Arabinoxylan-Based Microcapsules Being Loaded with Bee Products as Bioactive Food Components Are Able to Modulate the Cell Migration and Inflammatory Response—In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Honey-Loaded Microcapsules
2.3. Antioxidant Properties
2.3.1. Preparation of Extracts
2.3.2. Determination of Antioxidant Activity by Electron Paramagnetic Resonance (EPR) Spectroscopy
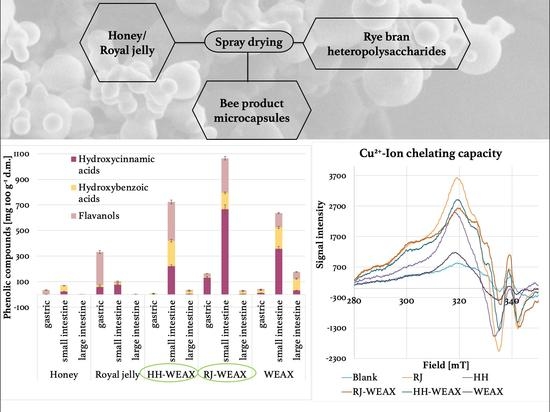
2.3.3. Determination of Cu2+-Ion Chelating Capacity by EPR Spectroscopy
2.4. Simulated Gastrointestinal Digestion
2.5. Cell Cultures
2.6. Cell Line-Based Studies
2.6.1. Cell Metabolic Activity
2.6.2. Cell Migration Assay
2.6.3. Detection of Intracellular Reactive Oxygen Species Generation in RAW 264.7 Cells
2.6.4. Determination of TNF-α, IL-6, and Nitric Oxide Secretion
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.1.1. Antioxidant Activity by Electron Paramagnetic Resonance (EPR) Spectroscopy
3.1.2. Cu2+-Ion Chelating Capacity of Microcapsules
3.2. Simulated Gastrointestinal Digestion
3.3. The Samples Encapsulation Influence on the Metabolic Activity and Migration of NIH-3T3 and HMEC-1 Cells
3.4. Immunomodulatory Properties of Microcapsules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilczyńska, A. Metody Oznaczania Aktywności Antyoksydacyjnej Miodów Pszczelich. Bromatol. Chem. Toksykol. 2009, 42, 870–874. [Google Scholar]
- Al-Waili, N.S.; Haq, A. Effect of Honey on Antibody Production Against Thymus-Dependent and Thymus-Independent Antigens in Primary and Secondary Immune Responses. J. Med. Food 2004, 7, 491–494. [Google Scholar] [CrossRef]
- Premratanachai, P.; Chanchao, C. Review of the Anticancer Activities of Bee Products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satarupa, R.; Subha, G. Physical, Chemical and Antioxidant Properties of Honey: A Review. Asian J. Chem. Pharm. Res. 2014, 2, 96–99. [Google Scholar]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major Royal Jelly Protein 3 Modulates Immune Responses in Vitro and in Vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, K.; Zhang, Y.-Z.; Zheng, Y.F.; Hu, F.L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 684–695. [Google Scholar]
- Caner, A.; Onal, M.G.; Silici, S. The Effect of Bee Bread (Perga) with Chemotherapy on MDA-MB-231 Cells. Mol. Biol. Rep. 2021, 48, 2299–2306. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 1259510. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic Fate of Polyphenols in the Human Superorganism. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4531–4538. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Han, X. Encapsulation of Food Ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hemar, Y. Nano- and Micro-Structured Assemblies for Encapsulation of Food Ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Khosravi-Darani, K.; Borazan, G.G.; Cui, J.; Pardakhty, A.; Yurdugul, S. Encapsulation of Food Ingredients Using Nanoliposome Technology. Int. J. Food Prop. 2008, 11, 833–844. [Google Scholar] [CrossRef]
