Saffron, Its Active Components, and Their Association with DNA and Histone Modification: A Narrative Review of Current Knowledge
Abstract
:1. Introduction
2. Interaction of Saffron with Linker Histone H1, B-DNA, and G-Quadruples DNA
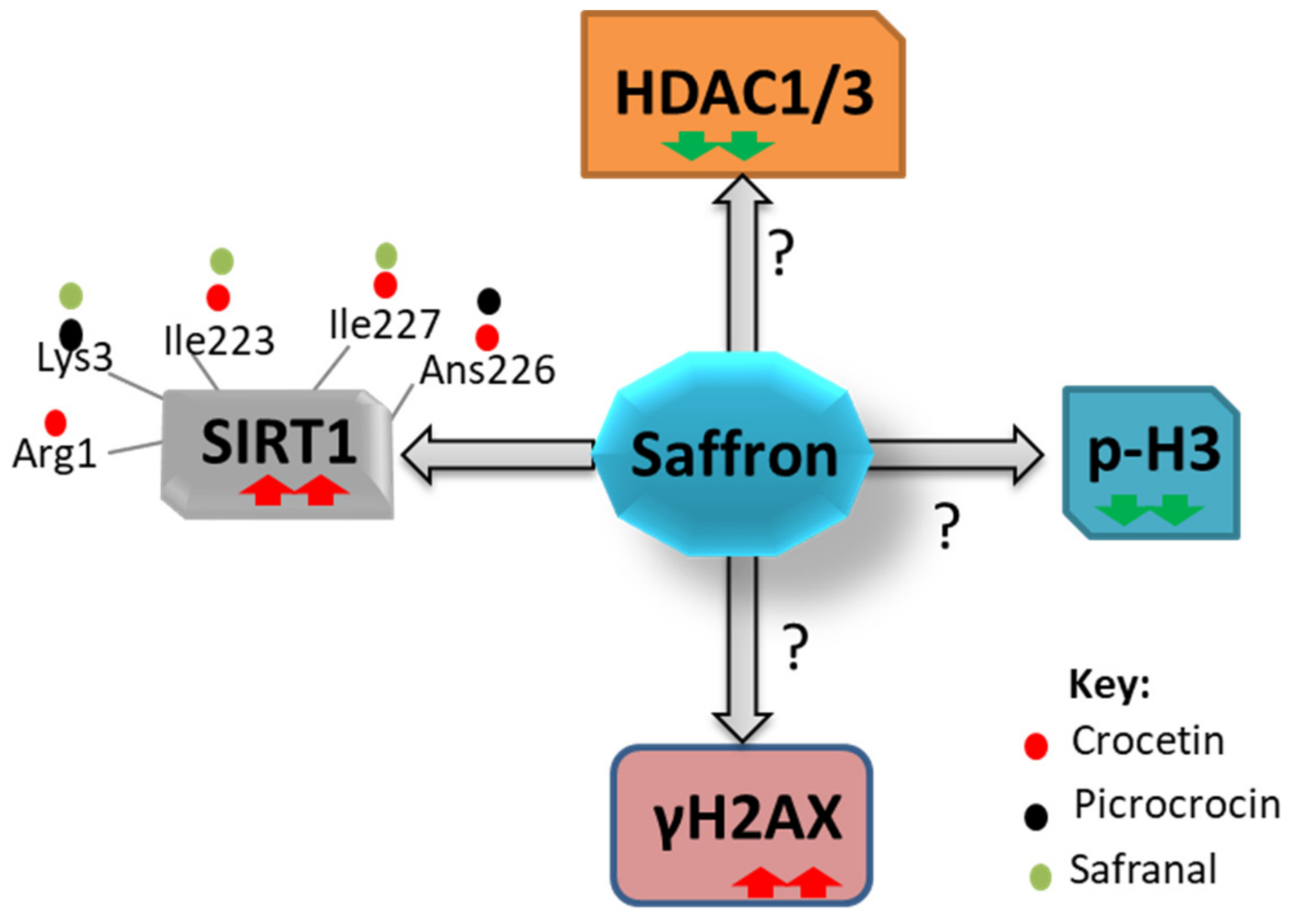
3. Saffron Modulates the Expression Levels of Histone Deacetylases, Phosphorylated H3, and γH2AX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledge
Conflicts of Interest
References
- You, J.S.; Jones, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Jose Bagur, M.; Alonso Salinas, G.L.; Jimenez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martinez-Tome, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother. Res. 2005, 19, 997–1000. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Tabatabaee, A.; Byrami, G. The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized guinea-pigs. Phytomedicine 2012, 19, 904–911. [Google Scholar] [CrossRef]
- Razavi, M.; Hosseinzadeh, H.; Abnous, K.; Motamedshariaty, V.S.; Imenshahidi, M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J. Basic Med. Sci. 2013, 16, 64–72. [Google Scholar]
- Amin, A.; Hamza, A.A.; Daoud, S.; Khazanehdari, K.; Hrout, A.A.; Baig, B.; Chaiboonchoe, A.; Adrian, T.E.; Zaki, N.; Salehi-Ashtiani, K. Saffron-Based Crocin Prevents Early Lesions of Liver Cancer: In vivo, In vitro and Network Analyses. Recent Pat. Anticancer Drug Discov. 2016, 11, 121–133. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Ma, Z.; Zhao, J. Crocin exerts anti-inflammatory and anti-catabolic effects on rat intervertebral discs by suppressing the activation of JNK. Int. J. Mol. Med. 2015, 36, 1291–1299. [Google Scholar] [CrossRef]
- Moallem, S.A.; Hosseinzadeh, H.; Farahi, S. A study of acute and chronic anti-nociceptive and anti-inflammatory effects of thiamine in mice. Iran Biomed J. 2008, 12, 173–178. [Google Scholar]
- Xiong, Y.; Wang, J.; Yu, H.; Zhang, X.; Miao, C. Anti-asthma potential of crocin and its effect on MAPK signaling pathway in a murine model of allergic airway disease. Immunopharmacol. Immunotoxicol. 2015, 37, 236–243. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum. Psychopharmacol. 2014, 29, 517–527. [Google Scholar] [CrossRef]
- Ghasemi, T.; Abnous, K.; Vahdati, F.; Mehri, S.; Razavi, B.M.; Hosseinzadeh, H. Antidepressant Effect of Crocus sativus Aqueous Extract and its Effect on CREB, BDNF, and VGF Transcript and Protein Levels in Rat Hippocampus. Drug Res. 2015, 65, 337–343. [Google Scholar] [CrossRef]
- Zamani Taghizadeh Rabe, S.; Sahebari, M.; Mahmoudi, Z.; Hosseinzadeh, H.; Haghmorad, D.; Tabasi, N.; Rastin, M.; Khazaee, M.; Mahmoudi, M. Inhibitory effect of Crocus sativus L. ethanol extract on adjuvant-induced arthritis. Food Agric. Immunol. 2015, 26, 170–180. [Google Scholar] [CrossRef]
- Mousavi, M.; Baharara, J.; Shahrokhabadi, K. The Synergic Effects of Crocus Sativus L. and Low Frequency Electromagnetic Field on VEGFR2 Gene Expression in Human Breast Cancer Cells. Avicenna J. Med. Biotechnol. 2014, 6, 123–127. [Google Scholar]
