Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Herb Materials
2.3. Preparation of C3G and P3G-Rich Fraction of Black Rice Germ and Bran
2.4. Total Phenolic Content and Total Flavonoid Content
2.5. Total Anthocyanin Content
- A = (A520nm − A700nm) pH 1.0 − (A520nm − A700nm) pH 4.5
- MW = molecular weight of C3G
- DF = dilution Factor
- ε = molar extinction coefficient = L × mol–1 × cm–1
- L= cell path length (1 cm)
2.6. Identification of Active Anthocyanin Compounds in BR Extract Using High-Performance Liquid Chromatography (HPLC) Technique
2.7. Cell Cultures
2.8. Cell Viability Assay
2.9. Determination of Cytokine Secretion by Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Expression of NLRP3, IL-6, IL-1β, and IL-18 Genes by Reverse Transcription-Polymerase Chain Reaction (RT-qPCR) Analysis
2.11. Nuclear Extraction and NF-kB Activity Assay
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
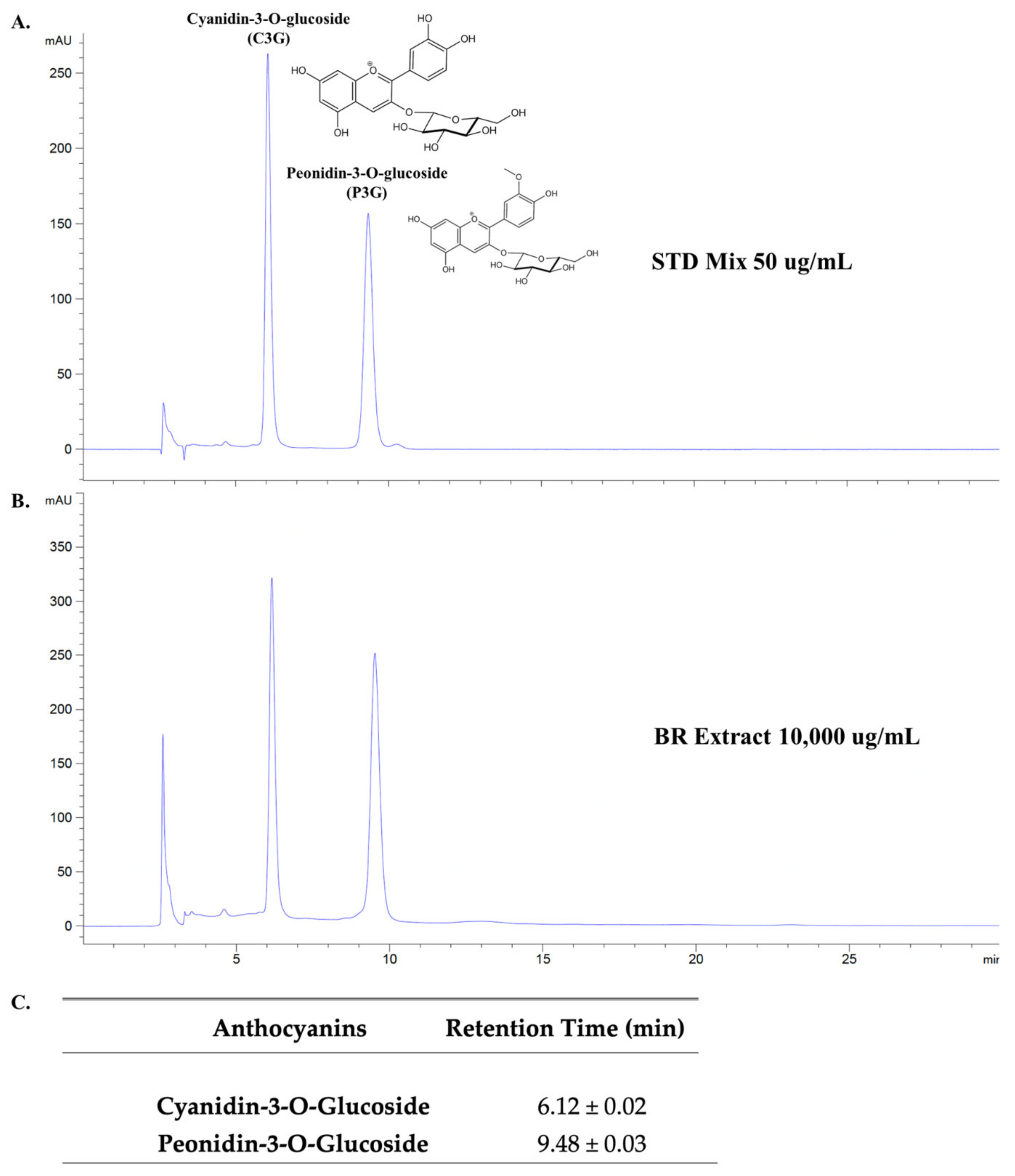
3.1. Anthocyanin-Rich Fractions Prepared from Black Rice Germ and Bran (BR Extract) Contain Peonidin-3-O-glucoside (P3G) and Cyanidin-3-O-glucoside (C3G) as Major Anthocyanin Compounds
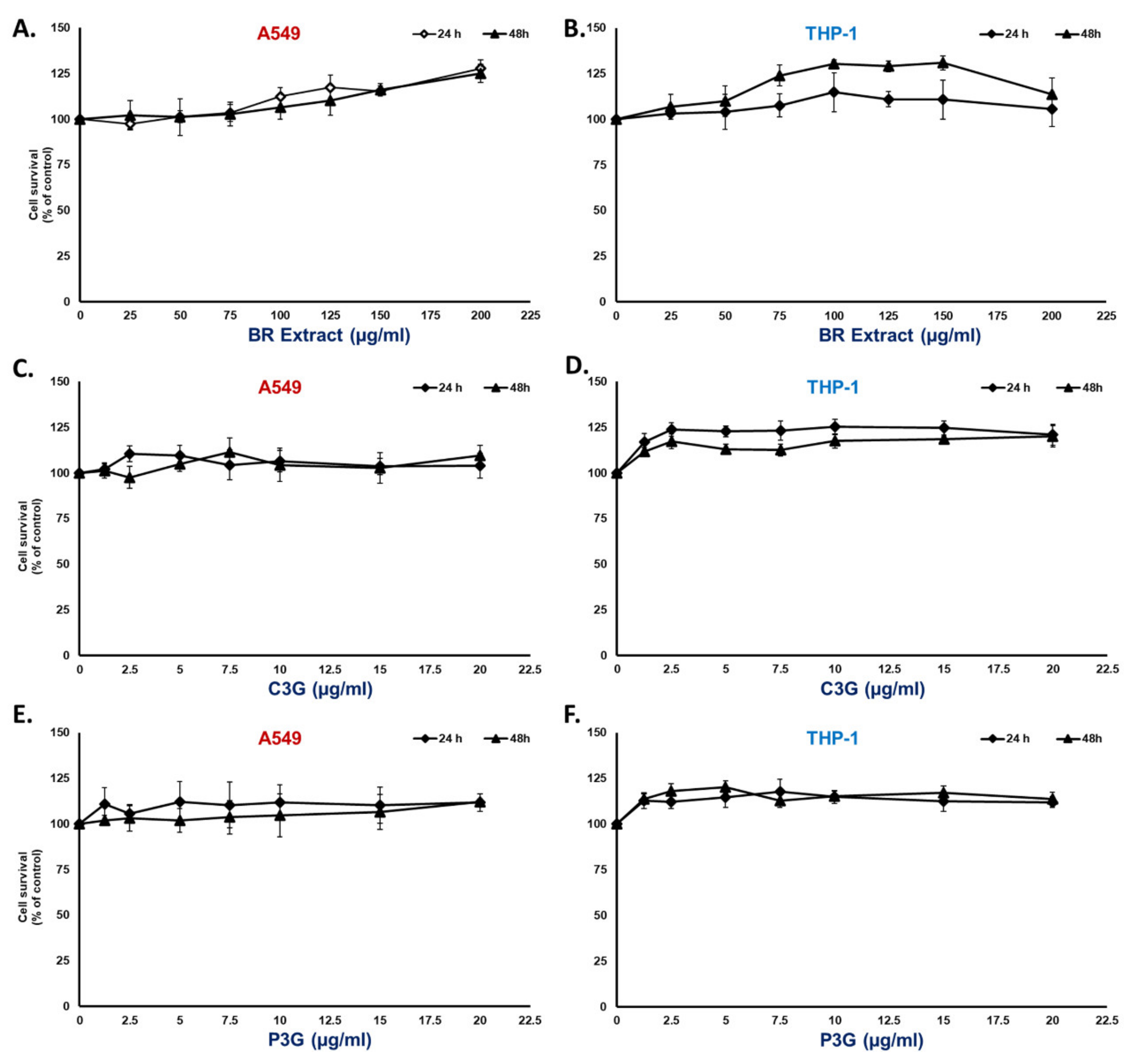
3.2. BR Extract and Its Active Compounds, P3G and C3G, Displayed No Cytotoxicity in A549 Lung Cells and THP-1 Macrophages
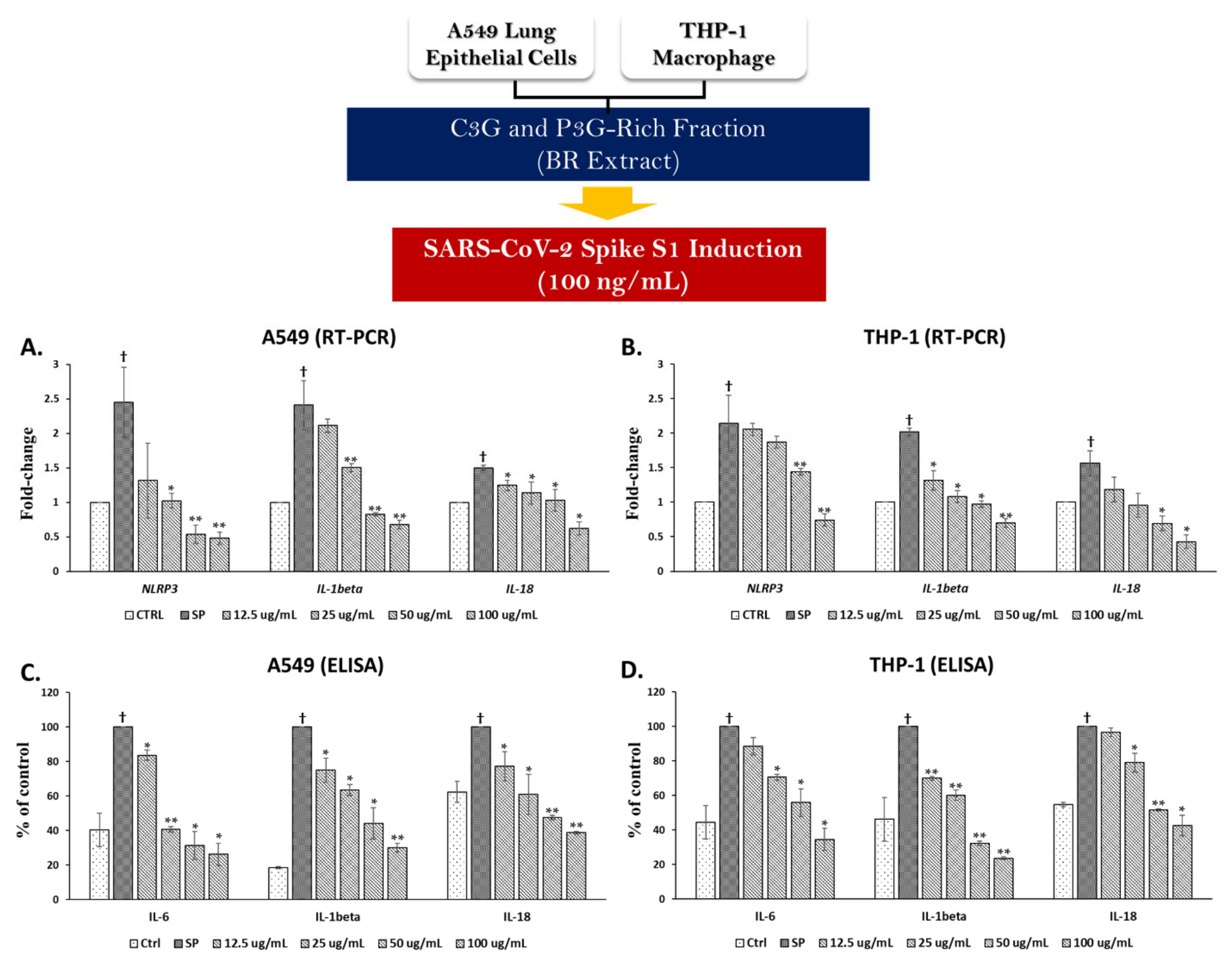
3.3. Spike Glycoprotein S1 (SP) Induced an Inflammatory Response by Upregulating the Inflammatory Gene Expressions and Increasing Cytokine Secretions in A549 Cells and THP-1 Macrophages
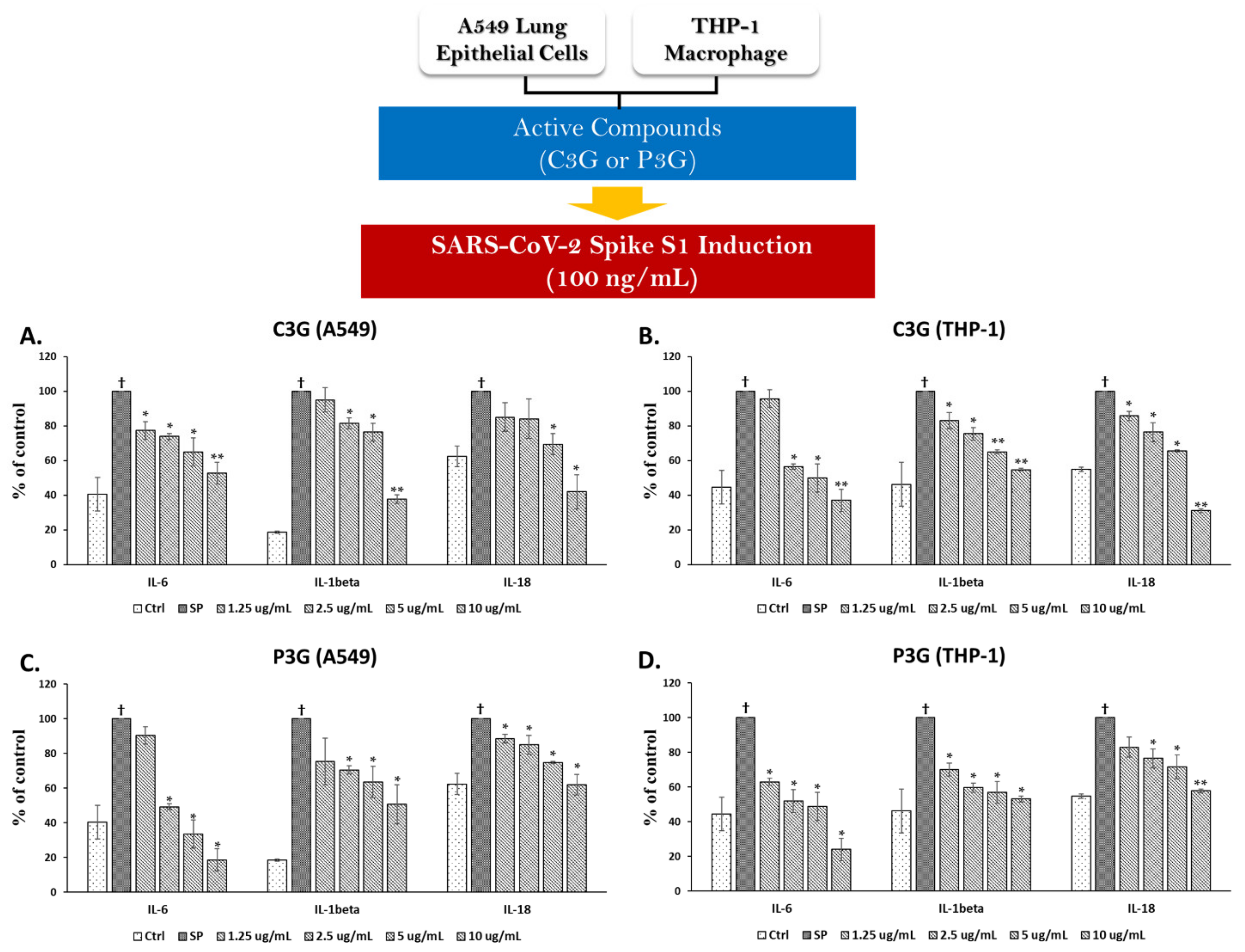
