Murtha, A.; Brandon, D.; Seed, P.C. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight 2019, 4, e127806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Herath, S.; Williams, E.J.; Lilly, S.T.; Gilbert, R.O.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 2007, 134, 683–693. [Google Scholar] [CrossRef]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota—New player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef]
- Fang, R.-L.; Chen, L.-X.; Shu, W.-S.; Yao, S.-Z.; Wang, S.-W.; Chen, Y.-Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581. [Google Scholar] [PubMed]
- Rizzo, A.E.; Gordon, J.C.; Berard, A.R.; Burgener, A.D.; Avril, S. The Female Reproductive Tract Microbiome—Implications for Gynecologic Cancers and Personalized Medicine. J. Pers. Med. 2021, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Walther-António, M.R.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R. Postmenopause as a key factor in the composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213. [Google Scholar] [CrossRef]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 2019, 25, 298–325. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Allan, J.A.; Waterhouse, M.A.; Ross, T.; Beagley, K.W.; Knox, C.L. Microorganisms within human follicular fluid: Effects on IVF. PLoS ONE 2013, 8, e59062. [Google Scholar] [CrossRef] [Green Version]
- Halis, G.; Arici, A. Endometriosis and inflammation in infertility. Ann. N. Y. Acad. Sci. 2004, 1034, 300–315. [Google Scholar] [CrossRef]
- López-Moreno, A.; Aguilera, M. Vaginal probiotics for reproductive health and related dysbiosis: Systematic review and meta-analysis. J. Clin. Med. 2021, 10, 1461. [Google Scholar] [CrossRef]
- Fuochi, V.; Li Volti, G.; Furneri, P.M. Commentary: Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2017, 8, 1815. [Google Scholar] [CrossRef]
- Russo, R.; Karadja, E.; De Seta, F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef. Microbes 2019, 10, 19–26. [Google Scholar] [CrossRef]
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The seminal microbiome in health and disease. Nat. Rev. Urol. 2019, 16, 703–721. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirsalehian, A.; Gilani, M.A.S.; Bahador, A.; Talebi, M. Asymptomatic infection with Mycoplasma hominis negatively affects semen parameters and leads to male infertility as confirmed by improved semen parameters after antibiotic treatment. Urology 2017, 100, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Marques, P.I.; Cavadas, B.; Damião, I.; Almeida, V.; Barros, N.; Barros, A.; Carvalho, F.; Gomes, S.; Seixas, S. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am. J. Reprod. Immunol. 2018, 79, e12838. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nolvak, H.; Preem, J.-K. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Kästle, J.; Coutinho, T.; Amorim, A.; Campos, G.; Santos, V.; Marques, L.; Timenetsky, J.; De Farias, S. Qualitative analysis of the vaginal microbiota of healthy cattle and cattle with genital-tract. Gen. Mol. Res. 2015, 14, 6518–6528. [Google Scholar] [CrossRef] [Green Version]
- Shpigel, N.; Adler-Ashkenazy, L.; Scheinin, S.; Goshen, T.; Arazi, A.; Pasternak, Z.; Gottlieb, Y. Characterization and identification of microbial communities in bovine necrotic vulvovaginitis. Vet. J. 2017, 219, 34–39. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, H.; Fu, K.; Pang, B.; Yang, Y.; Liu, Y.; Tian, W.; Cao, R. Characterization of the cervical bacterial community in dairy cows with metritis and during different physiological phases. Theriogenology 2018, 108, 306–313. [Google Scholar] [CrossRef]
- Saleh, M.; Haraki Nezhad, M.T.; Salmani, V. Detection of some bacterial causes of abortion in Afshari sheep using real time PCR detection and sensitivity assessment of campylobacter primers. Agric. Biotechnol. J. 2014, 6, 107–120. [Google Scholar]
- Younis, N.; Mahasneh, A. Probiotics and the envisaged role in treating human infertility. Middle East Fertil. Soc. J. 2020, 25, 33. [Google Scholar] [CrossRef]
