Maternal One-Carbon Supplement Reduced the Risk of Non-Alcoholic Fatty Liver Disease in Male Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Diet Composition
2.3. Intraperitoneal Injected Glucose Tolerance Test (IPGTT)
2.4. UPLC-MS/MS
2.5. SAM-Dependent Methyltransferase Activity
2.6. DNA Methyltransferase Activity
2.7. Genomic DNA Methylation Level
2.8. Antibodies
2.9. Real-Time PCR (RT-PCR)
2.10. Whole Genome Bisulfite Sequencing (WGBS)
2.11. Statistical Analysis
3. Results
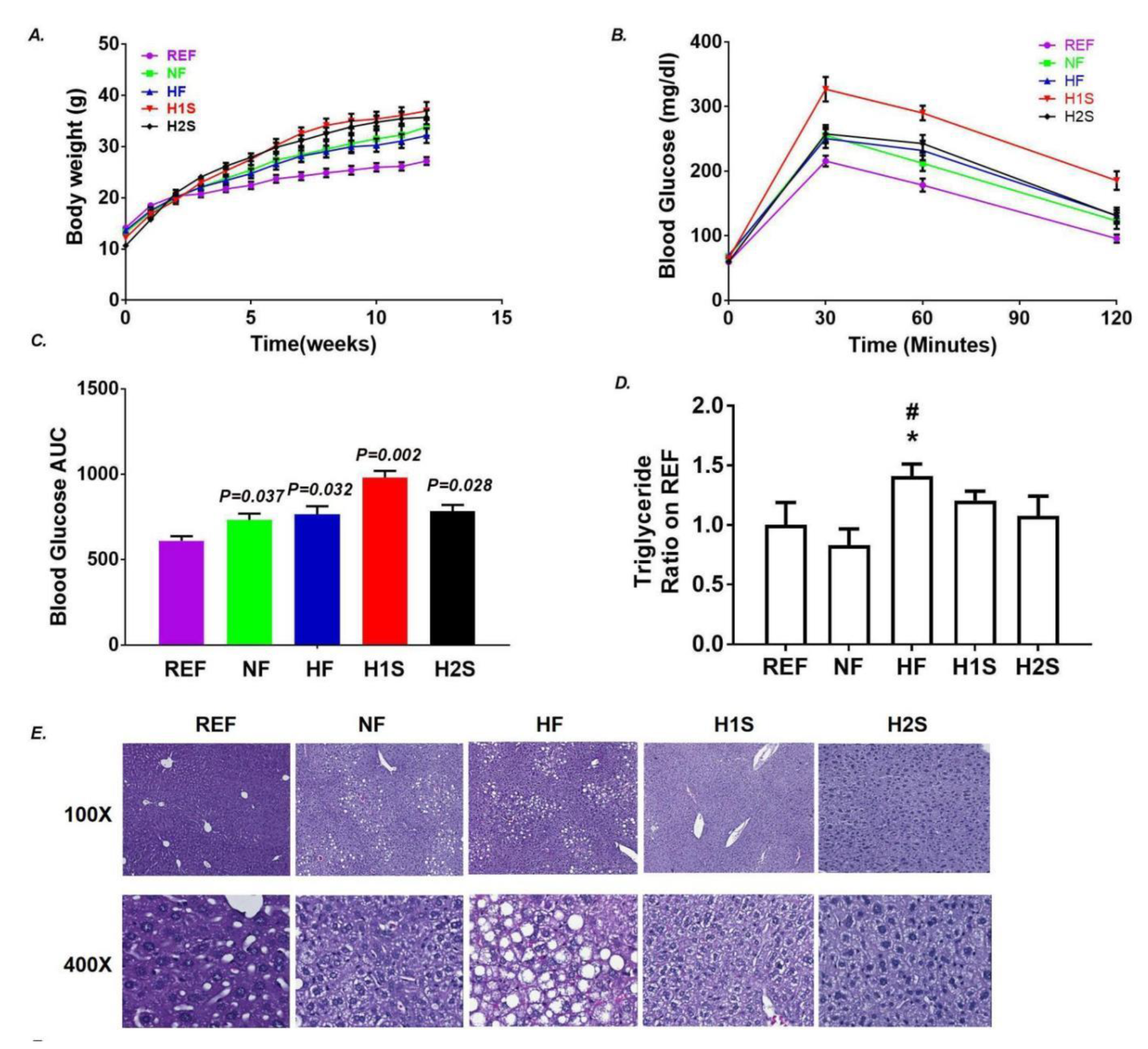
3.1. Maternal One-Carbon Supplementation Allowed Offspring to Avoid NAFLD Promoted by a Maternal HF Diet but Not Obesity and Glucose Intolerance
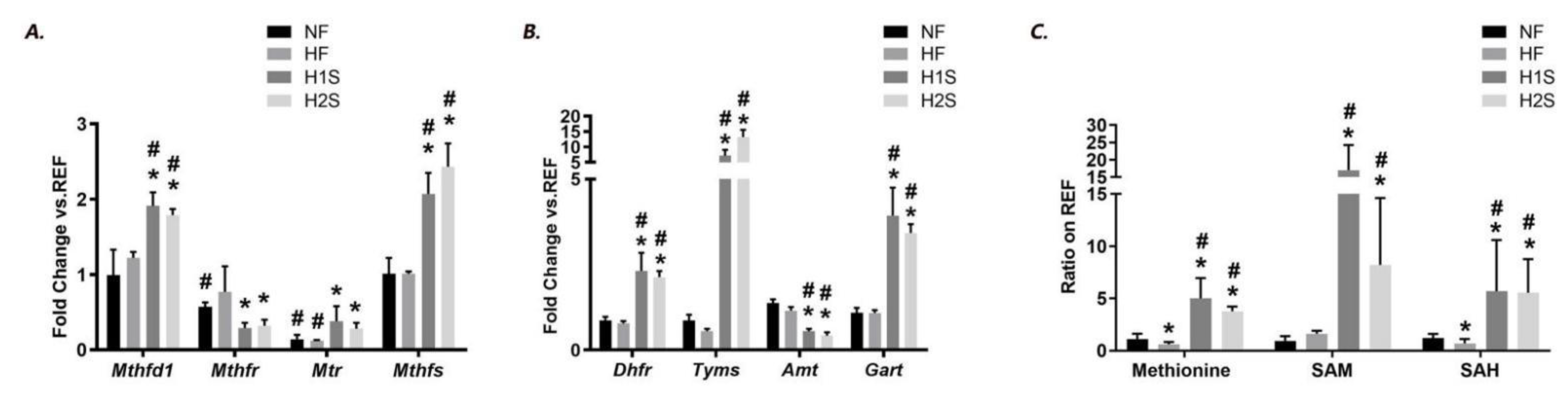
3.2. Maternal One-Carbon Supplementation Corrected and Overloaded the Disrupted Methionine Cycle by Maternal HF Diet
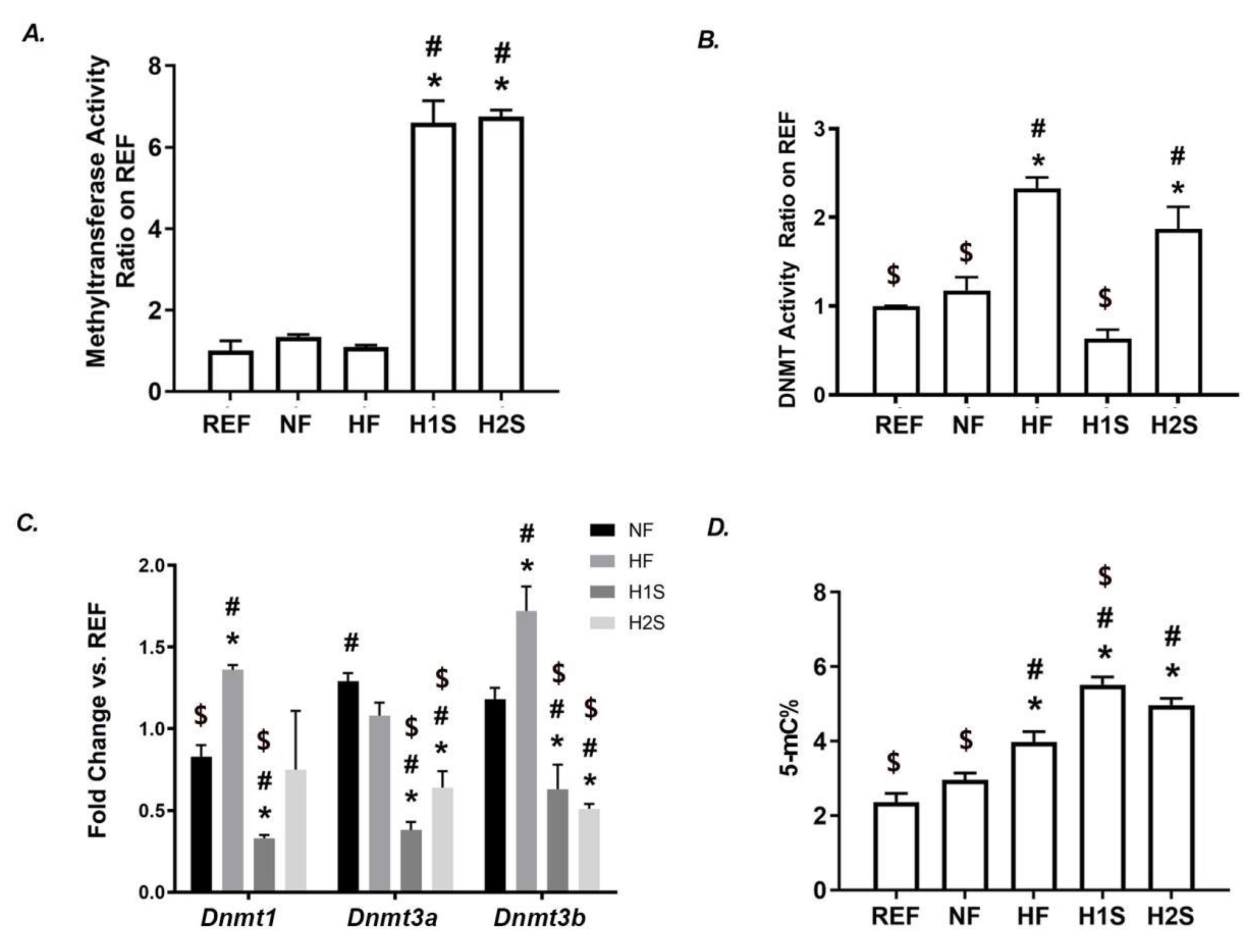
3.3. Maternal One-Carbon Supplementation Overactivated the SAM-Transferase Activity but Did Not Correct the Genomic DNA Hypermethylation Caused by a Maternal HF Diet
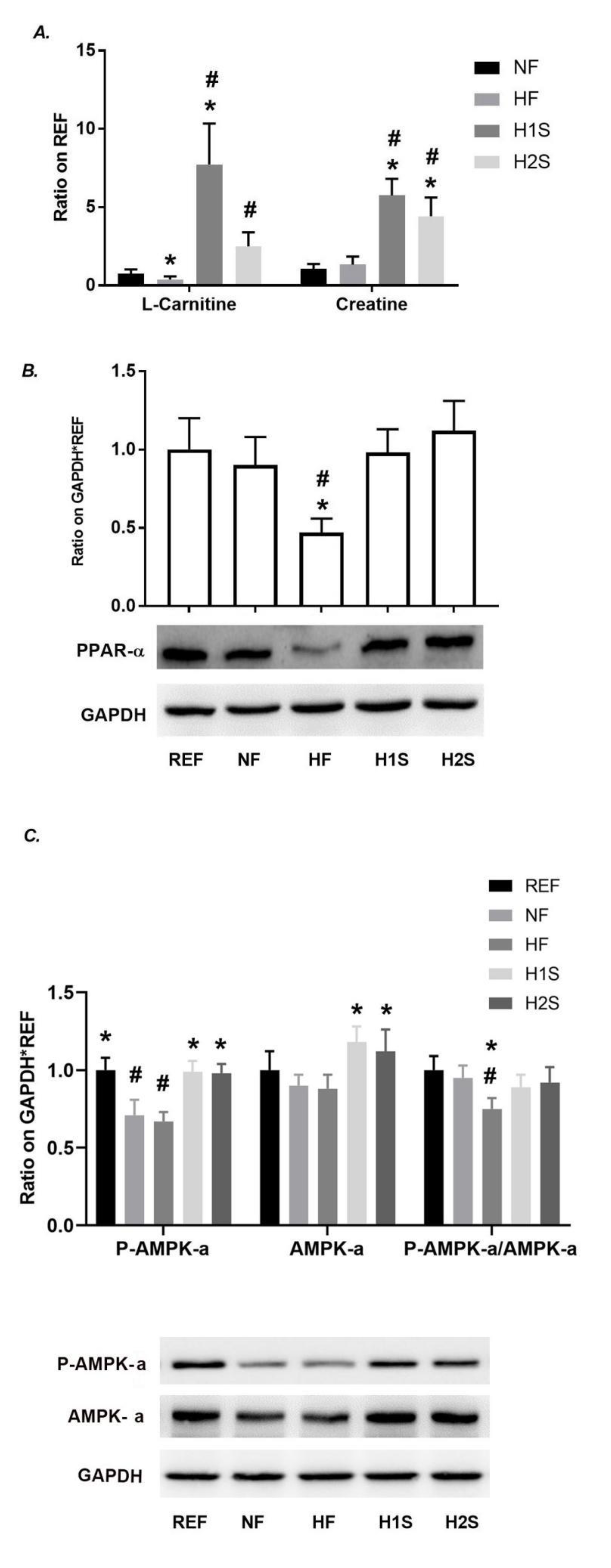
3.4. Maternal One-Carbon Supplementation Promoted Methyl Group Delivery to L-Carnitine and Normalized PPARα Expression and the AMPK Signaling Activation
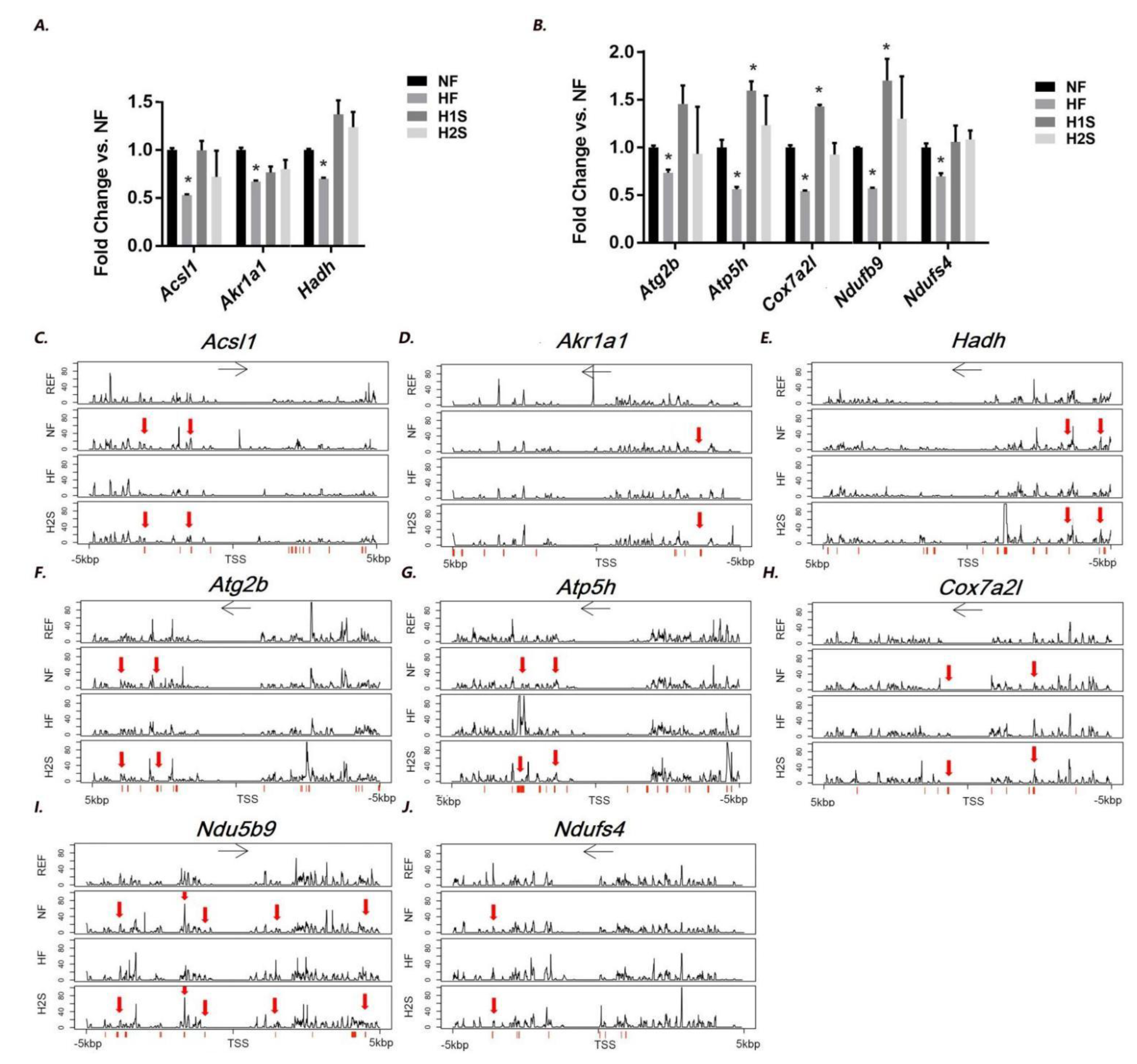
3.5. Genes Involved in Lipid Metabolism and Oxidative Phosphorylation, Whose Hepatic Expression Was Associated with DNA Methylation Modification, Were Identified in the Offspring Liver
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zheng, L.; Wang, M.; Du, Y.; Jiang, J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ Open 2020, 10, e036663. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006, 40 (Suppl. S1), S5–S10. [Google Scholar] [CrossRef]