3.4. BR Extract and the Active Anthocyanins C3G and P3G Exhibited Anti-Inflammatory Properties upon SP Induction in A549 Cells and THP-1 Macrophages
3.4.1. Effects of BR Extract on SP Induction in A549 Cells and THP-1 Macrophages
3.4.2. Effects of C3G and P3G on SP Induction in A549 Cells and THP-1 Macrophages
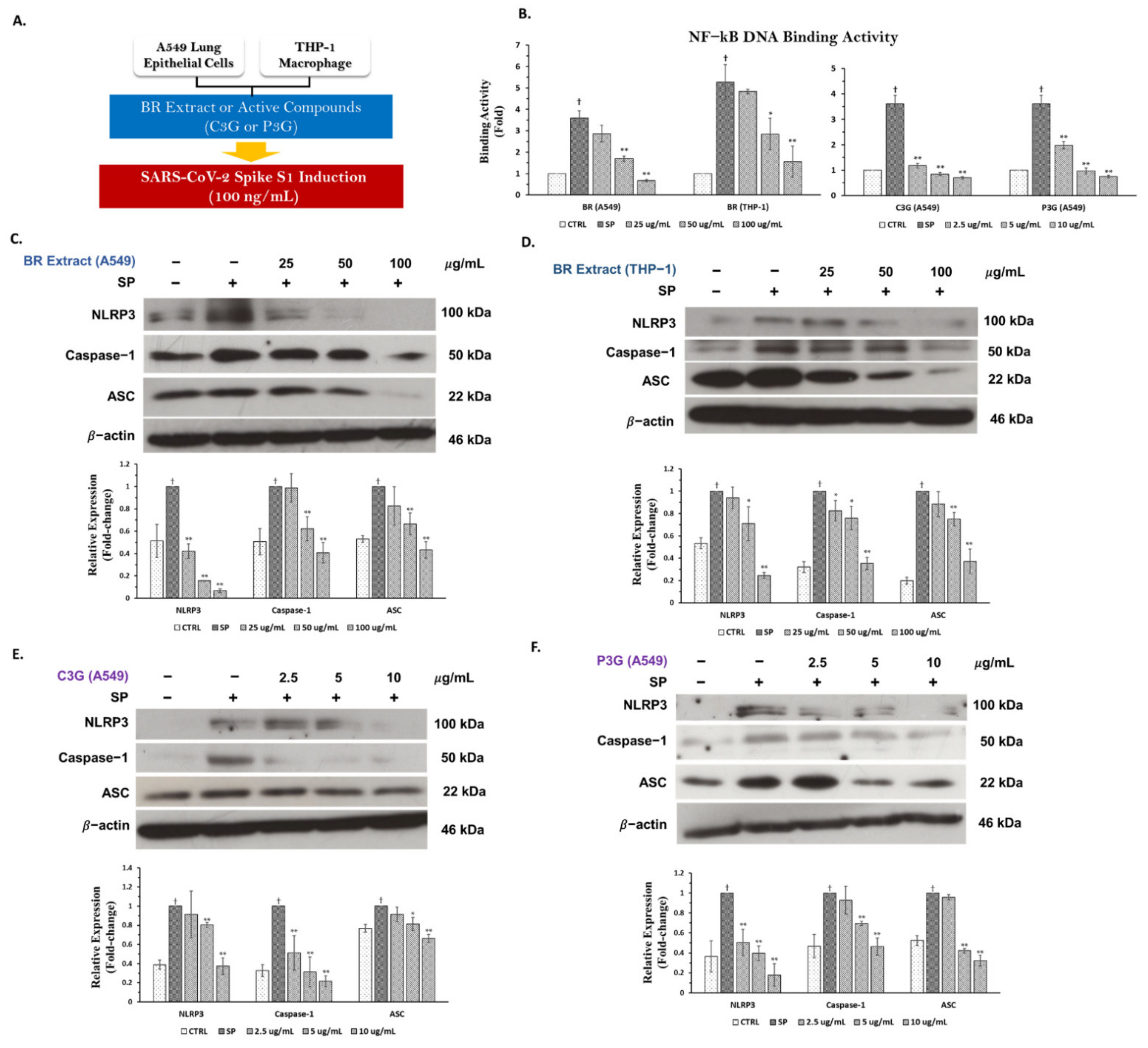
3.5. BR Extract and Its Active Anthocyanins, C3G and P3G, Exhibited Anti-Inflammatory Properties through Inhibition of NF-kB Transcriptional Activity and the NLRP3 Inflammasome Pathway in A549 and THP-1 Cell Lines
3.5.1. Inhibitory Effects of BR Extract, C3G, and P3G on NF-kB Transcriptional Activity in A549 Cells and THP-1 Macrophages
3.5.2. Inhibitory Effects of BR Extract, C3G, and P3G on NLRP3 Inflammasome Pathway in A549 Cells and THP-1 Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.T.; Bieneman, A.P. The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3. Front. Immunol. 2022, 13, 831763. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D. Immunology of COVID-19: Current state of the science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, 68563. [Google Scholar] [CrossRef]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: Potential role of angiotensin-converting enzyme inhibitor (perindopril). Front. Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018, 355, 93–102. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Semmarath, W.; Seesen, M.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Siviroj, P.; Limtrakul, P. The association between frailty indicators and blood-based biomarkers in early-old community dwellers of Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3457. [Google Scholar] [CrossRef] [Green Version]