- Lopez-Moreno, A.; Aguilera, M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients 2020, 12, 757. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Li, Y.; Wu, Q.J.; Zhang, P.; Li, W.T.; Mao, Z.Y.; Wu, D.M.; Jiang, X.M.; Zhuo, Y.; Fang, Z.F. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota. J. Anim. Sci. 2019, 97, 3426–3439. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Matsubayashi, H.; Takaya, Y.; Nishiyama, R.; Yamaguchi, K.; Takeuchi, T.; Ishikawa, T. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am. J. Reprod. Immunol. 2017, 78, e12719. [Google Scholar] [CrossRef] [PubMed]
- McQueen, D.B.; Bernardi, L.A.; Stephenson, M.D. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil. Steril. 2014, 101, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Liu, Y.; Zheng, S.; Zhao, W.; Wu, D.; Lei, L.; Chen, G. Confirmation of chronic endometritis in repeated implantation failure and success outcome in IVF-ET after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am. J. Reprod. Immunol. 2019, 82, e13177. [Google Scholar] [CrossRef]
- Weinreb, E.B.; Cholst, I.N.; Ledger, W.J.; Danis, R.B.; Rosenwaks, Z. Should all oocyte donors receive prophylactic antibiotics for retrieval? Fertil. Steril. 2010, 94, 2935–2937. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 17, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef]
- Trush, E.A.; Poluektova, E.A.; Beniashvilli, A.G.; Shifrin, O.S.; Poluektov, Y.M.; Ivashkin, V.T. The evolution of human probiotics: Challenges and prospects. Probiotics Antimicrob. Proteins 2020, 12, 1291–1299. [Google Scholar] [CrossRef]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef]
- Niu, C.; Cheng, C.; Liu, Y.; Huang, S.; Fu, Y.; Li, P. Transcriptome profiling analysis of bovine vaginal epithelial cell response to an isolated lactobacillus strain. Msystems 2019, 4, e00268-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenoll, E.; Moreno, I.; Sanchez, M.; Garcia-Grau, I.; Silva, A.; Gonzalez-Monfort, M.; Genoves, S.; Vilella, F.; Seco-Durban, C.; Simon, C.; et al. Selection of New Probiotics for Endometrial Health. Front. Cell. Infect. Microbiol. 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.; Iqbal, S.; Selami, F.; Odhiambo, J.; Wang, Y.; Gänzle, M.; Dunn, S.; Zebeli, Q. Intravaginal administration of lactic acid bacteria modulated the incidence of purulent vaginal discharges, plasma haptoglobin concentrations, and milk production in dairy cows. Res. Vet. Sci. 2014, 96, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Poutahidis, T.; Springer, A.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS ONE 2014, 9, e84877. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Wang, W.; Liu, W.; Gatlin III, D.M.; Zhang, Y.; Yao, B.; Ringø, E. Identification of highly-adhesive gut Lactobacillus strains in zebrafish (Danio rerio) by partial rpoB gene sequence analysis. Aquaculture 2012, 370, 150–157. [Google Scholar] [CrossRef]
- Valcarce, D.; Genovés, S.; Riesco, M.; Martorell, P.; Herráez, M.; Ramón, D.; Robles, V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes 2017, 8, 193–206. [Google Scholar] [CrossRef]
- Sashihara, T.; Sueki, N.; Furuichi, K.; Ikegami, S. Effect of growth conditions of Lactobacillus gasseri OLL2809 on the immunostimulatory activity for production of interleukin-12 (p70) by murine splenocytes. Int. J. Food Microbiol. 2007, 120, 274–281. [Google Scholar] [CrossRef]
- Inatomi, T.; Otomaru, K. Effect of dietary probiotics on the semen traits and antioxidative activity of male broiler breeders. Sci. Rep. 2018, 8, 5874. [Google Scholar] [CrossRef] [Green Version]
- Jeżewska-Frąckowiak, J.; Seroczyńska, K.; Banaszczyk, J.; Jedrzejczak, G.; Żylicz-Stachula, A.; Skowron, P.M. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar] [CrossRef] [Green Version]
- Pericolini, E.; Gabrielli, E.; Ballet, N.; Sabbatini, S.; Roselletti, E.; Cayzeele Decherf, A.; Pélerin, F.; Luciano, E.; Perito, S.; Jüsten, P. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence 2017, 8, 74–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buggio, L.; Somigliana, E.; Borghi, A.; Vercellini, P. Probiotics and vaginal microecology: Fact or fancy? BMC Womens Health 2019, 19, 25. [Google Scholar] [CrossRef]