- Gibbs, F. Encapsulation in the Food Industry: A Review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Jedlińska, A.; Samborska, K.; Wieczorek, A.; Wiktor, A.; Ostrowska-Ligęza, E.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; Tułodziecki, M.; et al. The Application of Dehumidified Air in Rapeseed and Honeydew Honey Spray Drying—Process Performance and Powders Properties Considerations. J. Food Eng. 2019, 245, 80–87. [Google Scholar] [CrossRef]
- Suhag, Y.; Nanda, V. Optimisation of Process Parameters to Develop Nutritionally Rich Spray-Dried Honey Powder with Vitamin C Content and Antioxidant Properties. Int. J. Food Sci. Technol. 2015, 50, 1771–1777. [Google Scholar] [CrossRef]
- Samborska, K.; Gajek, P.; Kaminska-Dworznicka, A. Spray Drying of Honey: The Effect of Drying Aids on Powder Properties. Polish J. Food Nutr. Sci. 2015, 65, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of Food Ingredients Using Carbohydrate Based Delivery Systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-Based Micro/Nanocarriers for Oral Colon-Targeted Drug Delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Sotelo-Cruz, N.; Micard, V.; Rascón-Chu, A.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Canett-Romero, R. Enzymatically CrossLinked Arabinoxylan Microspheres as Oral Insulin Delivery System. Int. J. Biol. Macromol. 2019, 126, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ouyang, W.; Jones, M.; Metz, T.; Martoni, C.; Haque, T.; Cohen, R.; Lawuyi, B.; Prakash, S. Preparation and Characterization of Novel Polymeric Microcapsules for Live Cell Encapsulation and Therapy. Cell Biochem. Biophys. 2007, 47, 159–167. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a Useful Natural Polymer for Microencapsulation and Therapeutic Applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Kowalska, G.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Wiktorska, M.; Gumul, D.; Orczykowska, M.; Dędek, K. Influence of Rye Bran Heteropolysaccharides on the Physicochemical and Antioxidant Properties of Honeydew Honey Microcapsules. Food Bioprod. Process. 2021, 130, 171–181. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and Nature of Ferulic Acid Substitution of Cell-Wall Polysaccharides in Graminaceous Plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Marquez-Escalante, J.; Campa-Mada, A.C.; Rascón-Chu, A.; López-Franco, Y.L.; Lizardi-Mendoza, J. Enzymatic CrossLinking of Ferulated Arabinoxylan: Effect of Laccase or Peroxidase Catalysis on the Gel Characteristics. Food Sci. Biotechnol. 2019, 28, 311–318. [Google Scholar] [CrossRef]
- Rosicka-Kaczmarek, J.; Komisarczyk, A.; Nebesny, E. Heteropolysaccharide Preparations from Rye and Wheat Bran as Sources of Antioxidants. J. Cereal Sci. 2018, 81, 37–43. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Possemiers, S.; Druart, C.; van de Wiele, T.; de Backer, F.; Cani, P.D.; Larondelle, Y.; Delzenne, N.M. Prebiotic Effects of Wheat Arabinoxylan Related to the Increase in Bifidobacteria, Roseburia and Bacteroides/Prevotella in Diet-Induced Obese Mice. PLoS ONE 2011, 6, e20944. [Google Scholar] [CrossRef] [Green Version]
