Choline Kinetics in Neonatal Liver, Brain and Lung—Lessons from a Rodent Model for Neonatal Care
Abstract
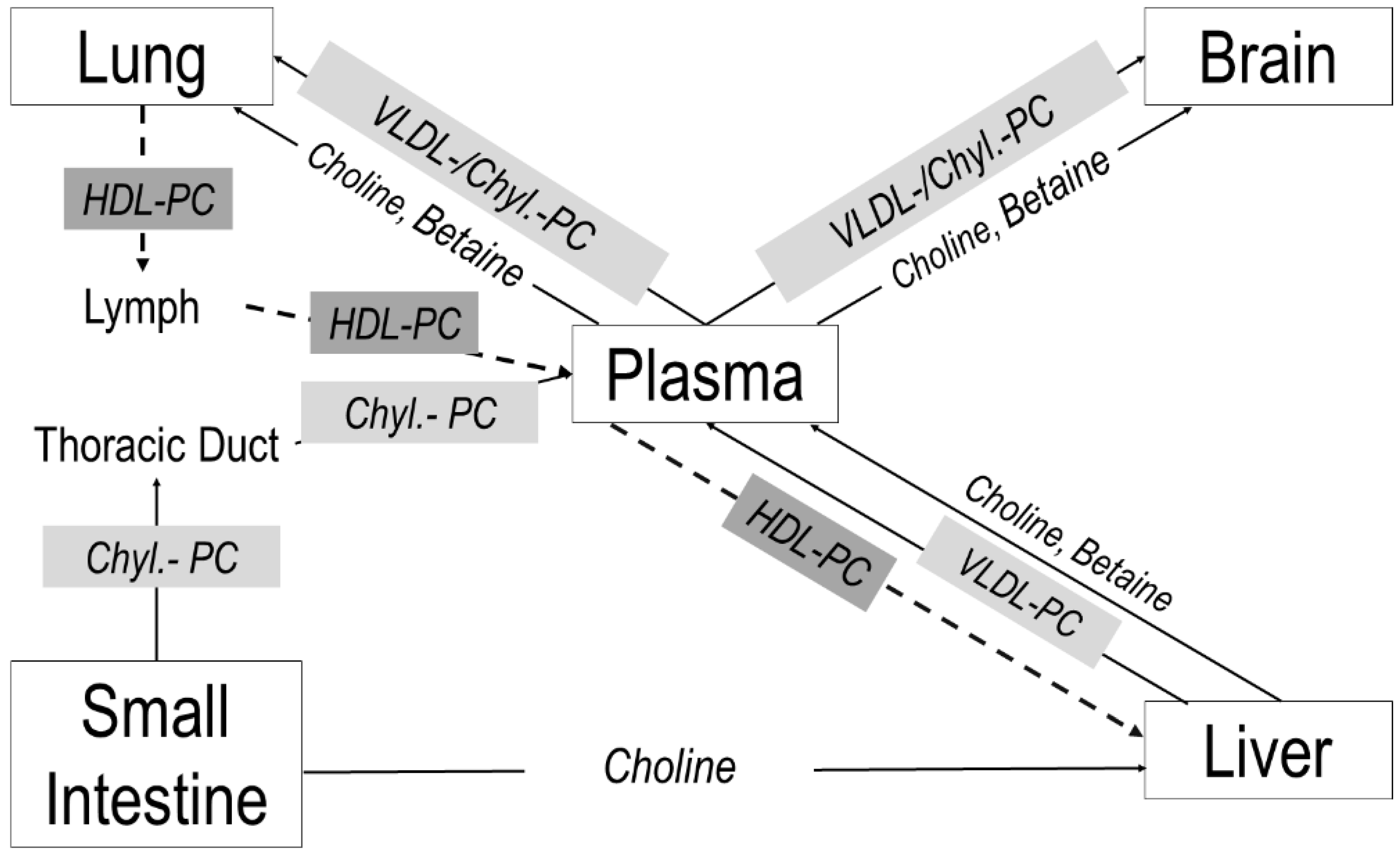
:1. Introduction
1.1. Impact of Choline Metabolism on Preterm Infant Development
1.2. Targeting Choline Metabolism
2. Materials and Methods
2.1. Materials
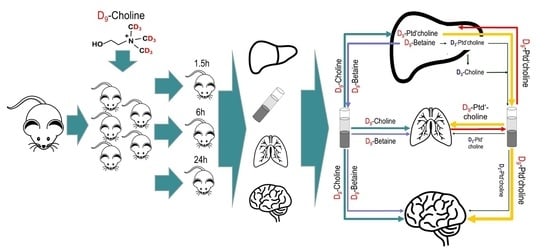
2.2. Animal Experiments
2.3. Blood Plasma and Tissue Extraction
2.4. Analysis of Choline, Water Soluble Choline Metabolites and Phospholipids
2.5. Statistics
3. Results
3.1. Pool Sizes of Unlabeled Choline and Its Water-Soluble Metabolites