- Bohbot, J.; Cardot, J. Vaginal impact of the oral administration of total freeze-dried culture of LCR 35 in healthy women. Infect. Dis. Obstet. Gynecol. 2012, 2012, 503648. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.A.; Meyn, L.A.; Murray, P.J.; Busse, B.; Hillier, S.L. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J. Infect. Dis. 2009, 199, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Hemmerling, A.; Harrison, W.; Schroeder, A.; Park, J.; Korn, A.; Shiboski, S.; Foster-Rosales, A.; Cohen, C.R. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex. Transm. Dis. 2010, 37, 745–750. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.M.; Fayez, M.; Elsohaby, I.; Ghoneim, I.; Al-Marri, T.; Kandeel, M.; ElGioushy, M. Isolation and characterization of vaginal Lactobacillus spp. in dromedary camels (Camelus dromedarius): In vitro evaluation of probiotic potential of selected isolates. PeerJ 2020, 8, e8500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-M.; Park, Y.J. Probiotics in the prevention and treatment of postmenopausal vaginal infections. J. Menopausal Med. 2017, 23, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef] [Green Version]
- Coudeyras, S.; Jugie, G.; Vermerie, M.; Forestier, C. Adhesion of human probiotic Lactobacillus rhamnosus to cervical and vaginal cells and interaction with vaginosis-associated pathogens. Infect. Dis. Obstet. Gynecol. 2008, 2008, 549640. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Marcone, V.; Calzolari, E.; Bertini, M. Effectiveness of vaginal administration of Lactobacillus rhamnosus following conventional metronidazole therapy: How to lower the rate of bacterial vaginosis recurrences. New Microbiol. 2008, 31, 429. [Google Scholar] [PubMed]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Cayzeele-Decherf, A.; Pélerin, F.; Jüsten, P. Saccharomyces cerevisiae CNCM I-3856 as a natural breakthrough for vaginal health: A clinical study. Med. J. Obstet. Gynecol. 2017, 5, 1112. [Google Scholar]
- Ahmadzadeh, L.; Hosseinkhani, A.; Kia, H.D. Effect of supplementing a diet with monensin sodium and Saccharomyces Cerevisiae on reproductive performance of Ghezel ewes. Anim. Reprod. Sci. 2018, 188, 93–100. [Google Scholar] [CrossRef]
- Genis, S.; Sanchez-Chardi, A.; Bach, A.; Fabregas, F.; Aris, A. A combination of lactic acid bacteria regulates Escherichia coli infection and inflammation of the bovine endometrium. J. Dairy Sci. 2017, 100, 479–492. [Google Scholar] [CrossRef]
- Deng, Q.; Odhiambo, J.; Farooq, U.; Lam, T.; Dunn, S.; Ametaj, B. Intravaginal probiotics modulated metabolic status and improved milk production and composition of transition dairy cows. J. Anim. Sci. 2016, 94, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Kritas, S.; Marubashi, T.; Filioussis, G.; Petridou, E.; Christodoulopoulos, G.; Burriel, A.; Tzivara, A.; Theodoridis, A.; Pískoriková, M. Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C-3102). J. Anim. Sci. 2015, 93, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Hashem, N.M.; Gonzalez-Bulnes, A. Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances. Animals 2021, 11, 1932. [Google Scholar] [CrossRef]
- Deng, F.; McClure, M.; Rorie, R.; Wang, X.; Chai, J.; Wei, X.; Lai, S.; Zhao, J. The vaginal and fecal microbiomes are related to pregnancy status in beef heifers. J. Anim. Sci. Biotechnol. 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizubuchi, H.; Yajima, T.; Aoi, N.; Tomita, T.; Yoshikai, Y. Isomalto-oligosaccharides polarize Th1-like responses in intestinal and systemic immunity in mice. J. Nutr. 2005, 135, 2857–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, K.; Liu, L.; Mo, T.; Pan, H.; Liu, H. Preparation, characterization, and antioxidant activity of an isomaltooligosaccharide–iron complex (IIC). J. Carbohydr. Chem. 2015, 34, 430–443. [Google Scholar] [CrossRef]