- Peña-Sanhueza, D.; Inostroza-Blancheteau, C.; Ribera-Fonseca, A.; Reyes-Díaz, M. Anthocyanins in berries and their potential use in human health. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; IntechOpen: London, UK, 2017; pp. 155–172. [Google Scholar]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef]
- Limtrakul, P.; Semmarath, W.; Mapoung, S. Anthocyanins and proanthocyanidins in natural pigmented rice and their bioactivities. Phytochem. Hum. Health 2019, 1, 86962. [Google Scholar]
- Dias, A.L.d.S.; Pachikian, B.; Larondelle, Y.; Quetin-Leclercq, J. Recent advances on bioactivities of black rice. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 470–476. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.-S.; Zhang, X.-H.; Yang, S.-N.; Liu, D.; Diao, C.-R.; Wang, H.; Zheng, F.-P. Cyanidin inhibits EMT induced by oxaliplatin via targeting the PDK1–PI3K/Akt signaling pathway. Food Funct. 2019, 10, 592–601. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [Green Version]
- Seesen, M.; Semmarath, W.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Ongprasert, K.; Chittrakul, J.; Siviroj, P. Combined black rice germ, bran supplement and exercise intervention modulate aging biomarkers and improve physical performance and lower-body muscle strength parameters in aging population. Int. J. Environ. Res. Public Health 2020, 17, 2931. [Google Scholar] [CrossRef]
- Messaoudi, O.; Gouzi, H.; El-Hoshoudy, A.N.; Benaceur, F.; Patel, C.; Goswami, D.; Boukerouis, D.; Bendahou, M. Berries anthocyanins as potential SARS-CoV–2 inhibitors targeting the viral attachment and replication; molecular docking simulation. Egypt J. Pet. 2021, 30, 33–43. [Google Scholar] [CrossRef]