3.2. Pool Sizes of Unlabeled Choline-Containing Phospholipids
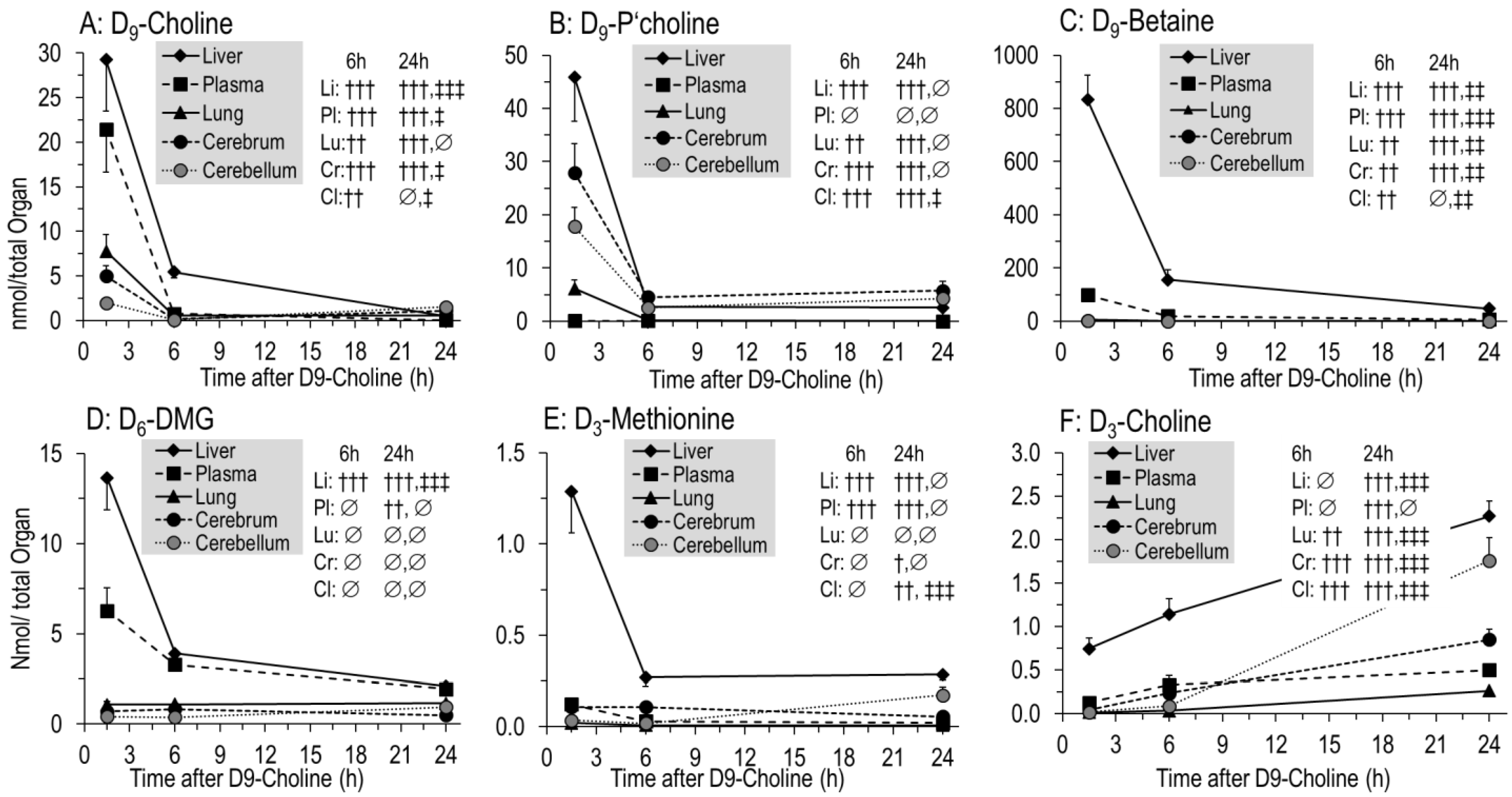
3.3. Kinetics of D9-Choline and Its Water-Soluble Deuterated Metabolites
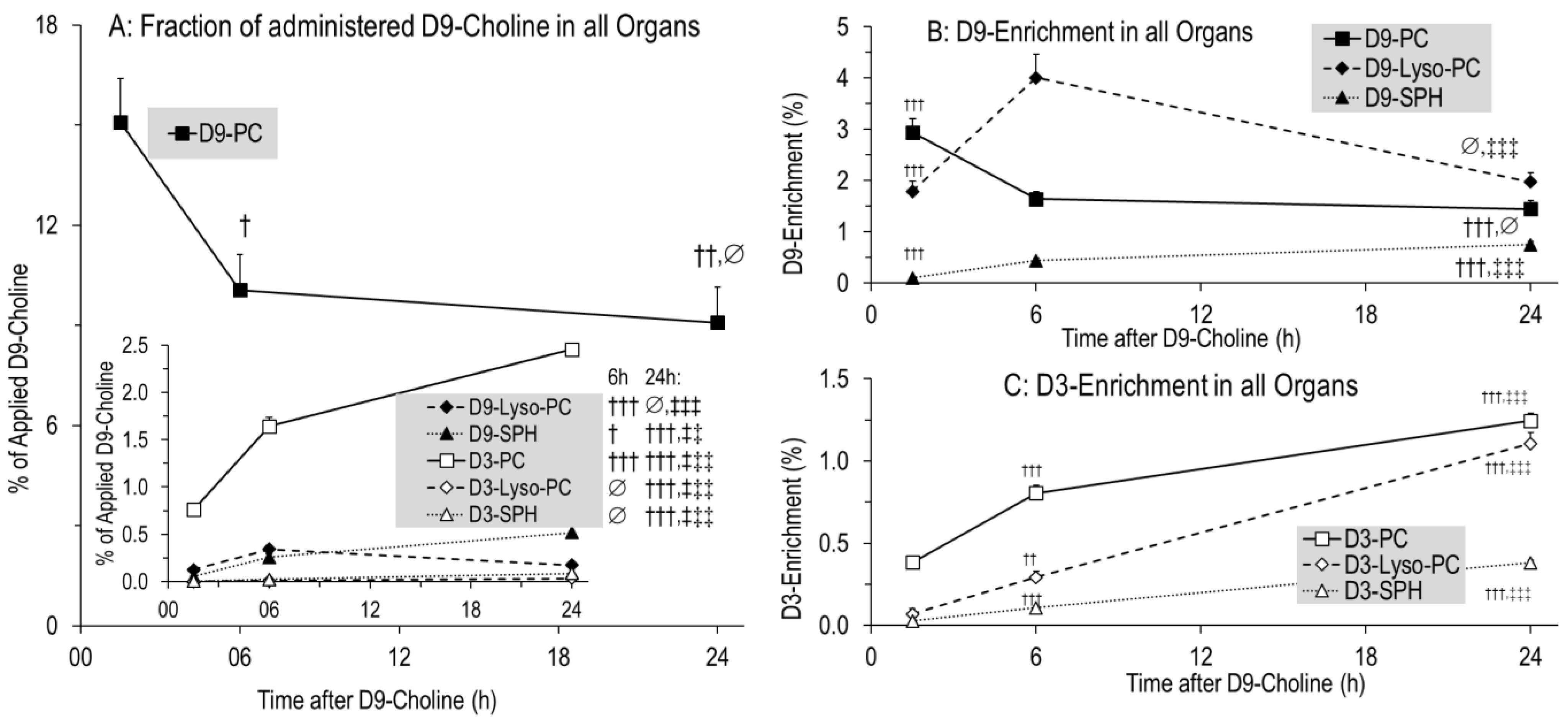
3.4. Kinetics of Phospholipids Containing D9- or D3-Choline
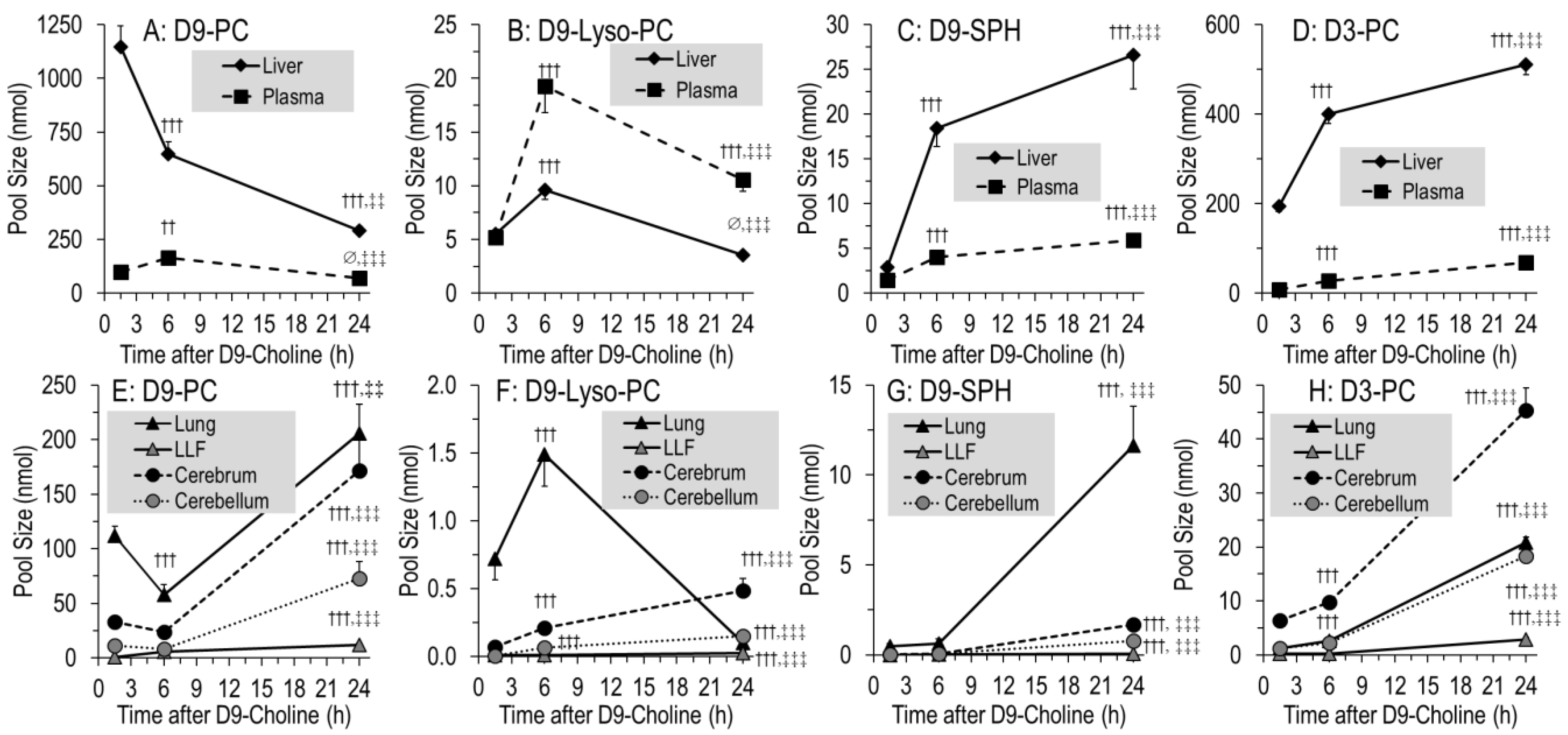
3.5. Organ-Specific Kinetics of Phospholipids Containing D9- or D3-Choline
3.6. Kinetics of Newly Synthesized PC Sub-Groups
4. Discussion
4.1. Overall Concentrations and Pools of Choline and Choline Metabolites in Targeted Organs
4.2. Overall Kinetics of D9-Choline, Its Water-Soluble Metabolites and D9-PC