- Kanzler, C.; Haase, P.T.; Schestkowa, H.; Kroh, L.W. Antioxidant Properties of Heterocyclic Intermediates of the Maillard Reaction and Structurally Related Compounds. J. Agric. Food Chem. 2016, 64, 7829–7837. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of Gastrointestinal PH Profiles in Normal Ambulant Human Subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. Standardised Static in vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basak, V.; Bahar, T.E.; Emine, K.; Yelda, K.; Mine, K.; Figen, S.; Rustem, N. Evaluation of Cytotoxicity and Gelatinases Activity in 3T3 Fibroblast Cell by Root Repair Materials. Biotechnol. Biotechnol. Equip. 2016, 30, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.; Lukeš, V. DFT/B3LYP Study of the Substituent Effect on the Reaction Enthalpies of the Individual Steps of Single Electron Transfer−Proton Transfer and Sequential Proton Loss Electron Transfer Mechanisms of Phenols Antioxidant Action. J. Phys. Chem. A 2006, 110, 12312–12320. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.; Lissi, E. Review of Methods to Determine Chain-Breaking Antioxidant Activity in Food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Zalibera, M.; Staško, A.; Šlebodová, A.; Jančovičová, V.; Čermáková, T.; Brezová, V. Antioxidant and Radical-Scavenging Activities of Slovak Honeys—An Electron Paramagnetic Resonance Study. Food Chem. 2008, 110, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Engeseth, N.J. Antioxidant Capacity of Honeys from Various Floral Sources Based on the Determination of Oxygen Radical Absorbance Capacity and Inhibition of in Vitro Lipoprotein Oxidation in Human Serum Samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Samborska, K.; Jedlińska, A.; Wiktor, A.; Derewiaka, D.; Wołosiak, R.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; et al. The Effect of Low-Temperature Spray Drying with Dehumidified Air on Phenolic Compounds, Antioxidant Activity, and Aroma Compounds of Rapeseed Honey Powders. Food Bioprocess Technol. 2019, 12, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Tomczyk, M.; Zaguła, G.; Tarapatskyy, M.; Kačániová, M.; Dżugan, M. The effect of honey variety on the quality of honey powder. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 949–954. [Google Scholar] [CrossRef]
- Čeksteryté, V.; Kurtinaitienė, B.; Venskutonis, P.R.; Pukalskas, A.; Kazernavičiūtė, R.; Balžekas, J. Evaluation of Antioxidant Activity and Flavonoid Composition in Differently Preserved Bee Products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Ecem Bayram, N.; Çebi, N.; Çelik, S.; Gerçek, Y.C.; Bayram, S.; Tanuğur Samancı, A.E.; Sağdıç, O.; Özkök, A. Turkish Royal Jelly: Amino Acid, Physicochemical, Antioxidant, Multi-Elemental, Antibacterial and Fingerprint Profiles by Analytical Techniques Combined with Chemometrics. J. Apic. Res. 2021, 60, 751–764. [Google Scholar] [CrossRef]