- Khalifa, I.; Nawaz, A.; Sobhy, R.; Althwab, S.A.; Barakat, H. Polyacylated anthocyanins constructively network with catalytic dyad residues of 3CLpro of 2019-nCoV than monomeric anthocyanins: A structural-relationship activity study with 10 anthocyanins using in-silico approaches. J. Mol. Graph. Model. 2020, 100, 107690. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin anti-aging assays of proanthocyanidin rich red rice extract, oryzanol and other phenolic compounds. Nat. Prod. Commun. 2018, 13, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Rani, R.; Pushkar, S.; Lal, S.; Srivastava, S.; Kumari, S.; Vinutha, T.; Dahuja, A.; Praveen, S.; Sachdev, A. Anthocyanin fingerprinting and dynamics in differentially pigmented exotic soybean genotypes using modified HPLC–DAD method. J. Food Meas. Charact. 2020, 14, 1966–1975. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-Induced THP-1 macrophages. Int. J. Biol. Sci 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Jin, X.; Wang, C.; Wu, W.; Liu, T.; Ji, B.; Zhou, F. Cyanidin-3-glucoside alleviates 4-hydroxyhexenal-induced NLRP3 inflammasome activation via JNK-c-Jun/AP-1 pathway in human retinal pigment epithelial cells. J. Immunol. Res. 2018, 2018, 5604610. [Google Scholar] [CrossRef] [Green Version]
- Paramel Varghese, G.; Folkersen, L.; Strawbridge, R.J.; Halvorsen, B.; Yndestad, A.; Ranheim, T.; Krohg-Sørensen, K.; Skjelland, M.; Espevik, T.; Aukrust, P. NLRP 3 inflammasome expression and activation in human atherosclerosis. J. Am. Heart Assoc. 