4.3. Overall and Hepatic Kinetics of D9-PC Precursors for De Novo PC Synthesis
4.4. 1.5–6 h Kinetics of D9-PC in the Lung Compared to the Liver
4.5. 1.5–6 h Kinetics of D9-PC in LLF
4.6. 1.5–6 h Kinetics of D9-PC in the Cerebrum and Cerebellum
4.7. 6–24 h Kinetics: D9-PC Accretion in the Lung and Brain
4.8. Organotypic Composition and Metabolism of D9-PC Subgroups
4.9. D9-Betaine Metabolism, Synthesis of Endogenous D3-PC, D3-Choline and D9-SPH
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernhard, W.; Poets, C.F.; Franz, A.R. Choline and choline-related nutrients in regular and preterm infant growth. Eur. J. Nutr. 2019, 58, 931–945. [Google Scholar] [CrossRef]
- Bernhard, W.; Raith, M.; Kunze, R.; Koch, V.; Heni, M.; Maas, C.; Abele, H.; Poets, C.F.; Franz, A.R. Choline concentrations are lower in postnatal plasma of preterm infants than in cord plasma. Eur. J. Nutr. 2015, 54, 733–741. [Google Scholar] [CrossRef]
- National Academy of Sciences. Dietary Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. Available online: https://www.ncbi.nlm.nih.gov/books/NBK114310/pdf/Bookshelf_NBK114310.pdf (accessed on 30 December 2021).