- Le Clair, C.; Abbi, T.; Sandhu, H.; Tappia, P.S. Impact of maternal undernutrition on diabetes and cardiovascular disease risk in adult offspring. Can. J. Physiol. Pharmacol. 2009, 87, 161–179. [Google Scholar] [CrossRef]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar]
- Metges, C.C. Early Nutrition and Later Obesity: Animal Models Provide Insights into Mechanisms. Adv. Exp. Med. Biol. 2009, 646, 105–112. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Ong, Z.Y. The fetal origins of obesity: Early origins of altered food intake. Endocr. Metab. Immune Disord.-Drug Targets 2011, 11, 189–197. [Google Scholar] [CrossRef]
- Rooney, K.; Ozanne, S. Maternal over-nutrition and offspring obesity predisposition: Targets for preventative interventions. Int. J. Obes. 2011, 35, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Simar, D.; Chen, H.; Lambert, K.; Mercier, J.; Morris, M. Interaction between maternal obesity and post-natal over-nutrition on skeletal muscle metabolism. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 269–276. [Google Scholar] [CrossRef]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1842, 507–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Huffman, S.L. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern. Child Nutr. 2012, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.-F.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity 2016, 24, 1546–1553. [Google Scholar] [CrossRef] [Green Version]
- Pietrobelli, A.; Agosti, M.; The MeNu Group. Nutrition in the First 1000 Days: Ten Practices to Minimize Obesity Emerging from Published Science. Int. J. Environ. Res. Public Health 2017, 14, 1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmurzynska, A.; Stachowiak, M.; Gawecki, J.; Pruszynska-Oszmalek, E.; Tubacka, M. Protein and folic acid content in the maternal diet determine lipid metabolism and response to high-fat feeding in rat progeny in an age-dependent manner. Genes Nutr. 2011, 7, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.A.; Lalitha, A.; Pavithra, D.; Padmavathi, I.J.; Ganeshan, M.; Rao, K.R.; Venu, L.; Balakrishna, N.; Shanker, N.H.; Reddy, S.U.; et al. Maternal dietary folate and/or vitamin B12 restrictions alter body composition (adiposity) and lipid metabolism in Wistar rat offspring. J. Nutr. Biochem. 2013, 24, 25–31. [Google Scholar] [CrossRef]
- McKay, J.; Xie, L.; Manus, C.; Langie, S.; Maxwell, R.J.; Ford, D.; Mathers, J.C. Metabolic effects of a high-fat diet post-weaning after low maternal dietary folate during pregnancy and lactation. Mol. Nutr. Food Res. 2014, 58, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Nathanielsz, P.W.; Yan, J.; Green, R.; Nijland, M.; Miller, J.W.; Wu, G.; McDonald, T.J.; Caudill, M.A. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol. Rep. 2015, 3, e12564. [Google Scholar] [CrossRef]
- Cordero, P.; Gomez-Uriz, A.M.; Campion, J.; Milagro, F.I.; Martinez, J.A. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2012, 8, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Cordero, P.; Milagro, F.I.; Campion, J.; Martinez, J.A. Maternal Methyl Donors Supplementation during Lactation Prevents the Hyperhomocysteinemia Induced by a High-Fat-Sucrose Intake by Dams. Int. J. Mol. Sci. 2013, 14, 24422–24437. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Xu, H.; Wu, J.; Li, J.; Zhou, Y.; Ding, Z.; Siwko, S.K.; Yuan, X.; Schalinske, K.L.; Alpini, G.; et al. Maternal high-fat diet disrupted one-carbon metabolism in offspring, contributing to nonalcoholic fatty liver disease. Liver Int. 2021, 41, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Alpha Target Genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef] [Green Version]
- Nohr, E.A.; Vaeth, M.; Baker, J.; Sørensen, T.I.; Olsen, J.; Rasmussen, K.M. Pregnancy outcomes related to gestational weight gain in women defined by their body mass index, parity, height, and smoking status. Am. J. Clin. Nutr. 2009, 90, 1288–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, K.M.; Catalano, P.M.; Yaktine, A.L. New guidelines for weight gain during pregnancy: What obstetrician/gynecologists should know. Curr. Opin. Obstet. Gynecol. 2009, 21, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse Offspring Fed a High-Fat Diet. Int. J. Mol. Sci. 2014, 15, 6298–6313. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Fu, Z.; Li, H.; Gu, X.; Cai, Z.; Xu, P.; Cui, X.; You, L.; Wang, X.; Zhu, L.; et al. High folate intake contributes to the risk of large for gestational age birth and obesity in male offspring. J. Cell. Physiol. 2018, 233, 9383–9389. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, X.; Li, T.; He, J.; Huang, L.; Ouyang, Z.; He, L.; Wei, T.; He, Q. Improved Sp1 and Betaine Homocysteine-S-Methyltransferase Expression and Homocysteine Clearance Are Involved in the Effects of Zinc on Oxidative Stress in High-Fat-Diet-Pretreated Mice. Biol. Trace Element Res. 2017, 184, 436–441. [Google Scholar] [CrossRef]