- Umigai, N.; Tanaka, J.; Tsuruma, K.; Shimazawa, M.; Hara, H. Crocetin, a carotenoid derivative, inhibits VEGF-induced angiogenesis via suppression of p38 phosphorylation. Curr. Neurovasc. Res. 2012, 9, 102–109. [Google Scholar] [CrossRef]
- Bie, X.; Chen, Y.; Zheng, X.; Dai, H. The role of crocetin in protection following cerebral contusion and in the enhancement of angiogenesis in rats. Fitoterapia 2011, 82, 997–1002. [Google Scholar] [CrossRef]
- Higashino, S.; Sasaki, Y.; Giddings, J.C.; Hyodo, K.; Sakata, S.F.; Matsuda, K.; Horikawa, Y.; Yamamoto, J. Crocetin, a carotenoid from Gardenia jasminoides Ellis, protects against hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Phytother. Res. 2014, 28, 1315–1319. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H.; Javadpour, Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010, 24, 990–994. [Google Scholar] [CrossRef]
- Liu, M.; Amini, A.; Ahmad, Z. Safranal and its analogs inhibit Escherichia coli ATP synthase and cell growth. Int. J. Biol. Macromol. 2017, 95, 145–152. [Google Scholar] [CrossRef]
- Eslami, M.; Bayat, M.; Mozaffari Nejad, A.S.; Sabokbar, A.; Anvar, A.A. Effect of polymer/nanosilver composite packaging on long-term microbiological status of Iranian saffron (Crocus sativus L.). Saudi J. Biol. Sci. 2016, 23, 341–347. [Google Scholar] [CrossRef] [PubMed]
- De Monte, C.; Bizzarri, B.; Gidaro, M.C.; Carradori, S.; Mollica, A.; Luisi, G.; Granese, A.; Alcaro, S.; Costa, G.; Basilico, N.; et al. Bioactive compounds of Crocus sativus L. and their semi-synthetic derivatives as promising anti-Helicobacter pylori, anti-malarial and anti-leishmanial agents. J. Enzyme Inhib. Med. Chem. 2015, 30, 1027–1033. [Google Scholar] [CrossRef]
- Razavi, B.M.; Hosseinzadeh, H. Saffron as an antidote or a protective agent against natural or chemical toxicities. Daru 2015, 23, 31. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Shaterzadeh, H.; Forouzanfar, F.; Hosseinzadeh, H. Neuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemia. Folia Neuropathol. 2017, 55, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Samini, F.; Azimi-Nezhad, M.; Farkhondeh, T. Anti-oxidative effects of safranal on immobilization-induced oxidative damage in rat brain. Neurosci. Lett. 2017, 659, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Rahimi, A. Effect of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic Acid treatment in anesthetized rats. Planta Med. 2008, 74, 1441–1445. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Avicenna’s (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): A review. Phytother. Res. 2013, 27, 475–483. [Google Scholar] [CrossRef]
- Hemmati, M.; Zohoori, E.; Mehrpour, O.; Karamian, M.; Asghari, S.; Zarban, A.; Nasouti, R. Anti-atherogenic potential of jujube, saffron and barberry: Anti-diabetic and antioxidant actions. EXCLI J. 2015, 14, 908–915. [Google Scholar] [CrossRef]
- Nader, M.; Chahine, N.; Salem, C.; Chahine, R. Saffron (Crocus sativus) pretreatment confers cardioprotection against ischemia-reperfusion injuries in isolated rabbit heart. J. Physiol. Biochem. 2016, 72, 711–719. [Google Scholar] [CrossRef]
