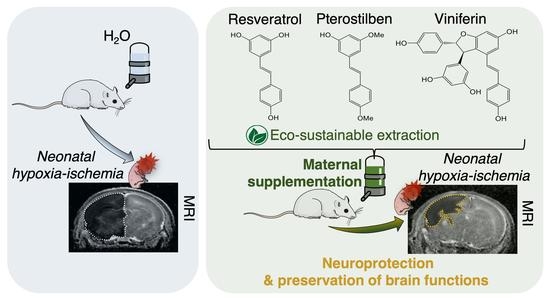
Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Chemistry
3.2. Comparison of the Neuroprotective Effects between RSV and Cocktail Maternal Supplementation for Two Weeks before the HI Event in Neonates
3.3. Can Maternal Polyphenolic Supplementation Be Also Curative?
3.4. Is the Neuroprotection Still Effective over Time?
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawn, J.E.; Blencowe, H.; Oza, S.; You, D.; Lee, A.C.; Waiswa, P.; Lalli, M.; Bhutta, Z.; Barros, A.J.; Christian, P.; et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy—Where to from Here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef] [Green Version]
- Vannucci, R.C.; Perlman, J.M. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997, 100, 1004–1014. [Google Scholar] [PubMed]
- Gonzalez, F.F.; Miller, S.P. Does perinatal asphyxia impair cognitive function without cerebral palsy? Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F454–F459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Gonzalez, F.F. Erythropoietin: A novel therapy for hypoxic-ischaemic encephalopathy? Dev. Med. Child. Neurol. 2015, 57 (Suppl. 3), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Pauliah, S.S.; Shankaran, S.; Wade, A.; Cady, E.B.; Thayyil, S. Therapeutic Hypothermia for Neonatal Encephalopathy in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e58834. [Google Scholar] [CrossRef] [Green Version]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumes, H.; Dumont, U.; Sanchez, S.; Mazuel, L.; Blanc, J.; Raffard, G.; Chateil, J.F.; Pellerin, L.; Bouzier-Sore, A.K. Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2020, 41, 342–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loren, D.J.; Seeram, N.P.; Schulman, R.N.; Holtzman, D.M. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr. Res. 2005, 57, 858–864. [Google Scholar] [CrossRef] [Green Version]
- West, T.; Atzeva, M.; Holtzman, D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Isac, S.; Panaitescu, A.M.; Spataru, A.; Iesanu, M.; Totan, A.; Udriste, A.; Cucu, N.; Peltecu, G.; Zagrean, L.; Zagrean, A.M. Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neurosci. Lett. 2017, 653, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Aden, U.; Fredholm, B.B.; Hagberg, H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr. Res. 1995, 38, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Arteaga, O.; Revuelta, M.; Uriguen, L.; Alvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karalis, F.; Soubasi, V.; Georgiou, T.; Nakas, C.T.; Simeonidou, C.; Guiba-Tziampiri, O.; Spandou, E. Resveratrol ameliorates hypoxia/ischemia-induced behavioral deficits and brain injury in the neonatal rat brain. Brain Res. 2011, 1425, 98–110. [Google Scholar] [CrossRef]
- Dumont, U.; Sanchez, S.; Repond, C.; Beauvieux, M.C.; Chateil, J.F.; Pellerin, L.; Bouzier-Sore, A.K.; Roumes, H. Neuroprotective Effect of Maternal Resveratrol Supplementation in a Rat Model of Neonatal Hypoxia-Ischemia. Front. Neurosci. 2020, 14, 616824. [Google Scholar] [CrossRef] [PubMed]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.F.; Pellerin, L.; Beauvieux, M.C.; Bouzier-Sore, A.K.; Roumes, H. Maternal consumption of piceatannol: A nutritional neuroprotective strategy against hypoxia-ischemia in rat neonates. Brain Res. 2019, 1717, 86–94. [Google Scholar] [CrossRef]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.F.; Deffieux, D.; Quideau, S.; Pellerin, L.; Beauvieux, M.C.; Bouzier-Sore, A.K.; Roumes, H. Maternal alcoholism and neonatal hypoxia-ischemia: Neuroprotection by stilbenoid polyphenols. Brain Res. 2020, 1738, 146798. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Song, T.; Yang, L.; Wang, X.; Yang, C.; Jiang, Y. Neuroprotective actions of pterostilbene on hypoxic-ischemic brain damage in neonatal rats through upregulation of heme oxygenase-1. Int. J. Dev. Neurosci. 2016, 54, 22–31. [Google Scholar] [CrossRef]
- Zeng, Q.; Lian, W.; Wang, G.; Qiu, M.; Lin, L.; Zeng, R. Pterostilbene induces Nrf2/HO-1 and potentially regulates NF-kappaB and JNK-Akt/mTOR signaling in ischemic brain injury in neonatal rats. 3 Biotech 2020, 10, 192. [Google Scholar] [CrossRef]
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Rioux Bilan, A. Trans epsilon viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS ONE 2019, 14, e0212663. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Gélinas, A.; Beaulieu, J.; Renaud, J.; Tardif-Pellerin, E.; Guillard, J.; Martinoli, M.-G. Anti-Apoptotic and Anti-Inflammatory Role of Trans ε-Viniferin in a Neuron-Glia Co-Culture Cellular Model of Parkinson’s Disease. Foods 2021, 10, 586. [Google Scholar] [CrossRef]
- Turner, R.C.; DiPasquale, K.; Logsdon, A.F.; Tan, Z.; Naser, Z.J.; Huber, J.D.; Rosen, C.L.; Lucke-Wold, B.P. The role for infarct volume as a surrogate measure of functional outcome following ischemic stroke. J. Syst. Integr. Neurosci. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.P.; Ramaswamy, V.; Michelson, D.; Barkovich, A.J.; Holshouser, B.; Wycliffe, N.; Glidden, D.V.; Deming, D.; Partridge, J.C.; Wu, Y.W.; et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 2005, 146, 453–460. [Google Scholar] [CrossRef]
- Rutherford, M.A.; Azzopardi, D.; Whitelaw, A.; Cowan, F.; Renowden, S.; Edwards, A.D.; Thoresen, M. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005, 116, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Macaya, A.; DeVivo, D.; Kenyon, N.; Janec, E.M. Neonatal hypoxic-ischemic or excitotoxic striatal injury results in a decreased adult number of substantia nigra neurons. Neuroscience 1992, 50, 559–569. [Google Scholar] [CrossRef]
- Hatch, B.; Healey, D.M.; Halperin, J.M. Associations between birth weight and attention-deficit/hyperactivity disorder symptom severity: Indirect effects via primary neuropsychological functions. J. Child. Psychol. Psychiatry 2014, 55, 384–392. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.-K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 773. https://doi.org/10.3390/nu14040773
Roumes H, Sanchez S, Benkhaled I, Fernandez V, Goudeneche P, Perrin F, Pellerin L, Guillard J, Bouzier-Sore A-K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients. 2022; 14(4):773. https://doi.org/10.3390/nu14040773
Chicago/Turabian StyleRoumes, Hélène, Stéphane Sanchez, Imad Benkhaled, Valentin Fernandez, Pierre Goudeneche, Flavie Perrin, Luc Pellerin, Jérôme Guillard, and Anne-Karine Bouzier-Sore. 2022. "Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia" Nutrients 14, no. 4: 773. https://doi.org/10.3390/nu14040773