2016, 5, e003031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Pompimon, W.; Limtrakul, P. Crebanine, an aporphine alkaloid, sensitizes TNF-α-induced apoptosis and suppressed invasion of human lung adenocarcinoma cells A549 by blocking NF-κB-regulated gene products. Tumor Biol. 2014, 35, 8615–8624. [Google Scholar] [CrossRef] [PubMed]
- Barilli, A.; Visigalli, R.; Ferrari, F.; Bianchi, M.G.; Dall’Asta, V.; Rotoli, B.M. Immune-Mediated Inflammatory Responses of Alveolar Epithelial Cells: Implications for COVID-19 Lung Pathology. Biomedicines 2022, 10, 618. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, X.; Zheng, X.; Luo, S.; Xu, S.; Weng, J. Targeting inflammation and cytokine storm in COVID-19. Pharmacol. Res. 2020, 159, 105051. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R.; Bahadoram, M.; Alghasi, A. COVID-19: A Case for Inhibiting NLRP3 Inflammasome, Suppression of Inflammation with Curcumin? Basic Clin. Pharmacol. Toxicol. 2021, 128, 37–45. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, E.-C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef]
- Fan, H.; Lou, F.; Fan, J.; Li, M.; Tong, Y. The emergence of powerful oral anti-COVID-19 drugs in the post-vaccine era. Lancet Microbe 2022, 3, 1. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.-M.; Shi, J.; Tomas-Barberan, F.; Datta, N.; Singanusong, R.; Chen, S. Flavonoids in food and their health benefits. Plant. Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef]