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhard, W.; Full, A.; Arand, J.; Maas, C.; Poets, C.F.; Franz, A.R. Choline supply of preterm infants: Assessment of dietary intake and pathophysiological considerations. Eur. J. Nutr. 2013, 52, 1269–1278. [Google Scholar] [CrossRef]
- Hollenbeck, C.B. An introduction to the nutrition and metabolism of choline. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 100–113. [Google Scholar] [CrossRef]
- Lockman, P.R.; Allen, D.D. The transport of choline. Drug Dev. Ind. Pharm. 2002, 28, 749–771. [Google Scholar] [CrossRef]
- Li, Z.; Agellon, L.B.; Vance, D.E. Choline redistribution during adaptation to choline deprivation. J. Biol. Chem. 2007, 282, 10283–10289. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H. Dietary choline: Biochemistry, physiology, and pharmacology. Annu. Rev. Nutr. 1981, 1, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Agellon, L.B.; Vance, D.E. Phosphatidylcholine homeostasis and liver failure. J. Biol. Chem. 2005, 280, 37798–37802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchman, A.L.; Dubin, M.; Jenden, D.; Moukarzel, A.; Roch, M.H.; Rice, K.; Gornbein, J.; Ament, M.E.; Eckhert, C.D. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 1992, 102, 1363–1370. [Google Scholar] [CrossRef]
- Gyamfi, M.A.; Damjanov, I.; French, S.; Wan, Y.J. The pathogenesis of ethanol versus methionine and choline deficient diet-induced liver injury. Biochem. Pharm. 2008, 75, 981–995. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Kuipers, F.; Havinga, R.; Ando, H.; Vance, D.E.; Jacobs, R.L.; van der Veen, J.N. Impaired hepatic phosphatidylcholine synthesis leads to cholestasis in Mice challenged with a high-fat diet. Hepatol. Commun. 2019, 3, 262–276. [Google Scholar] [CrossRef]
- Bernhard, W.; Maas, C.; Shunova, A.; Mathes, M.; Böckmann, K.; Bleeker, C.; Vek, J.; Poets, C.F.; Schleicher, E.; Franz, A.R. Transport of long-chain polyunsaturated fatty acids in preterm infant plasma is dominated by phosphatidylcholine. Eur. J. Nutr. 2018, 57, 2105–2112. [Google Scholar] [CrossRef]
- Bates, S.R.; Tao, J.Q.; Yu, K.J.; Borok, Z.; Crandall, E.D.; Collins, H.L.; Rothblat, G.H. Expression and biological activity of ABCA1 in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Agellon, L.B.; Vance, D.E. A role for high density lipoproteins in hepatic phosphatidylcholine homeostasis. Biochim. Biophys. Acta 2007, 1771, 893–900. [Google Scholar] [CrossRef]
- Zeisel, S.H. The fetal origins of memory: The role of dietary choline in optimal brain development. J. Pediatr. 2006, 149 (Suppl. S5), 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, S.H. The supply of choline is important for fetal progenitor cells. Semin. Cell Dev. Biol. 2011, 22, 624–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, H.K.; Dickerson, J.W.; Waterlow, J.C. Changes in content and composition of brain phospholipids in malnourished children. Am. J. Clin. Nutr. 1979, 32, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A. Brain Injury in the Preterm Infant: New Horizons for Pathogenesis and Prevention. Pediatr. Neurol. 2015, 53, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Pynn, C.J.; Jaworski, A.; Rau, G.A.; Hohlfeld, J.M.; Freihorst, J.; Poets, C.F.; Stoll, D.; Postle, A.D. Mass spectrometric analysis of surfactant metabolism in human volunteers using deuteriated choline. Am. J. Respir. Crit. Care Med. 2004, 170, 54–58. [Google Scholar] [CrossRef]
- Pynn, C.J.; Henderson, N.G.; Clark, H.; Koster, G.; Bernhard, W.; Postle, A.D. Specificity and rate of human and mouse liver and plasma hosphatidylcholine synthesis analyzed In Vivo. J. Lipid Res. 2011, 52, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olofsson, S.O.; Gustafson, A.; Svanberg, U. Studies on human serum high-density lipoproteins (HDL). IV. Isolation of lipoprotein families after incubation of HDL. Scand. J. Clin. Lab. Investig. 1975, 35, 363–371. [Google Scholar] [CrossRef]
- Bernhard, W.; Böckmann, K.; Maas, C.; Mathes, M.; Hövelmann, J.; Shunova, A.; Hund, V.; Schleicher, E.; Poets, C.F.; Franz, A.R. Combined choline and DHA supplementation, a randomized controlled trial. Eur. J. Nutr. 2020, 59, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W. Choline in cystic fibrosis: Relations to pancreas insufficiency, enterohepatic cycle, PEMT and intestinal microbiota. Eur. J. Nutr. 2021, 60, 1737–1759. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Vance, D.E. Physiological roles of phosphatidylethanolamine N-methyltransferase. Biochim. Biophys. Acta 2013, 1831, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Gesche, J.; Fehrenbach, H.; Koslowski, R.; Ohler, F.M.; Pynn, C.J.; Griese, M.; Poets, C.F.; Bernhard, W. rhKGF stimulates lung surfactant production in neonatal rats In Vivo. Pediatr. Pulmonol. 2011, 46, 882–895. [Google Scholar] [CrossRef]