- Goyal, S.N.; Arora, S.; Sharma, A.K.; Joshi, S.; Ray, R.; Bhatia, J.; Kumari, S.; Arya, D.S. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine 2010, 17, 227–232. [Google Scholar] [CrossRef]
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Yusof, B.N.; Noor, S.M. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.P.E.; Stasinopoulou, M.; Konstandi, O.A.; Kenoutis, C.; Kakazanis, Z.I.; Rizakou, A.; Kostomitsopoulos, N.; Valsami, G. Crocus sativus L. aqueous extract reduces atherogenesis, increases atherosclerotic plaque stability and improves glucose control in diabetic atherosclerotic animals. Atherosclerosis 2018, 268, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Farshid, A.A.; Tamaddonfard, E.; Moradi-Arzeloo, M.; Mirzakhani, N. The effects of crocin, insulin and their co-administration on the heart function and pathology in streptozotocin-induced diabetic rats. Avicenna J. Phytomed. 2016, 6, 658–670. [Google Scholar] [PubMed]
- Konstantopoulos, P.; Doulamis, I.P.; Tzani, A.; Korou, M.L.; Agapitos, E.; Vlachos, I.S.; Pergialiotis, V.; Verikokos, C.; Mastorakos, G.; Katsilambros, N.L.; et al. Metabolic effects of Crocus sativus and protective action against non-alcoholic fatty liver disease in diabetic rats. Biomed. Rep. 2017, 6, 513–518. [Google Scholar] [CrossRef]
- Mashmoul, M.; Azlan, A.; Mohtarrudin, N.; Mohd Yusof, B.N.; Khaza’ai, H.; Khoo, H.E.; Farzadnia, M.; Boroushaki, M.T. Protective effects of saffron extract and crocin supplementation on fatty liver tissue of high-fat diet-induced obese rats. BMC Complement. Altern. Med. 2016, 16, 401. [Google Scholar] [CrossRef] [PubMed]
- Omidi, A.; Riahinia, N.; Montazer Torbati, M.B.; Behdani, M.A. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J. Phytomed. 2014, 4, 330–336. [Google Scholar]
- Lari, P.; Abnous, K.; Imenshahidi, M.; Rashedinia, M.; Razavi, M.; Hosseinzadeh, H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol. Ind. Health 2015, 31, 367–376. [Google Scholar] [CrossRef]
- Nahid, K.; Fariborz, M.; Ataolah, G.; Solokian, S. The effect of an Iranian herbal drug on primary dysmenorrhea: A clinical controlled trial. J. Midwifery Womens Health 2009, 54, 401–404. [Google Scholar] [CrossRef]
- Patel, S.; Sarwat, M.; Khan, T.H. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): The molecular perspective. Crit. Rev. Oncol. Hematol. 2017, 115, 27–35. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta 2014, 1845, 20–30. [Google Scholar] [CrossRef]
- Colapietro, A.; Mancini, A.; D’Alessandro, A.M.; Festuccia, C. Crocetin and Crocin from Saffron in Cancer Chemotherapy and Chemoprevention. Anti-Cancer Agents Med. Chem. 2019, 19, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent Advances on the Anticancer Properties of Saffron (Crocus sativus L.) and Its Major Constituents. Molecules 2020, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, R.; Mollaei, H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 2017, 69, 1419–1427. [Google Scholar] [CrossRef]