- Zarei, M.; Fazlara, A.; Tulabifard, N. Effect of Thermal Treatment on Physicochemical and Antioxidant Properties of Honey. Heliyon 2019, 5, e01894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkmen, N.; Sari, F.; Poyrazoglu, E.S.; Velioglu, Y.S. Effects of Prolonged Heating on Antioxidant Activity and Colour of Honey. Food Chem. 2006, 95, 653–657. [Google Scholar] [CrossRef]
- Wang, X.H.; Gheldof, N.; Engeseth, N.J. Effect of Processing and Storage on Antioxidant Capacity of Honey. J. Food Sci. 2006, 69, 96–101. [Google Scholar] [CrossRef]
- Braghini, F.; Biluca, F.C.; Gonzaga, L.V.; Kracik, A.S.; Vieira, C.R.W.; Vitali, L.; Micke, G.A.; Costa, A.C.O.; Fett, R. Impact of Short-term Thermal Treatment on Stingless Bee Honey (Meliponinae): Quality, Phenolic Compounds and Antioxidant Capacity. J. Food Process. Preserv. 2019, 43, e13954. [Google Scholar] [CrossRef]
- Aka, J.P.; Courtois, F.; Louarme, L.; Nicolas, J.; Billaud, C. Modelling the Interactions between Free Phenols, l-Ascorbic Acid, Apple Polyphenoloxidase and Oxygen during a Thermal Treatment. Food Chem. 2013, 138, 1289–1297. [Google Scholar] [CrossRef]
- Rakić, S.; Petrović, S.; Kukić, J.; Jadranin, M.; Tešević, V.; Povrenović, D.; Šiler-Marinković, S. Influence of Thermal Treatment on Phenolic Compounds and Antioxidant Properties of Oak Acorns from Serbia. Food Chem. 2007, 104, 830–834. [Google Scholar] [CrossRef]
- Jiang, D.; Chiaro, C.; Maddali, P.; Prabhu, K.S.; Peterson, D.G. Identification of Hydroxycinnamic Acid−Maillard Reaction Products in Low-Moisture Baking Model Systems. J. Agric. Food Chem. 2009, 57, 9932–9943. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu(II), Zn(II), and Fe(II) by Tannin Constituents of Selected Edible Nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Davy, B.; Wilson, J.; Melby, C.L. Antioxidant Properties of Cereal Products. J. Food Sci. 2002, 67, 2600–2603. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G. Physicochemical Characterization and Antioxidant Activity of 17 Commercial Moroccan Honeys. Int. J. Food Sci 2014, 65, 449–457. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in Vitro Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds, Minerals, and Antioxidant Capacity of Mimosa Scabrella Bentham Honeydew Honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Giampieri, F.; Zhang, J.; Ansary, J.; Pacetti, M.; Quiles, J.L.; Simal-Gandara, J.; Battino, M. Effect of In Vitro Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds and Antioxidant Activity of Manuka Honey. eFood 2019, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Suhag, Y.; Nayik, G.A.; Karabagias, I.K.; Nanda, V. Development and Characterization of a Nutritionally Rich Spray-Dried Honey Powder. Foods 2021, 10, 162. [Google Scholar] [CrossRef]
- He, R.; Ye, J.; Wang, L.; Sun, P. Preparation and Evaluation of Microcapsules Encapsulating Royal Jelly Sieve Residue: Flavor and Release Profile. Appl. Sci. 2020, 10, 8126. [Google Scholar] [CrossRef]