- Raith, M.; Schaal, K.; Koslowski, R.; Fehrenbach, H.; Poets, C.F.; Schleicher, E.; Bernhard, W. Effects of recombinant human keratinocyte growth factor on surfactant, plasma, and liver phospholipid homeostasis in hyperoxic neonatal rats. J. Appl. Physiol. 2012, 112, 1317–1328. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Schmiedl, A.; Koster, G.; Orgeig, S.; Acevedo, C.; Poets, C.F.; Postle, A.D. Developmental changes in rat surfactant lipidomics in the context of species variability. Pediatr. Pulmonol. 2007, 42, 794–804. [Google Scholar] [CrossRef]
- Johnson, D.; Roofe, P.G. Blood constituents of normal newborn rats and those exposed to low oxygen tension during gestation; weight of newborn and litter size also considered. Anat. Rec. 1965, 153, 303–309. [Google Scholar] [CrossRef]
- Shmukler, M. Density of Blood. The Physics Factbook-An Encyclopedia of Scientific Essays. 2004. Available online: https://hypertextbook.com/facts/2004/MichaelShmukler.shtml (accessed on 28 July 2021).
- Bligh., E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1956, 226, 497–507. [Google Scholar] [CrossRef]
- Baldwin, D.R.; Wise, R.; Andrews, J.M.; Honeybourne, D. Microlavage: A technique for determining the volume of epithelial lining fluid. Thorax 1991, 46, 658–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhard, W.; Raith, M.; Koch, V.; Kunze, R.; Maas, C.; Abele, H.; Poets, C.F.; Franz, A.R. Plasma phospholipids indicate impaired fatty acid homeostasis in preterm infants. Eur. J. Nutr. 2014, 53, 1533–1547. [Google Scholar] [CrossRef]
- Bernhard, W.; Raith, M.; Koch, V.; Maas, C.; Abele, H.; Poets, C.F.; Franz, A.R. Developmental changes in polyunsaturated fetal plasma phospholipids and feto-maternal plasma phospholipid ratios and their association with bronchopulmonary dysplasia. Eur. J. Nutr. 2016, 55, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metabolism 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Byard, R.W.; Simmer, K.; Gibson, R.A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 1994, 60, 189–194. [Google Scholar] [CrossRef]
- Dombrowsky, H.; Clark, G.T.; Rau, G.A.; Bernhard, W.; Postle, A.D. Molecular species compositions of lung and pancreas phospholipids in the cftr(tm1HGU/tm1HGU) cystic fibrosis mouse. Pediatr. Res. 2003, 53, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Lange, R.; Graepler-Mainka, U.; Engel, C.; Machann, J.; Hund, V.; Shunova, A.; Hector, A.; Riethmüller, J. Choline Supplementation in Cystic Fibrosis-The Metabolic and Clinical Impact. Nutrients 2019, 11, 656. [Google Scholar] [CrossRef] [Green Version]
- Maas, C.; Franz, A.R.; Shunova, A.; Mathes, M.; Bleeker, C.; Poets, C.F.; Schleicher, E.; Bernhard, W. Choline and polyunsaturated fatty acids in preterm infants’ maternal milk. Eur. J. Nutr. 2017, 56, 1733–1742. [Google Scholar] [CrossRef]
- Shunova, A.; Böckmann, K.A.; Minarski, M.; Franz, A.R.; Wiechers, C.; Poets, C.F.; Bernhard, W. Choline content of term and preterm infant formulae compared to expressed breast milk-How do we justify the discrepancies? Nutrients 2020, 12, 3815. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.; Mar, M.H.; Ranasinghe, A.; Swenberg, J.A.; Zeisel, S.H. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 2002, 74, 4734–4740. [Google Scholar] [CrossRef] [PubMed]
- Stocker, J.T.; Dehner, L.P.; Husain, A.N. Means and standard deviations of weights and measurements of lifeborn infants by body weight (Appendix 28-29). In Stocker & Dehner’s Pediatric Pathology, 2nd ed.; Lippinkott Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 1302–1304. [Google Scholar]
- Burg, M.B.; Peters, E.M. Effects of glycine betaine and glycerophosphocholine on thermal stability of ribonuclease. Am. J. Physiol. 1998, 274, F762–F765. [Google Scholar] [CrossRef] [PubMed]
- Slow, S.; Lever, M.; Cambers, S.T.; George, P.M. Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol. Res. 2009, 58, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Hamad, D.; García-Pérez, A.; Ferraris, J.D.; Peters, E.M.; Burg, M.B. Induction of gene expression by heat shock versus osmotic stress. Am. J. Physiol. 1994, 267, F28–F34. [Google Scholar] [CrossRef]
- Grothe, J.; Riethmüller, J.; Tschürtz, S.M.; Raith, M.; Pynn, C.J.; Stoll, D.; Bernhard, W. Plasma phosphatidylcholine alterations in cystic fibrosis patients, impaired metabolism and correlation with lung function and inflammation. Cell Physiol. Biochem. 2015, 35, 1437–1453. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.R.; Tao, J.Q.; Collins, H.L.; Francone, O.L.; Rothblat, G.H. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L980–L989. [Google Scholar] [CrossRef]
- The LipidWeb—Plasma Lipoproteins. Available online: https//www.lipidhome.co.uk/lipids/simple/lipoprot/index.htm (accessed on 29 April 2020).