- Ashktorab, H.; Soleimani, A.; Singh, G.; Amin, A.; Tabtabaei, S.; Latella, G.; Stein, U.; Akhondzadeh, S.; Solanki, N.; Gondre-Lewis, M.C.; et al. Saffron: The Golden Spice with Therapeutic Properties on Digestive Diseases. Nutrients 2019, 11, 943. [Google Scholar] [CrossRef]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran. J. Basic Med. Sci. 2019, 22, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, X.Y.; Ouyang, T.; Qu, Y.; Luo, T.; Wang, H.Q. Synergistic anticancer effect of combined crocetin and cisplatin on KYSE-150 cells via p53/p21 pathway. Cancer Cell Int. 2017, 17, 98. [Google Scholar] [CrossRef]
- Premkumar, K.; Thirunavukkarasu, C.; Abraham, S.K.; Santhiya, S.T.; Ramesh, A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum. Exp. Toxicol. 2006, 25, 79–84. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef]
- Marangoni, D.; Falsini, B.; Piccardi, M.; Ambrosio, L.; Minnella, A.M.; Savastano, M.C.; Bisti, S.; Maccarone, R.; Fadda, A.; Mello, E.; et al. Functional effect of Saffron supplementation and risk genotypes in early age-related macular degeneration: A preliminary report. J. Transl. Med. 2013, 11, 228. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ziaee, T.; Sadeghi, A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine 2008, 15, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Skladnev, N.V.; Ganeshan, V.; Kim, J.Y.; Burton, T.J.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Widespread brain transcriptome alterations underlie the neuroprotective actions of dietary saffron. J. Neurochem. 2016, 139, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Oppong-Twene, P.; Maecker, H.T.; Chirumamilla, L.; Kibreab, A.; Nabi, E.; Laiyemo, A.; Brim, H. Saffron as an Adjuvant Therapy in Ulcerative Colitis Patients. Inflamm. Bowel Dis. 2022, 28, S18. [Google Scholar] [CrossRef]
- Banskota, S.; Brim, H.; Kwon, Y.H.; Singh, G.; Sinha, S.R.; Wang, H.; Khan, W.I.; Ashktorab, H. Saffron Pre-Treatment Promotes Reduction in Tissue Inflammatory Profiles and Alters Microbiome Composition in Experimental Colitis Mice. Molecules 2021, 26, 3351. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Haileselassie, Y.; Ji, A.R.; Maecker, H.T.; Sinha, S.R.; Brim, H.; Habtezion, A.; Ashktorab, H. Protective Effect of Saffron in Mouse Colitis Models Through Immune Modulation. Dig. Dis. Sci. 2021, 67, 2922–2935. [Google Scholar] [CrossRef]
- Wolffe, A.P.; Matzke, M.A. Epigenetics: Regulation through repression. Science 1999, 286, 481–486. [Google Scholar] [CrossRef]
- Rashid, M.; Shah, S.G.; Verma, T.; Chaudhary, N.; Rauniyar, S.; Patel, V.B.; Gera, P.B.; Smoot, D.; Ashaktorab, H.; Dalal, S.N.; et al. Tumor-specific overexpression of histone gene, H3C14 in gastric cancer is mediated through EGFR-FOXC1 axis. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194703. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Histone variants on the move: Substrates for chromatin dynamics. Nat. Rev. Mol. Cell Biol. 2017, 18, 115–126. [Google Scholar] [CrossRef]