- Xu, L.; Huang, D.; Hu, Q.; Wu, J.; Wang, Y.; Feng, J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br. J. Nutr. 2015, 113, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Bakir, M.B.; Salama, M.A.; Refaat, R.; Ali, M.A.; Khalifa, E.A.; Kamel, M. Evaluating the therapeutic potential of one-carbon donors in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2019, 847, 72–82. [Google Scholar] [CrossRef]
- Bouzidi, A.; Magnifico, M.C.; Paiardini, A.; Macone, A.; Boumis, G.; Giardina, G.; Rinaldo, S.; Liberati, F.R.; Lauro, C.; Limatola, C.; et al. Cytosolic serine hydroxymethyltransferase controls lung adenocarcinoma cells migratory ability by modulating AMP kinase activity. Cell Death Dis. 2020, 11, 1012. [Google Scholar] [CrossRef]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, J.N.; Leclerc, G.J.; Leclerc, G.M.; Barredo, J.C. AMPK and Akt Determine Apoptotic Cell Death following Perturbations of One-Carbon Metabolism by Regulating ER Stress in Acute Lymphoblastic Leukemia. Mol. Cancer Ther. 2011, 10, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Papadopoli, D.J.; Ma, E.H.; Roy, D.; Russo, M.; Bridon, G.; Avizonis, D.; Jones, R.G.; St-Pierre, J. Methotrexate elicits pro-respiratory and anti-growth effects by promoting AMPK signaling. Sci. Rep. 2020, 10, 8187. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Hadi, A.; Miraghajani, M.; Symonds, M.E.; Sheikhi, A.; Ghaedi, E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 151, 104554. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, F.B.; Coswig, V.S.; Galliano, L.M. Comment on ‘The effect of (l-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials’. Obes. Rev. 2016, 18, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Pooyandjoo, M.; Nouhi, M.; Shab-Bidar, S.; Djafarian, K.; Olyaeemanesh, A. The effect of (L-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 970–976. [Google Scholar] [CrossRef]
- McCann, M.; De la Rosa, M.G.; Rosania, G.; Stringer, K. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Tryndyak, V.P.; Bagnyukova, T.V.; Melnyk, S.; Montgomery, B.; Ross, S.A.; Latendresse, J.R.; Rusyn, I.; Beland, F.A. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J. Hepatol. 2009, 51, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Moody, L.; Wang, H.; Jung, P.M.; Chen, H.; Pan, Y.-X. Maternal and Post-Weaning High-Fat Diets Produce Distinct DNA Methylation Patterns in Hepatic Metabolic Pathways within Specific Genomic Contexts. Int. J. Mol. Sci. 2019, 20, 3229. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Sloboda, D.M.; Saffery, R.; Joo, E.; Vickers, M.H. Maternal nutritional history modulates the hepatic IGF–IGFBP axis in adult male rat offspring. Endocrine 2013, 46, 70–82. [Google Scholar] [CrossRef]
- Wankhade, U.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Front. Endocrinol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.-J.; Luo, S.-M.; Lin, F.; Liang, Q.-X.; Huang, L.; Wei, Y.-C.; Hou, Y.; Han, Z.-M.; Schatten, H.; Sun, Q.-Y. DNA Methylation in Oocytes and Liver of Female Mice and Their Offspring: Effects of High-Fat-Diet–Induced Obesity. Environ. Health Perspect. 