- Keskin, M.; Keskin, Ş.; Kolayli, S. Preparation of alcohol free propolis-alginate microcapsules, characterization and release property. Lwt 2019, 108, 89–96. [Google Scholar] [CrossRef]
- Keskin, Ş.; Trusheva, B.; Popova, M.; Kolaylı, S.; Bankova, V. Pollen beads: A new carrier for propolis active compounds. Comb. Chem. High Throughput Screen. 2010, 24, 1688–1695. [Google Scholar] [CrossRef]
- Argyri, K.; Komaitis, M.; Kapsokefalou, M. Iron Decreases the Antioxidant Capacity of Red Wine under Conditions of in Vitro Digestion. Food Chem. 2006, 96, 281–289. [Google Scholar] [CrossRef]
- Bobrich, A.; Fanning, K.J.; Rychlik, M.; Russell, D.; Topp, B.; Netzel, M. Phytochemicals in Japanese Plums: Impact of Maturity and Bioaccessibility. Food Res. Int. 2014, 65, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Brudzynski, K.A. Current Perspective on Hydrogen Peroxide Production in Honey. A Review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound Repair at a Glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir Honeydew Honey Flavonoids Inhibit TNF-α-Induced MMP-9 Expression in Human Keratinocytes: A New Action of Honey in Wound Healing. Arch. Dermatol. Res. 2013, 305, 619–627. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Ranzato, E.; Martinotti, S.; Burlando, B. Epithelial Mesenchymal Transition Traits in Honey-Driven Keratinocyte Wound Healing: Comparison among Different Honeys. Wound Repair Regen. 2012, 20, 778–785. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Yun, H.; Park, H.; Kim, S.Y.; Lee, K.G.; Han, S.M.; Cho, Y. Royal Jelly Enhances Migration of Human Dermal Fibroblasts and Alters the Levels of Cholesterol and Sphinganine in an In Vitro Wound Healing Model. Nutr. Res. Pract. 2010, 4, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic Acid/Alginate and an Assorted Polymers Loaded with Honey, Plant, and Marine Compounds for Progressive Wound Healing—Know-How. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Lu, L.; Reinach, P.S.; Kao, W. Corneal Epithelial Wound Healing. Exp. Biol. Med. 2001, 226, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Glucose Transporter 1 Expression in Corneal Wound Repair under High Serum Glucose Level. Jpn. J. Ophthalmol. 2000, 44, 470–474. [Google Scholar] [CrossRef]
- Knight, A. The Therapeutic Effects of Honey. Plymouth Stud. Sci. 2013, 6, 375–385. [Google Scholar]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal Activity of Different Honeys against Pathogenic Bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and Bioactivity of Royal Jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K.A. Potent Antibacterial Protein in Royal Jelly. Purification and Determination of the Primary Structure of Royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Hou, W.; Hu, S.; Su, Z.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J.; Gao, P. Myricetin Attenuates LPS-Induced Inflammation in RAW 264.7 Macrophages and Mouse Models. Future Med. Chem. 2018, 10, 2253–2264. [Google Scholar] [CrossRef]