- Shah, S.G.; Mandloi, T.; Kunte, P.; Natu, A.; Rashid, M.; Reddy, D.; Gadewal, N.; Gupta, S. HISTome2: A database of histone proteins, modifiers for multiple organisms and epidrugs. Epigenetics Chromatin 2020, 13, 31. [Google Scholar] [CrossRef]
- Metere, A.; Graves, C.E. Factors Influencing Epigenetic Mechanisms: Is There A Role for Bariatric Surgery? High Throughput 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Metere, A.; Chiesa, C.; Di Cosimo, C.; Fierro, G.; Giacomelli, L.; Pietraforte, D. A novel approach to study oxidative stress in thyroid diseases: A preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 646–652. [Google Scholar] [PubMed]
- Shukla, S.; Penta, D.; Mondal, P.; Meeran, S.M. Epigenetics of Breast Cancer: Clinical Status of Epi-drugs and Phytochemicals. Adv. Exp. Med. Biol. 2019, 1152, 293–310. [Google Scholar] [CrossRef]
- Curran, K.M.; Bracha, S.; Wong, C.P.; Beaver, L.M.; Stevens, J.F.; Ho, E. Sulforaphane absorption and histone deacetylase activity following single dosing of broccoli sprout supplement in normal dogs. Vet. Med. Sci. 2018, 4, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scire, A.; Armeni, T. Side Effects of Curcumin: Epigenetic and Antiproliferative Implications for Normal Dermal Fibroblast and Breast Cancer Cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Brait, M.; Ford, J.G.; Papaiahgari, S.; Garza, M.A.; Lee, J.I.; Loyo, M.; Maldonado, L.; Begum, S.; McCaffrey, L.; Howerton, M.; et al. Association between lifestyle factors and CpG island methylation in a cancer-free population. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2984–2991. [Google Scholar] [CrossRef]
- Shah, S.; Rashid, M.; Verma, T.; Gupta, S. Chapter 8—Chromatin, histones, and histone modifications in health and disease. In Genome Plasticity in Health and Disease; Forero, D.A., Patrinos, G.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 109–135. [Google Scholar] [CrossRef]
- Simpson, R.T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry 1978, 17, 5524–5531. [Google Scholar] [CrossRef]
- Hamiche, A.; Schultz, P.; Ramakrishnan, V.; Oudet, P.; Prunell, A. Linker histone-dependent DNA structure in linear mononucleosomes. J. Mol. Biol. 1996, 257, 30–42. [Google Scholar] [CrossRef]
- Bednar, J.; Horowitz, R.A.; Grigoryev, S.A.; Carruthers, L.M.; Hansen, J.C.; Koster, A.J.; Woodcock, C.L. Nucleosomes, linker DNA, and linker histone form a unique structural motif that directs the higher-order folding and compaction of chromatin. Proc. Natl. Acad. Sci. USA 1998, 95, 14173–14178. [Google Scholar] [CrossRef]