2014, 122, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirola, C.J.; Gianotti, T.F.; Burgueño, A.L.; Rey-Funes, M.; Loidl, C.F.; Mallardi, P.; Martino, J.S.; Castaño, G.O.; Sookoian, S. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut 2012, 62, 1356–1363. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Jonas, W.; Ernst, S.B.; Kammel, A.; Jähnert, M.; Schürmann, A. Diet-dependent Alterations of Hepatic Scd1 Expression are Accompanied by Differences in Promoter Methylation. Horm. Metab. Res. 2013, 45, 786–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sookoian, S.; Rosselli, M.S.; Gemma, C.; Burgueño, A.L.; Gianotti, T.F.; Castaño, G.O.; Pirola, C.J. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: Impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1alpha promoter. Hepatology 2010, 52, 1992–2000. [Google Scholar] [CrossRef]
| Maternal Diet | Offspring Diet | ||
|---|---|---|---|
| Pre-Pregnancy | Pregnancy/Lactation | after Weaning | |
| REF (n = 15) | NF | NF | NF |
| NF (n = 15) | NF | NF | HF |
| HF (n = 15) | HF | HF | HF |
| H1S (n = 15) | HF | HF + H1S | HF |
| H2S (n = 15) | HF | HF + H2S | HF |
| HF vs. NF | H2S vs. NF | |||||
|---|---|---|---|---|---|---|
| Hypo-Met DMR | Hyper-Met DMR | Total | Hypo-Met DMR | Hyper-Met DMR | Total | |
| Acsl1 | 21 (100%) | 0 (0%) | 21 | 0 (0%) | 2 (100%) | 2 |
| Akr1a1 | 0 (0%) | 4 (100%) | 4 | 0 (0%) | 30 (100%) | 30 |
| Hadh | 0 (0%) | 1 (100%) | 1 | 17 (68%) | 8 (32%) | 25 |
| Atg2b | 4 (33.33%) | 8 (66.67%) | 12 | 6 (26.09%) | 17 (73.91%) | 23 |
| Atp5h | 0 (0%) | 144 (100%) | 144 | 0 (0%) | 20 (100%) | 20 |
| Cox7a2l | 2 (7.69%) | 24 (92.31%) | 26 | 0 (0%) | 0 (0%) | 0 |
| Ndufb9 | 12 (27.27%) | 32 (72.72%) | 44 | 0 (0%) | 72 (100%) | 72 |