- Tonks, A. Honey Stimulates Inflammatory Cytokine Production from Monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Battino, M. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: A comparison with Manuka honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef] [Green Version]
- Martos, I.; Ferreres, F.; Tomás-Barberán, F.A. Identification of Flavonoid Markers for the Botanical Origin of Eucalyptus Honey. J. Agric. Food Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive Compounds and Health-Promoting Properties of Royal Jelly: A Review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Abdelaliem, Y.F.; Esmail, A.H.M.; Mohdaly, A.A.A.; Ramadan, M.F. Evaluation of Egyptian Honeys and Their Floral Origins: Phenolic Compounds, Antioxidant Activities, and Antimicrobial Characteristics. Environ. Sci. Pollut. Res. 2020, 27, 20748–20756. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, D.; Vucevic, D.; Chinou, I.; Colic, M. Royal Jelly Fatty Acids Modulate Proliferation and Cytokine Production by Human Peripheral Blood Mononuclear Cells. Eur. Food Res. Technol. 2014, 238, 881–887. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless Bee Honey Protects against Lipopolysaccharide Induced-Chronic Subclinical Systemic Inflammation and Oxidative Stress by Modulating Nrf2, NF-ΚB and P38 MAPK. Nutr. Metab. 2019, 16, 15. [Google Scholar] [CrossRef]
- Hawkes, J.S.; Gibson, R.A.; Roberton, D.; Makrides, M. Effect of Dietary Nucleotide Supplementation on Growth and Immune Function in Term Infants: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2006, 60, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic Acid, Phenolic Acids, and Flavonoids in Malaysian Honey Extracts Demonstrate in Vitro Anti-Inflammatory Activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef]
- Woo, K.J.; Jeong, Y.J.; Inoue, H.; Park, J.W.; Kwon, T.K. Chrysin Suppresses Lipopolysaccharide-Induced Cyclooxygenase-2 Expression through the Inhibition of Nuclear Factor for IL-6 (NF-IL6) DNA-Binding Activity. FEBS Lett. 2005, 579, 705–711. [Google Scholar] [CrossRef] [Green Version]






| mg 100 g−1 d.m. | Honey | Royal Jelly | HH-WEAX | RJ-WEAX | WEAX | |
|---|---|---|---|---|---|---|
| caffeic acid | gastric | 0.00 ± 0.00 A,a | 0.11 ± 0.01 A,a | 0.12 ± 0.01 B,a | 0.21 ± 0.01B,a | 0.50 ± 0.02 a |
| small intestine | 0.02 ± 0.01 A,b | 0.90 ± 0.03 A,b | 8.15 ± 0.24 B,b | 9.31 ± 0.28 B,b | 9.36 ± 0.28 b | |
| large intestine | 0.07 ± 0.03 A,c | 0.01 ± 0.00 A,c | 0.20 ± 0.02 B,c | 0.32 ± 0.05 B,c | 1.92 ± 0.06 c | |
| chlorogenic acid | gastric | 0.03 ± 0.00 Aa | 0.04 ± 0.01 A,a | 0.52 ± 0.02 B,a | 0.44 ± 0.01 B,a | 1.25 ± 0.04 a |
| small intestine | 0.91 ± 0.03 A,b | 0.87 ± 0.03 A,b | 1.74 ± 0.05 B,b | 2.45 ± 0.07 B,b | 2.85 ± 0.09 b | |
| large intestine | 0.01 ± 0.00 A,c | 0.00 ± 0.00 A,c | 0.56 ± 0.02 B,a | 0.69 ± 0.02 B,c | 24.85 ± 0.75 c | |