- Nilsson, Å.; Duan, R.D. Pancreatic and mucosal enzymes in choline phospholipid digestion. Am. J. Physiol. Gastrointest Liver Physiol. 2019, 316, G425–G445. [Google Scholar] [CrossRef]
- Bernhard, W. Regulation of Surfactant-Associated Phospholipid Synthesis and Secretion. In Fetal and Neonatal Physiology, 5th ed.; Richard, A., Polin, R.A., Steven, H., Abman, S.A., Rowitch, D.H., William, E., Benitz, W.E., Eds.; Elsevier: Amsterdam, NL, USA, 2016; pp. 813–824. [Google Scholar]
- Goss, K.C.W.; Goss, V.M.; Townsend, J.P.; Koster, G.; Clark, H.W.; Postle, A.D. Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants, implications for choline and PUFA metabolism. Am. J. Clin. Nutr. 2020, 112, 1438–1447. [Google Scholar] [CrossRef]
- Böckmann, K.A.; von Stumpff, A.; Bernhard, W.; Shunova, A.; Minarski, M.; Frische, B.; Warmann, S.; Schleicher, E.; Poets, C.F.; Franz, A.R. Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants. Eur. J. Nutr. 2021, 60, 861–872. [Google Scholar] [CrossRef]
- Koletzko, B.; Bergmann, K.; Brenna, J.T.; Calder, P.C.; Campoy, C.; Clandinin, M.T.; Colombo, J.; Daly, M.; Decsi, T.; Demmelmair, H.; et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 2020, 111, 10–16. [Google Scholar] [CrossRef]
- Aureli, M.; Schiumarini, D.; Loberto, N.; Bassi, R.; Tamanini, A.; Mancini, G.; Tironi, M.; Munari, S.; Cabrini, G.; Dechecchi, M.C.; et al. Unravelling the role of sphingolipids in cystic fibrosis lung disease. Chem. Phys. Lipids 2016, 200, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassmé, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Mastrigt, E.; Zweekhorst, S.; Bol, B.; Tibboel, J.; van Rosmalen, J.; Samsom, J.N.; Kroon, A.A.; de Jongste, J.C.; Reiss, I.K.M.; Post, M.; et al. Ceramides in tracheal aspirates of preterm infants: Marker for bronchopulmonary dysplasia. PLoS ONE 2018, 13, e0185969. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Muñoz, K.D.; Xu, J.; Voynow, J.A. Tracheal aspirate VEGF and sphingolipid metabolites in the preterm infant with later development of bronchopulmonary dysplasia. Pediatr. Pulmonol. 2018, 53, 1046–1052. [Google Scholar] [CrossRef]



| (A) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water-Soluble | Organ Weight | Choline | Phospho-choline | CDP-Choline | All PC Precursors | GPC | Betaine | DMG | Choline Derivatives | TMAO | Methionine |
| Organ | mg | Pool Size (nmol) | |||||||||
| Liver | 831 ± 21 | 194 ± 13 | 98 ± 8 | 1.0 ± 0.2 | 293 ± 19 | 109 ± 8 | 1631 ± 125 | 39 ± 2 | 1657 ± 125 | 1.0 ± 0.3 | 25 ± 3 |
| Plasma 1 | 2151 ± 26 | 75 ± 7 ††† | 3.8 ± 0.6 ††† | 0.4 ± 0.1 | 79 ± 7 ††† | 9 ± 1 ††† | 242 ± 22 ††† | 16 ± 1 | 252 ± 23 ††† | 3.0 ± 0.8 ††† | 4.4 ± 0.3 ††† |