- Syed, S.H.; Goutte-Gattat, D.; Becker, N.; Meyer, S.; Shukla, M.S.; Hayes, J.J.; Everaers, R.; Angelov, D.; Bednar, J.; Dimitrov, S. Single-base resolution mapping of H1-nucleosome interactions and 3D organization of the nucleosome. Proc. Natl. Acad. Sci. USA 2010, 107, 9620–9625. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Bathaie, S.Z.; Taghikhani, M.; Moosavi-Movahedi, A.A. The effect of carotenoids obtained from saffron on histone H1 structure and H1-DNA interaction. Int. J. Biol. Macromol. 2005, 36, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, R.; Bathaie, S.Z.; Ashrafi, M. Interaction of safranal and picrocrocin with ctDNA and their preferential mechanisms of binding to GC- and AT-rich oligonucleotides. DNA Cell Biol. 2008, 27, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bathaie, S.Z.; Bolhasani, A.; Hoshyar, R.; Ranjbar, B.; Sabouni, F.; Moosavi-Movahedi, A.A. Interaction of saffron carotenoids as anticancer compounds with ctDNA, Oligo (dG.dC)15, and Oligo (dA.dT)15. DNA Cell Biol. 2007, 26, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Nekludova, L.; Pabo, C.O. Distinctive DNA conformation with enlarged major groove is found in Zn-finger-DNA and other protein-DNA complexes. Proc. Natl. Acad. Sci. USA 1994, 91, 6948–6952. [Google Scholar] [CrossRef]
- Timsit, Y. DNA structure and polymerase fidelity. J. Mol. Biol. 1999, 293, 835–853. [Google Scholar] [CrossRef]
- Lu, X.J.; Shakked, Z.; Olson, W.K. A-form conformational motifs in ligand-bound DNA structures. J. Mol. Biol. 2000, 300, 819–840. [Google Scholar] [CrossRef]
- Hoshyar, R.; Bathaie, S.Z.; Kyani, A.; Mousavi, M.F. Is there any interaction between telomeric DNA structures, G-quadruplex and I-motif, with saffron active metabolites? Nucleosides Nucleotides Nucleic Acids 2012, 31, 801–812. [Google Scholar] [CrossRef]
- Lin, S.J.; Ford, E.; Haigis, M.; Liszt, G.; Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004, 18, 12–16. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Lu, J.; Rodova, M.; Lezi, E.; Crafter, A.B.; Swerdlow, R.H. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim. Biophys. Acta 2010, 1802, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, M.; Fischhuber, K.; Poloske, D.; Mechtler, K.; Heiss, E.H. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020, 29, 101393. [Google Scholar] [CrossRef]
- Dai, H.; Case, A.W.; Riera, T.V.; Considine, T.; Lee, J.E.; Hamuro, Y.; Zhao, H.; Jiang, Y.; Sweitzer, S.M.; Pietrak, B.; et al. Crystallographic structure of a small molecule SIRT1 activator-enzyme complex. Nat. Commun. 2015, 6, 7645. [Google Scholar] [CrossRef]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.M. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar] [CrossRef]
- Krishnaswamy, V.K.D.; Alugoju, P.; Periyasamy, L. Multifaceted targeting of neurodegeneration with bioactive molecules of saffron (Crocus sativus): An insilco evidence-based hypothesis. Med. Hypotheses 2020, 143, 109872. [Google Scholar] [CrossRef]
- Nezamdoost, Z.; Saghebjoo, M.; Hoshyar, R.; Hedayati, M.; Keska, A. High-Intensity Training and Saffron: Effects on Breast Cancer-related Gene Expression. Med. Sci. Sports Exerc. 2020, 52, 1470–1476. [Google Scholar] [CrossRef]