- Shawan, M.M.A.K.; Halder, S.K.; Hasan, M.A. Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: An in silico molecular modeling approach in battling the COVID-19 outbreak. Bull. Natl. Res. Cent. 2021, 45, 27. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Bao, J.; Beta, T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015, 172, 630–639. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef]
- Zhang, Q.; Luna-Vital, D.; de Mejia, E.G. Anthocyanins from colored maize ameliorated the inflammatory paracrine interplay between macrophages and adipocytes through regulation of NF-κB and JNK-dependent MAPK pathways. J. Funct. Foods 2019, 54, 175–186. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Lepiarz-Raba, I.; Al-Hindawi, A.A. Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation 2021, 44, 1865–1877. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef]
- Sohn, K.M.; Lee, S.-G.; Kim, H.J.; Cheon, S.; Jeong, H.; Lee, J.; Kim, I.S.; Silwal, P.; Kim, Y.J.; Paik, S. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 2020, 35, e343. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. J. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Xie, S.; Huang, B.; Qian, Y.; Chen, K.; Niu, Y.; Shen, H.-M.; Cai, J.; Li, P. Myricetin inhibits NLRP3 inflammasome activation via reduction of ROS-dependent ubiquitination of ASC and promotion of ROS-independent NLRP3 ubiquitination. Toxicol. Appl. Pharmacol. 2019, 365, 19–29. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, X.; Feng, X.-L.; Xu, L.; Han, J.-B.; Yu, D.; Zou, Q.-C.; Liu, Q.; Li, X.; Ma, G. Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. EBioMedicine 2022, 75, 103803. [Google Scholar] [CrossRef]