- Guo, Y.; Du, X.; Bian, Y.; Wang, S. Chronic unpredictable stress-induced reproductive deficits were prevented by probiotics. Reprod. Biol. 2020, 20, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Dardmeh, F.; Alipour, H.; Gazerani, P.; Van der Horst, G.; Brandsborg, E.; Nielsen, H.I. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLoS ONE 2017, 12, e0185964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wen, M.; Pan, K.; Shen, K.; Yang, R.; Liu, Z. Protective effects of dietary co-administration of probiotic Lactobacillus casei on CP-induced reproductive dysfunction in adult male Kunming mice. Vet. Res. 2012, 5, 110–119. [Google Scholar]
- Tremellen, K.; Pearce, K. Probiotics to improve testicular function (Andrology 5: 439–444, 2017)—A comment on mechanism of action and therapeutic potential of probiotics beyond reproduction. Andrology 2017, 5, 1052–1053. [Google Scholar] [CrossRef] [Green Version]
- Ommati, M.M.; Li, H.; Jamshidzadeh, A.; Khoshghadam, F.; Retana-Márquez, S.; Lu, Y.; Farshad, O.; Nategh Ahmadi, M.H.; Gholami, A.; Heidari, R. The crucial role of oxidative stress in non-alcoholic fatty liver disease-induced male reproductive toxicity: The ameliorative effects of Iranian indigenous probiotics. Naunyn-Schmiedebergs Arch. Pharmacol. 2022, 395, 247–265. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Consumption of a high-fat diet alters the seminal fluid and gut microbiomes in male mice. Reprod. Fertil. Dev. 2017, 29, 1602–1612. [Google Scholar] [CrossRef]
- Nader-Macías, M.E.F.; De Gregorio, P.R.; Silva, J.A. Probiotic lactobacilli in formulas and hygiene products for the health of the urogenital tract. Pharmacol. Res. Perspect. 2021, 9, e00787. [Google Scholar] [CrossRef]
- Mattia, A.; Merker, R. Regulation of probiotic substances as ingredients in foods: Premarket approval or “generally recognized as safe” notification. Clin. Infect. Dis. 2008, 46, S115–S118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldassarre, M.E.; Palladino, V.; Amoruso, A.; Pindinelli, S.; Mastromarino, P.; Fanelli, M.; Di Mauro, A.; Laforgia, N. Rationale of Probiotic Supplementation during Pregnancy and Neonatal Period. Nutrients 2018, 10, 1693. [Google Scholar] [CrossRef] [Green Version]
- Kuitunen, M.; Kukkonen, K.; Savilahti, E. Pro-and prebiotic supplementation induces a transient reduction in hemoglobin concentration in infants. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki-Maulden, G.L. Effect of dietary modulation of intestinal microbiota on reproduction and early growth. Theriogenology 2008, 70, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.E.; Fraser, C.M.; Palumbo, F.; Ravel, J.; Rowthorn, V.; Schwartz, J. Probiotics: Achieving a better regulatory fit. Food Drug Law J. 2014, 69, 237. [Google Scholar] [PubMed]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Wagner, I.; Suhajda, Á.; Tobak, T.; Harasztos, A.H.; Vigh, T.; Sóti, P.L.; Pataki, H.; Molnár, K.; Marosi, G. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. eXPRESS Polym. Lett. 2014, 8, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhang, Y.; Song, J.; Chen, L.; Du, M.; Mao, X. Yogurt Enriched with Inulin Ameliorated Reproductive Functions and Regulated Gut Microbiota in Dehydroepiandrosterone-Induced Polycystic Ovary Syndrome Mice. Nutrients 2022, 14, 279. [Google Scholar] [CrossRef]
- Zakariaee, H.; Sudagar, M.; Hosseini, S.S.; Paknejad, H.; Baruah, K. In vitro Selection of Synbiotics and in vivo Investigation of Growth Indices, Reproduction Performance, Survival, and Ovarian Cyp19α Gene Expression in Zebrafish Danio rerio. Front. Microbiol. 2021, 12, 758758. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
| Probiotic Species (Reference) | Properties/Function |
|---|---|
| Lactobacillus rhamnosus (E21 and L3) Lactobacillus helveticus (P7, P12, S7, and U13) Lactobacillus salivarius (N30) [50] |
|
| Lactobacillus strain (SQ0048) [51] |
|
| Lactobacillus reuteri RC14 Lactobacillus rhamnosus GR1 [6] |
|
| Lactobacillus rhamnosus BPL005 [52] |
|
| Lactobacillus buchneri (DSM 32407) [14,53] |
|
| Lactobacillus reuteri ATCC PTA 6475 [54] |
|
| Lactobacillus rhamnosus CICC6141 Lactobacillus casei BL23v [55] |
|
| Lactobacillus rhamnosus CECT8361 Bifidobacterium longum CECT7347 [56] |
|
| Lactobacillus gasseri OLL2809 [57] |