- Morimoto, S.; Tsuda, M.; Bunch, H.; Sasanuma, H.; Austin, C.; Takeda, S. Type II DNA Topoisomerases Cause Spontaneous Double-Strand Breaks in Genomic DNA. Genes 2019, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Al-Hrout, A.; Chaiboonchoe, A.; Khraiwesh, B.; Murali, C.; Baig, B.; El-Awady, R.; Tarazi, H.; Alzahmi, A.; Nelson, D.R.; Greish, Y.E.; et al. Safranal induces DNA double-strand breakage and ER-stress-mediated cell death in hepatocellular carcinoma cells. Sci. Rep. 2018, 8, 16951. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Angelucci, A.; Llorens, S.; Mattei, V.; Gravina, G.L.; Alonso, G.L.; Festuccia, C. Crocetin Extracted from Saffron Shows Antitumor Effects in Models of Human Glioblastoma. Int. J. Mol. Sci. 2020, 21, 423. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Farrukh, A.; Murali, C.; Soleimani, A.; Praz, F.; Graziani, G.; Brim, H.; Ashktorab, H. Saffron and Its Major Ingredients’ Effect on Colon Cancer Cells with Mismatch Repair Deficiency and Microsatellite Instability. Molecules 2021, 26, 3855. [Google Scholar] [CrossRef] [PubMed]
- Fragkos, M.; Jurvansuu, J.; Beard, P. H2AX is required for cell cycle arrest via the p53/p21 pathway. Mol. Cell Biol. 2009, 29, 2828–2840. [Google Scholar] [CrossRef] [PubMed]
- Geierstanger, B.H.; Wemmer, D.E. Complexes of the minor groove of DNA. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 463–493. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Gezici, S. Comparative anticancer activity analysis of saffron extracts and a principle component, crocetin for prevention and treatment of human malignancies. J. Food Sci. Technol. 2019, 56, 5435–5443. [Google Scholar] [CrossRef]
- Gainer, J.L.; Sheehan, J.P.; Larner, J.M.; Jones, D.R. Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. J. Neurosurg. 2017, 126, 460–466. [Google Scholar] [CrossRef]
- Hosseini, A.; Mousavi, S.H.; Ghanbari, A.; Homaee Shandiz, F.; Raziee, H.R.; Pezeshki Rad, M.; Mousavi, S.H. Effect of saffron on liver metastases in patients suffering from cancers with liver metastases: A randomized, double blind, placebo-controlled clinical trial. Avicenna J. Phytomed. 2015, 5, 434–440. [Google Scholar]
- Mir, M.A.; Ganai, S.A.; Mansoor, S.; Jan, S.; Mani, P.; Masoodi, K.Z.; Amin, H.; Rehman, M.U.; Ahmad, P. Isolation, purification and characterization of naturally derived Crocetin beta-d-glucosyl ester from Crocus sativus L. against breast cancer and its binding chemistry with ER-alpha/HDAC2. Saudi J. Biol. Sci. 2020, 27, 975–984. [Google Scholar] [CrossRef]
- Chahine, N.; Hanna, J.; Makhlouf, H.; Duca, L.; Martiny, L.; Chahine, R. Protective effect of saffron extract against doxorubicin cardiotoxicity in isolated rabbit heart. Pharm. Biol. 2013, 51, 1564–1571. [Google Scholar] [CrossRef]
- Abdullaev, F.I.; Frenkel, G.D. The effect of saffron on intracellular DNA, RNA and protein synthesis in malignant and non-malignant human cells. Biofactors 1992, 4, 43–45. [Google Scholar] [PubMed]
- Kanakis, C.D.; Tarantilis, P.A.; Pappas, C.; Bariyanga, J.; Tajmir-Riahi, H.A.; Polissiou, M.G. An overview of structural features of DNA and RNA complexes with saffron compounds: Models and antioxidant activity. J. Photochem. Photobiol. B 2009, 95, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Gullu, N.; Kobelt, D.; Brim, H.; Rahman, S.; Timm, L.; Smith, J.; Soleimani, A.; Di Marco, S.; Bisti, S.; Ashktorab, H.; et al. Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells. Cells 2020, 9, 1829. [Google Scholar] [CrossRef]