| Lung | 282 ± 4 | 92 ± 12 ††† | 29 ± 2 ††† | 0.2 ± 0.03 | 122 ± 13 ††† | 34 ± 2 ††† | 29 ± 2 †††, ‡‡‡ | 2.6 ± 0.2 | 30 ± 2 †††, ‡‡‡ | 1.6 ± 0.4 ††† | 3.8 ± 0.5 ††† |
| Cerebrum | 881 ± 37 | 230 ± 17 | 375 ± 31 ††† | 16 ± 2 | 621 ± 45 ††† | 55 ± 13 ††† | 21 ± 2 †††, ‡‡‡ | 7.7 ± 0.6 | 26 ± 2 †††, ‡‡‡ | 0.31 ± 0.07 | 24.6 ± 4.1 |
| Cerebellum | 268 ± 14 | 193 ± 29 | 329 ± 36 ††† | 13 ± 1 | 533 ± 63 ††† | 36 ± 7 ††† | 15 ± 2 †††, ‡‡‡ | 6.5 ± 0.9 | 20 ± 2 †††, ‡‡‡ | 0.22 ± 0.03 | 19.2 ± 4.9 |
| Total | 4411 ± 102 | 785 ± 36 | 834 ± 43 | 30 ± 2 | 1647 ± 68 | 243 ± 16 | 1939 ± 142 | 72 ± 3 | 1986 ± 143 # | 6.2 ± 1.4 | 77 ± 7 |
| (B) | |||||||||||
| B: Lipids | Organ Weight | PC | Lyso-PC | SPH | Choline Phospholipids | ||||||
| Organ | mg | Pool Size (µmol) | |||||||||
| Liver | 831 ± 21 | 19.9 ± 1.0 | 0.19 ± 0.010 | 2.71 ± 0.10 | 22.8 ± 1.0 | ||||||
| Plasma 1 | 2151 ± 26 | 4.7 ± 0.2 ††† | 0.44 ± 0.03 ††† | 0.39 ± 0.02 ††† | 5.5 ± 0.2 ††† | ||||||
| Lung | 282 ± 4 | 5.6 ± 0.3 ††† | 0.052 ± 0.004 †††, ‡‡‡ | 1.0 ± 0.1 †††, ‡‡‡ | 6.7 ± 0.3 ††† | ||||||
| LLF | - | 0.50 ± 0.04 ††† | 0.002 ± 0.001 ††† | 0.01 ± 0.00 †††, ‡‡‡ | 0.51 ± 0.05 ††† | ||||||
| Cerebrum | 881 ± 37 | 19.0 ± 1.6 | 0.063 ± 0.007 †††, ‡‡‡ | 0.87 ± 0.09 †††, ‡‡‡ | 20.0 ± 1.701 | ||||||
| Cerebellum | 268 ± 14 | 5.4 ± 0.6 ††† | 0.019 ± 0.003 †††, ‡‡‡ | 0.35 ± 0.03 ††† | 5.8 ± 0.601 ††† | ||||||
| Total | 4411 ± 102 | 55.2 ± 3.7 | 0.765 ± 0.049 | 5.3 ± 0.3 | 61.3 ± 3.9 | ||||||
| Time | Water-Soluble D9-Choline- Derived Metabolites | D9-Labeled Phospholipids | D3-Labeled Phospholipids |
|---|---|---|---|
| 1.5 h | 11.8 ± 1.5% | 15.3 ± 1.4% | 0.8 ± 0.1% |
| 6 h | 2.1 ± 0.3% ††† | 10.6 ± 1.1% ††† | 1.6 ± 0.1% ††† |
| 24 h | 1.0 ± 0.1% †††, ‡‡‡ | 9.7 ± 1.1% ††† | 2.5 ± 0.1% †††, ‡‡‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernhard, W.; Raith, M.; Shunova, A.; Lorenz, S.; Böckmann, K.; Minarski, M.; Poets, C.F.; Franz, A.R. Choline Kinetics in Neonatal Liver, Brain and Lung—Lessons from a Rodent Model for Neonatal Care. Nutrients 2022, 14, 720. https://doi.org/10.3390/nu14030720
Bernhard W, Raith M, Shunova A, Lorenz S, Böckmann K, Minarski M, Poets CF, Franz AR. Choline Kinetics in Neonatal Liver, Brain and Lung—Lessons from a Rodent Model for Neonatal Care. Nutrients. 2022; 14(3):720. https://doi.org/10.3390/nu14030720
Chicago/Turabian StyleBernhard, Wolfgang, Marco Raith, Anna Shunova, Stephan Lorenz, Katrin Böckmann, Michaela Minarski, Christian F. Poets, and Axel R. Franz. 2022. "Choline Kinetics in Neonatal Liver, Brain and Lung—Lessons from a Rodent Model for Neonatal Care" Nutrients 14, no. 3: 720. https://doi.org/10.3390/nu14030720