|
| Bacillus amyloliquefaciens [58] |
|
| Bacillus subtilis [11] Lactobacillus rhamnosus CECT8361 and Bifidobacterium longum CECT7347 [56] |
|
| Bacillus subtilis (DSM10) Bacillus clausii (DSM 8716) Bacillus coagulans (DSM 1) Bacillus amyloliquefaciens (DSM 7) [59] |
|
| Bifidobacterium lactis V9 [45] |
|
| Saccharomyces cerevisiae [60,61] |
|
| Specie (References) | Probiotic Treatment | Results |
|---|---|---|
| Studies on Women | ||
| Women [6] | Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14 |
|
| Pregnant women [7] | Tablets containing Bifidobacterium longum (5 × 106 CFU), Lactobacillus delbrueckii bulgaricus (5 × 105 CFU), and Streptococcus thermophilus (5 × 105 CFU) Two tablets twice a day from week 32 of pregnancy to delivery |
|
| Women [45] | Bifidobacterium lactis V9 |
|
| Women with vulvovaginal candidiasis [75] | Oral intake of Saccharomyces cerevisiae CNCM I-3856 (5 × 109 CFU/mL) Once a day for 56 days |
|
| Women [74] | Lactobacillus rhamnosus HN001(6 × 109 CFU/day) From 14−16 days of pregnancy to 6 months of breastfeeding |
|
| Women [12] | Lactobacillus-containing vaginal tablets |
|
| Studies on animals | ||
| Ewes [10] | A combination of 60% Lactobacillus crispatus, 20% Lactobacillus brevis, and 20% Lactobacillus gasseri at fluorogestone acetate sponge insertion |
|
| Late pregnant sows [11] | Different combinations of 0.5% isomaltooligosaccharide (IMO), 0.02% Bacillus subtilis, and 0.02% Bacillus licheniformis |
|
| Landrace × Yorkshire sows [41] | Basal diets supplemented with 0, 0.1%, 0.2%, or 0.4% Clostridium butyricum (4 × 108 CFU/kg) From day 90 of gestation to weaning at day 21 of lactation |
|
| Ghezel ewes [76] | Dietary supplementation with 30 mg/ewe/day of monensin (MS) or 4 × 109 of CFU/ewe/d Saccharomyces cerevisiae (SC) |
|
| Cows with signs of subclinical endometritis [14] | Intrauterine administration of the Lactobacillus buchneri DSM 32407 |
|
| Cows [77] | A combination of Lactobacillus rhamnosus, Pediococcus acidilactici, and Lactobacillus reuteri at a ratio of 25:25:2 |
|
| Dairy cows [78] | A combination of Lactobacillus sakei FUA3089, Pediococcus acidilactici FUA3138, and Pediococcus acidilactici FUA3140 (108−109 CFU/dose) Weekly from 2 weeks prepartum to 1 week postpartum |
|
| Late pregnant sows [79] | Dietary Bacillus |
|
| Late pregnant cows [53] | Intravaginal administration of Lactobacillus sakei FUA 3089, Pediococcus acidilactici FUA 3140, and Pediococcus acidilactici FUA 3138 (1010–1012 CFU/cow) Weekly from two weeks prepartum to 4 weeks postpartum |
|
| Specie (References) | Probiotic Treatment | Results |
|---|---|---|
| Studies on men | ||
| Idiopathic asthenozoospermia men [8] | Tablets containing Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophil (2 × 1011 CFU) |
|
| Men with idiopathic oligoasthenoteratospermia [9] et al., 2017 | A combination of Lactobacillus paracasei B21060 (5 × 109 cells) + arabinogalctan (1243 mg) + oligo-fructosaccharides (700 mg) + L-glutamine (500 mg) for 6 months |
|
| Asthenozoospermic men [56] | Lactobacillus rhamnosus CECT8361 and Bifidobacterium longum CECT7347 for six weeks |
|
| Studies on animals | ||
| Stressed mice [84] | Lactobacillus rhamnosus Gorbach–Goldin An oral dose of 0.3 mL/mouse (1 × 1010 cells/mL) |
|
| Brioler [58] | Dietary supplementation with Bacillus amyloliquefaciens |
|
| Mice [85] | Lactobacillus rhamnosus PB01 (DSM 14870) supplementation |
|
| Aging and/or obese mice [54] | Lactobacillus reuteri ATCC PTA 6475 (3.5 × 105 cell/mouse/day) |
|
| Mice exposed to cyclophosphamide toxicity [86] | Dietary administration of Lactobacillus casei (105, 106, 107, 108 CFU/g) for 8 weeks |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. https://doi.org/10.3390/nu14040902
Hashem NM, Gonzalez-Bulnes A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients. 2022; 14(4):902. https://doi.org/10.3390/nu14040902
Chicago/Turabian StyleHashem, Nesrein M., and Antonio Gonzalez-Bulnes. 2022. "The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function" Nutrients 14, no. 4: 902. https://doi.org/10.3390/nu14040902