| p-coumaric | gastric | 0.09 ± 0.00 A,a | 0.42 ± 0.01 A,a | 1.49 ± 0.04 B,a | 0.25 ± 0.01 B,a | 6.16 ± 0.13 a |
| small intestine | 0.59 ± 0.02 A,b | 0.73 ± 0.02 A,b | 3.24 ± 0.10 B,b | 5.14 ± 0.15 B,b | 14.96 ± 0.45 b | |
| large intestine | 0.09 ± 0.00 A,a | 0.12 ± 0.00 A,c | 0.92 ± 0.03 B,c | 0.90 ± 0.03 B,c | 2.52 ± 0.18 c | |
| ferulic acid | gastric | 0.12 ± 0.00 A,a | 0.00 ± 0.01 A,a | 1.15 ± 0.03 B,a | 0.22 ± 0.01 B,a | 2.22 ± 0.07 a |
| small intestine | 0.00 ± 0.00 A,b | 0.03 ± 0.01 A,b | 12.08 ± 0.36 B,b | 30.88 ± 0.93 B,b | 67.42 ± 2.02 b | |
| large intestine | 0.02 ± 0.00 A,c | 0.00 ± 0.00 A,a | 0.33 ± 0.01 B,c | 0.25 ± 0.02 B,a | 0.50 ± 0.02 c | |
| sinapic acid | gastric | 0.68 ± 0.02 A,a | 53.71 ± 1.61 A,a | 1.74 ± 0.05 B,a | 130.80 ± 3.92 B,a | 1.10 ± 0.03 a |
| small intestine | 21.66 ± 0.65 A,b | 34.34 ± 1.03 A,b | 307.67 ± 9.23 B,b | 486.89 ± 14.61 B,b | 280.49 ± 8.41 b | |
| large intestine | 0.14 ± 0.00 A,c | 0.46 ± 0.01 A,c | 1.86 ± 0.06 B,c | 1.68 ± 0.05 B,c | 1.45 ± 0.05 c | |
| galic acid | gastric | 0.79 ± 0.02 A,a | 8.35 ± 0.25 A,a | 4.98 ± 0.15 B,a | 5.92 ± 0.28 B,a | 0.65 ± 0.02 a |
| small intestine | 47.23 ± 1.42 A,b | 10.47 ± 0.41 A,b | 78.71 ± 2.36 B,b | 66.03 ± 1.98 B,b | 110.14 ± 3.30 b | |
| large intestine | 0.15 ± 0.00 A,c | 0.82 ± 0.05 A,c | 2.05 ± 0.09 B,c | 0.65 ± 0.02 B,c | 7.01 ± 0.21 c | |
| 3,4-dihydroxybenzoic acid | gastric | 0.55 ± 0.00 A,a | 4.09 ± 0.12 A,a | 0.85 ± 0.03 B,a | 2.96 ± 0.09 B,a | 0.72 ± 0.02 a |
| small intestine | 12.14 ± 0.36 A,b | 0.92 ± 0.03 A,b | 114.37 ± 3.43 B,b | 45.14 ± 1.35 B,b | 49.79 ± 1.49 b | |
| large intestine | 0.00 ± 0.00 A,a | 0.01 ± 0.02 A,c | 19.04 ± 0.57 B,c | 11.32 ± 0.34 B,c | 80.04 ± 2.40 c | |
| 4-hydroxybenzoic acid | gastric | 0.00 ± 0.00 A,a | 3.47 ± 0.10 A,a | 0.32 ± 0.01 B,a | 0.00 ± 0.00 B,a | 1.34 ± 0.04 a |
| small intestine | 0.02 ± 0.00 A,a | 0.01 ± 0.00 A,b | 5.03 ± 0.15 B,b | 11.82 ± 0.35 B,b | 7.93 ± 0.24 b | |
| large intestine | 0.09 ± 0.02 A,a | 0.03 ± 0.01 A,b | 0.16 ± 0.06 A,c | 0.06 ± 0.02 A,c | 0.09 ± 0.00 c | |
| 3-hydroxybenzoic acid | gastric | 0.00 ± 0.02 A,a | 0.00 ± 0.00 A,a | 0.02 ± 0.01 A,a | 0.02 ± 0.01 B,a | 0.01 ± 0.00 a |
| small intestine | 0.24 ± 0.01 A,b | 3.98 ± 0.12 A,b | 1.28 ± 0.04 B,b | 5.73 ± 0.17 B,b | 0.66 ± 0.02 b | |
| large intestine | 0.01 ± 0.00 A,a | 0.32 ± 0.02 A,a | 0.72 ± 0.02 B,c | 7.05 ± 0.21 B,c | 4.96 ± 0.15 c | |
| elagic acid | gastric | 2.00 ± 0.12 A,a | 3.49 ± 0.10 A,a | 1.24 ± 0.04 B,a | 3.16 ± 0.09 B,a | 3.38 ± 0.10 a |
| small intestine | 0.90 ± 0.03 A,b | 0.18 ± 0.01 A,b | 19.82 ± 0.59 B,b | 56.86 ± 1.71 B,b | 26.59 ± 0.80 b | |
| large intestine | 0.00 ± 0.00 A,a | 0.00 ± 0.00 A,c | 0.15 ± 0.00 B,c | 0.26 ± 0.01 B,c | 25.96 ± 0.78 b | |
| (+)-catechin | gastric | 0.12 ± 0.00 A,a | 72.53 ± 2.18 A,a | 0.05 ± 0.03 B,a | 10.12 ± 0.30 B,a | 0.77 ± 0.02 a |
| small intestine | 0.02 ± 0.00 A,b | 1.03 ± 0.03 A,b | 14.70 ± 0.44 B,b | 18.93 ± 0.57 B,b | 3.81 ± 0.11 b | |