- Amin, A.; Hamza, A.A.; Bajbouj, K.; Ashraf, S.S.; Daoud, S. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology 2011, 54, 857–867. [Google Scholar] [CrossRef]
- Warmerdam, D.O.; Kanaar, R. Dealing with DNA damage: Relationships between checkpoint and repair pathways. Mutat. Res. 2010, 704, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Hashemi Tayer, A.; Shakeri, H.; Sotoodeh Jahromi, A.; Moradzadeh, M.; Hojjat-Farsangi, M. Toxicity of Saffron Extracts on Cancer and Normal Cells: A Review Article. Asian Pac. J. Cancer Prev. 2020, 21, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fang, D. The Roles of SIRT1 in Cancer. Genes Cancer 2013, 4, 97–104. [Google Scholar] [CrossRef]
- Liu, T.F.; Yoza, B.K.; El Gazzar, M.; Vachharajani, V.T.; McCall, C.E. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J. Biol. Chem. 2011, 286, 9856–9864. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, A.; Scher, M.; Lee, D.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 2004, 16, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Vachharajani, V.T.; Liu, T.; Wang, X.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins Link Inflammation and Metabolism. J. Immunol. Res. 2016, 2016, 8167273. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Dou, H.; Lin, W.; Zou, L. Sirtuin 1 inhibits lipopolysaccharide-induced inflammation in chronic myelogenous leukemia k562 cells through interacting with the Toll-like receptor 4-nuclear factor kappa B-reactive oxygen species signaling axis. Cancer Cell Int. 2020, 20, 73. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Fievez, L.; Neyrinck, A.M.; Delzenne, N.M.; Bureau, F.; Vanbever, R. Sirtuin inhibition attenuates the production of inflammatory cytokines in lipopolysaccharide-stimulated macrophages. Biochem. Biophys Res. Commun. 2012, 420, 857–861. [Google Scholar] [CrossRef]
- Bajbouj, K.; Schulze-Luehrmann, J.; Diermeier, S.; Amin, A.; Schneider-Stock, R. The anticancer effect of saffron in two p53 isogenic colorectal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 69. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.; Brim, H.; Ashktorab, H. Saffron, Its Active Components, and Their Association with DNA and Histone Modification: A Narrative Review of Current Knowledge. Nutrients 2022, 14, 3317. https://doi.org/10.3390/nu14163317
Rashid M, Brim H, Ashktorab H. Saffron, Its Active Components, and Their Association with DNA and Histone Modification: A Narrative Review of Current Knowledge. Nutrients. 2022; 14(16):3317. https://doi.org/10.3390/nu14163317
Chicago/Turabian StyleRashid, Mudasir, Hassan Brim, and Hassan Ashktorab. 2022. "Saffron, Its Active Components, and Their Association with DNA and Histone Modification: A Narrative Review of Current Knowledge" Nutrients 14, no. 16: 3317. https://doi.org/10.3390/nu14163317