- Wilson, J.G.; Simpson, L.J.; Ferreira, A.-M.; Rustagi, A.; Roque, J.; Asuni, A.; Ranganath, T.; Grant, P.M.; Subramanian, A.; Rosenberg-Hasson, Y. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 2020, 5, e140289. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Jayasingha, J.A.C.C.; Kim, G.-Y. Anthocyanins from Hibiscus syriacus L. inhibit NLRP3 inflammasome in BV2 microglia cells by alleviating NF-κB-and ER stress-induced Ca2+ accumulation and mitochondrial ROS production. Oxid. Med. Cell. Longev. 2021, 2021, 1246491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lin, X.; Zhang, P.; Liu, Y.; Ling, W.; Guo, H. Upregulated NLRP3 inflammasome activation is attenuated by anthocyanins in patients with nonalcoholic fatty liver disease: A case-control and an intervention study. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101843. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.K.; Costa, R.S.; Silveira, T.N.; Caetano, B.C.; Horta, C.V.; Gutierrez, F.R.S.; da Matta Guedes, P.M.; Andrade, W.A.; De Niz, M.; Gazzinelli, R.T. Apoptosis-associated Speck–like protein containing a caspase recruitment domain inflammasomes mediate IL-1β response and host resistance to Trypanosoma cruzi infection. J. Immunol. 2013, 191, 3373–3383. [Google Scholar] [CrossRef] [Green Version]
- Groslambert, M.; Py, B.F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 2018, 11, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Patra, R.; Das, N.C.; Mukherjee, S. Targeting human TLRs to combat COVID-19: A solution? J. Med. Virol. 2021, 93, 615–617. [Google Scholar] [CrossRef]
- Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway de Macario, E.; Cappello, F.; Macario, A.J.; Marino Gammazza, A. The role of molecular chaperones in virus infection and implications for understanding and treating COVID-19. J. Clin. Med. 2020, 9, 3518. [Google Scholar] [CrossRef]


| Gene Product | Primer Sequence |
|---|---|
| NLRP3 | Forward: 5′ AAG GGC CAT GGA CTA TTT CC 3′ Reverse: 5′ GAC TCC ACC CGA TGA CAG TT 3′ |
| IL-6 | Forward: 5′-ATG AAC TCC TTC ACA AGC-3′ Reverse: 5′-GTT TTC TGC CAG TGC CTC TTT G-3′ |
| IL-1β | Forward, 5′ ATG ATG GCT TAT TAC AGT GGC AA 3′ Reverse, 5′ GTC GGA GAT TCG TAG CTG GA 3′ |
| IL-18 | Forward, 5′ AAA CTA TTT GTC GCA GGA ATA AAG AT 3′ Reverse, 5′ GCT TGC CAA AGT AAT CTG ATT CC 3′ |
| GADPH | Forward, 5′ TCA ACA GCG ACA CCC AC 3′ Reverse, 5′ GGG TCT CTC TCT TCC TCT TGT G 3′ |
| Phytochemicals | mg/g Extract |
|---|---|
| Total phenolic contents | 115.25 ± 10.55 |
| Total flavonoids | 68.23 ± 6.84 |
| Total anthocyanins | 105.24 ± 6.66 |
| Cyanidin-3-glucoside (C3G) | 33.04 ± 1.65 |
| Peonidin-3-glucoside (P3G) | 52.70 ± 1.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway. Nutrients 2022, 14, 2738. https://doi.org/10.3390/nu14132738
Semmarath W, Mapoung S, Umsumarng S, Arjsri P, Srisawad K, Thippraphan P, Yodkeeree S, Dejkriengkraikul P. Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway. Nutrients. 2022; 14(13):2738. https://doi.org/10.3390/nu14132738
Chicago/Turabian StyleSemmarath, Warathit, Sariya Mapoung, Sonthaya Umsumarng, Punnida Arjsri, Kamonwan Srisawad, Pilaiporn Thippraphan, Supachai Yodkeeree, and Pornngarm Dejkriengkraikul. 2022. "Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway" Nutrients 14, no. 13: 2738. https://doi.org/10.3390/nu14132738