| Ndufs4 | 0 (0%) | 10 (100%) | 10 | 0 (0%) | 3 (100%) | 3 |
| Total | 39 (14.89%) | 223 (85.11%) | 262 | 23 (13.14%) | 152 (86.86%) | 175 |
| # of HF-Induced DMRs | Recovered DMRs | Un-Recovered DMRs | Additional DMRsInduced by H2S | p Value HF vs. H2S (Fisher Exact Test) | |||
|---|---|---|---|---|---|---|---|
| # | % of HF-Induced | # | % of HF-Induced | ||||
| Acsl1 | 21 | 21 | 100 | 0 | 0 | 2 | 0 |
| Akr1a1 | 4 | 1 | 25 | 3 | 75 | 27 | 0 |
| Hadh | 1 | 0 | 0 | 1 | 100 | 24 | 0 |
| Atg2b | 12 | 12 | 100 | 0 | 0 | 23 | 0.054 |
| Atp5h | 144 | 124 | 99.31 | 1 | 0.69 | 19 | 0 |
| Cox7a2l | 26 | 24 | 100 | 0 | 0 | 0 | 0 |
| Ndufb9 | 44 | 41 | 93.18 | 3 | 6.82 | 69 | 0 |
| Ndufs4 | 10 | 10 | 100 | 0 | 0 | 3 | 0.051 |
| Total | 262 | 233 | 96.95 | 8 | 3.05 | 167 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Xu, H.; Wu, J.; Li, J.; Wang, X.; Liu, Z.; Kim, M.; Jeon, M.S.; Zhang, K.K.; Xie, L. Maternal One-Carbon Supplement Reduced the Risk of Non-Alcoholic Fatty Liver Disease in Male Offspring. Nutrients 2022, 14, 2545. https://doi.org/10.3390/nu14122545
Peng H, Xu H, Wu J, Li J, Wang X, Liu Z, Kim M, Jeon MS, Zhang KK, Xie L. Maternal One-Carbon Supplement Reduced the Risk of Non-Alcoholic Fatty Liver Disease in Male Offspring. Nutrients. 2022; 14(12):2545. https://doi.org/10.3390/nu14122545
Chicago/Turabian StylePeng, Hui, Huiting Xu, Jie Wu, Jiangyuan Li, Xian Wang, Zhimin Liu, Minjee Kim, Minsun S. Jeon, Ke K. Zhang, and Linglin Xie. 2022. "Maternal One-Carbon Supplement Reduced the Risk of Non-Alcoholic Fatty Liver Disease in Male Offspring" Nutrients 14, no. 12: 2545. https://doi.org/10.3390/nu14122545