| large intestine | 0.01 ± 0.00 A,c | 0.03 ± 0.00 A,c | 0.02 ± 0.00 B,a | 0.18 ± 0.01 B,c | 1.57 ± 0.05 c | |
| procyanidin B2 | gastric | 0.14 ± 0.00 A,a | 0.94 ± 0.03 A,a | 0.56 ± 0.02 B,a | 1.98 ± 0.06 B,a | 2.54 ± 0.08 a |
| small intestine | 0.00 ± 0.01 A,b | 0.00 ± 0.00 A,b | 1.84 ± 0.06 B,b | 1.80 ± 0.05 B,b | 4.76 ± 0.14 b | |
| large intestine | 0.00 ± 0.00 A,b | 0.00 ± 0.00 A,b | 0.94 ± 0.03 B,c | 0.52 ± 0.04 B,c | 4.29 ± 0.13 c | |
| (-)-epicatechin | gastric | 30.52 ± 0.92 A,a | 181.99 ± 5.46 A,a | 0.38 ± 0.01 B,a | 6.65 ± 0.20 B,a | 2.20 ± 0.07 a |
| small intestine | 0.00 ± 0.03 A,b | 0.00 ± 0.01 A,b | 264.30 ± 7.93 B,b | 186.00 ± 5.58 B,b | 72.34 ± 2.17 b | |
| large intestine | 0.00 ± 0.01 A,b | 0.01 ± 0.00 A,b | 9.57 ± 0.29 B,c | 6.86 ± 0.21 B,a | 18.63 ± 0.56 c | |
| quercetin-3-glucoside | gastric | 0.00 ± 0.01 A,a | 0.01 ± 0.00 A,a | 0.00 ± 0.00 A,a | 0.00 ± 0.01 A,a | 0.00 ± 0.00 a |
| small intestine | 1.01 ± 0.03 A,b | 0.00 ± 0.01 A,a,b | 1.67 ± 0.05 B,b | 3.81 ± 0.11 B,b | 2.30 ± 0.07 b | |
| large intestine | 0.04 ± 0.02 A,c | 0.00 ± 0.00 A,b | 0.00 ± 0.00 B,a | 0.00 ± 0.00 A,a | 0.00 ± 0.00 a | |
| quercetin-3-galactoside | gastric | 0.03 ± 0.00 A,a | 0.09 ± 0.01 A,a | 0.10 ± 0.01 B,a | 0.20 ± 0.01 B,a | 0.09 ± 0.00 a |
| small intestine | 0.13 ± 0.02 A,b | 0.05 ± 0.00 A,b | 0.98 ± 0.03 B,b | 1.37 ± 0.04 B,b | 1.10 ± 0.03 b | |
| large intestine | 0.02 ± 0.00 A,c | 0.13 ± 0.00 A,c | 1.20 ± 0.04 B,c | 1.13 ± 0.03 B,c | 2.81 ± 0.08 c | |
| Total | gastric | 35.07 ± 1.12 A,a | 329.24 ±9.88 A,a | 13.52 ± 0.41 B,a | 162.93 ± 4.89 B,a | 22.93 ± 0.69 a |
| small intestine | 84.84 ± 2.54 A,b | 53.50 ± 1.61 A,b | 835.58 ± 25.07 B,b | 932.16 ± 27.96 B,b | 654.50 ± 19.64 b | |
| large intestine | 0.74 ± 0.06 A,c | 1.87 ± 0.08 A,c | 37.72 ± 1.13 B,c | 31.87 ± 0.96 B,c | 176.60 ± 5.30 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, G.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Zakłos-Szyda, M.; Rohn, S.; Kanzler, C.; Wiktorska, M.; Niewiarowska, J. Arabinoxylan-Based Microcapsules Being Loaded with Bee Products as Bioactive Food Components Are Able to Modulate the Cell Migration and Inflammatory Response—In Vitro Study. Nutrients 2022, 14, 2529. https://doi.org/10.3390/nu14122529
Kowalska G, Rosicka-Kaczmarek J, Miśkiewicz K, Zakłos-Szyda M, Rohn S, Kanzler C, Wiktorska M, Niewiarowska J. Arabinoxylan-Based Microcapsules Being Loaded with Bee Products as Bioactive Food Components Are Able to Modulate the Cell Migration and Inflammatory Response—In Vitro Study. Nutrients. 2022; 14(12):2529. https://doi.org/10.3390/nu14122529
Chicago/Turabian StyleKowalska, Gabriela, Justyna Rosicka-Kaczmarek, Karolina Miśkiewicz, Małgorzata Zakłos-Szyda, Sascha Rohn, Clemens Kanzler, Magdalena Wiktorska, and Jolanta Niewiarowska. 2022. "Arabinoxylan-Based Microcapsules Being Loaded with Bee Products as Bioactive Food Components Are Able to Modulate the Cell Migration and Inflammatory Response—In Vitro Study" Nutrients 14, no. 12: 2529. https://doi.org/10.3390/nu14122529