Vitamin-D Deficiency and Supplementation Altered the Network of the Coronary Arteries in a Rodent Model—In Situ Video Microscopic Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Animals
2.2. Chemicals
2.3. Chronic Treatment of the Rats
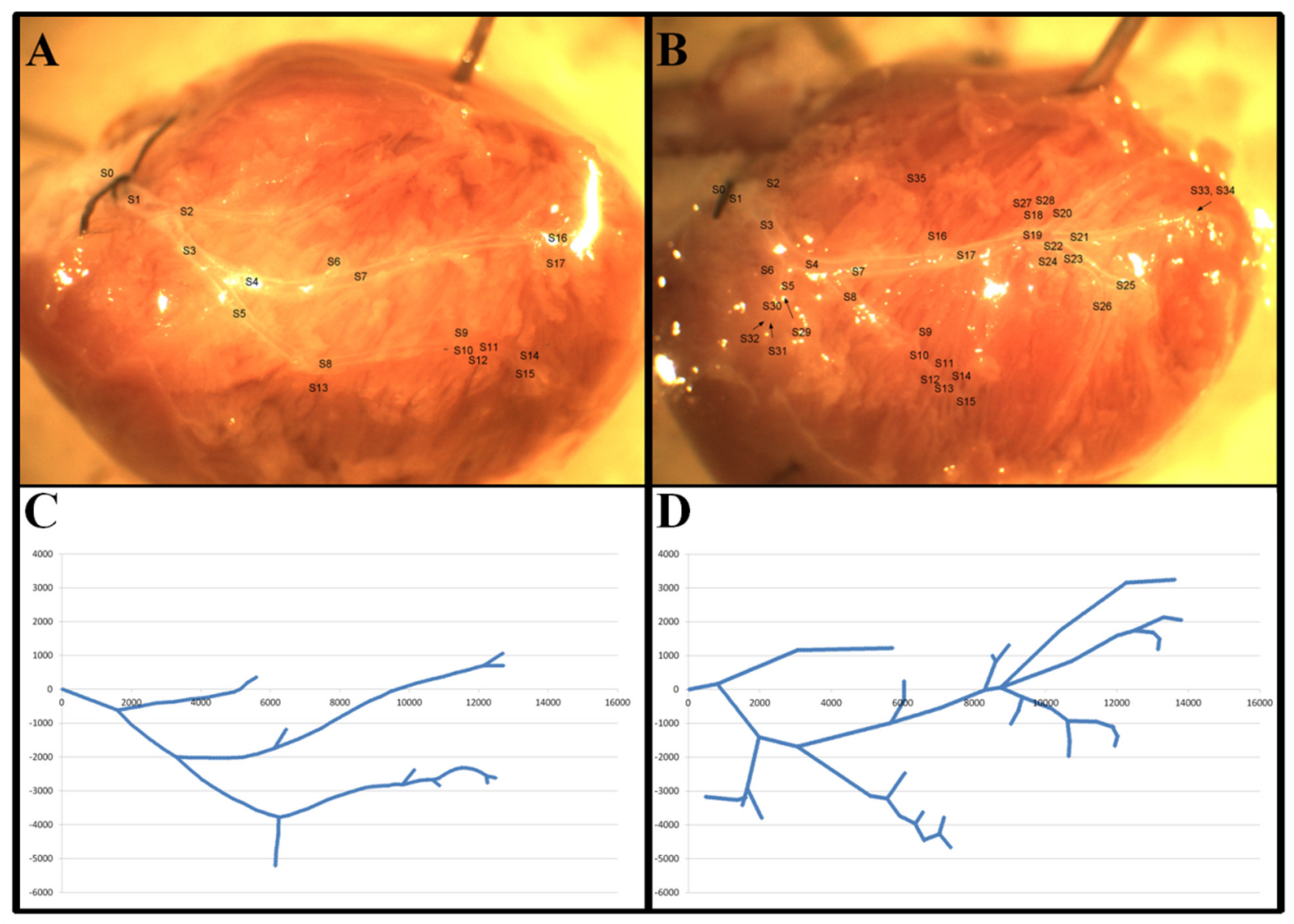
2.4. Preparation and Recording of LAD Coronary Networks
2.5. The Coordinate System and Geometric Analysis
2.6. Segment Analysis
2.7. Branching Analysis
2.8. Analysis of 50 µm-Long Vascular Ring Units
2.9. Network Anomalies
2.10. Statistical Analysis
3. Results
3.1. Body-Weight, Heart-Weight and Blood-Pressure Data
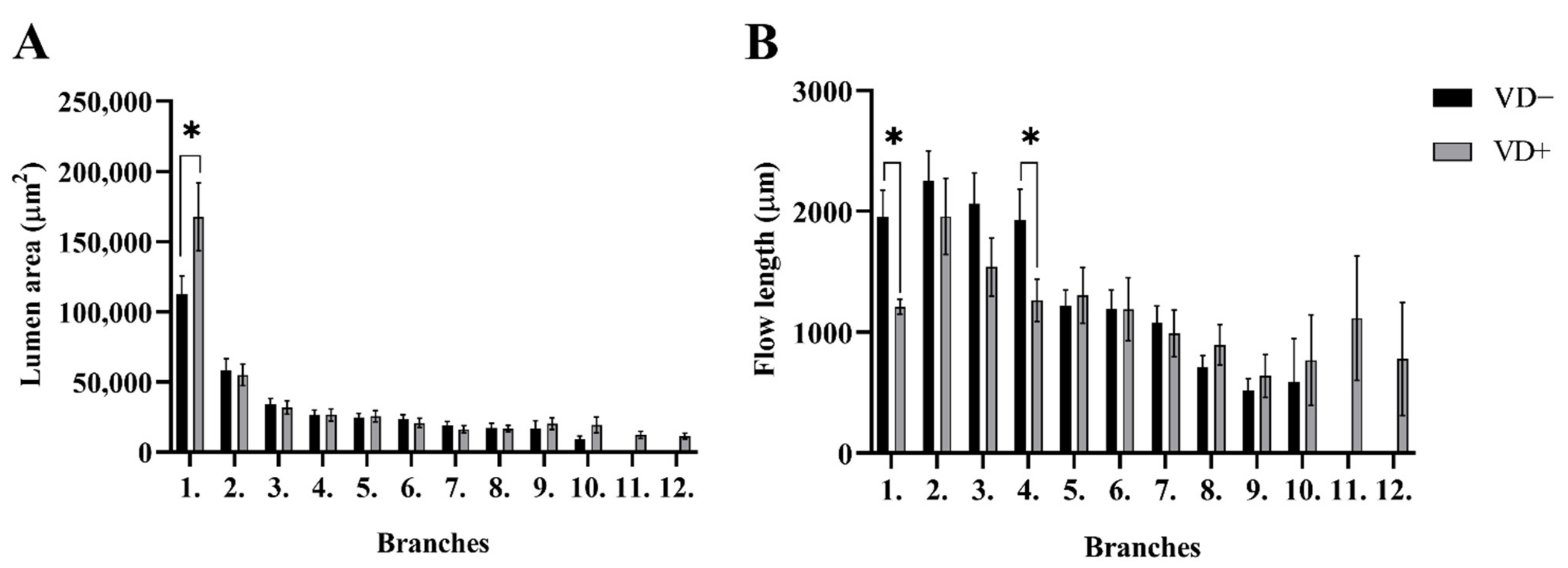
3.2. Segment Analysis
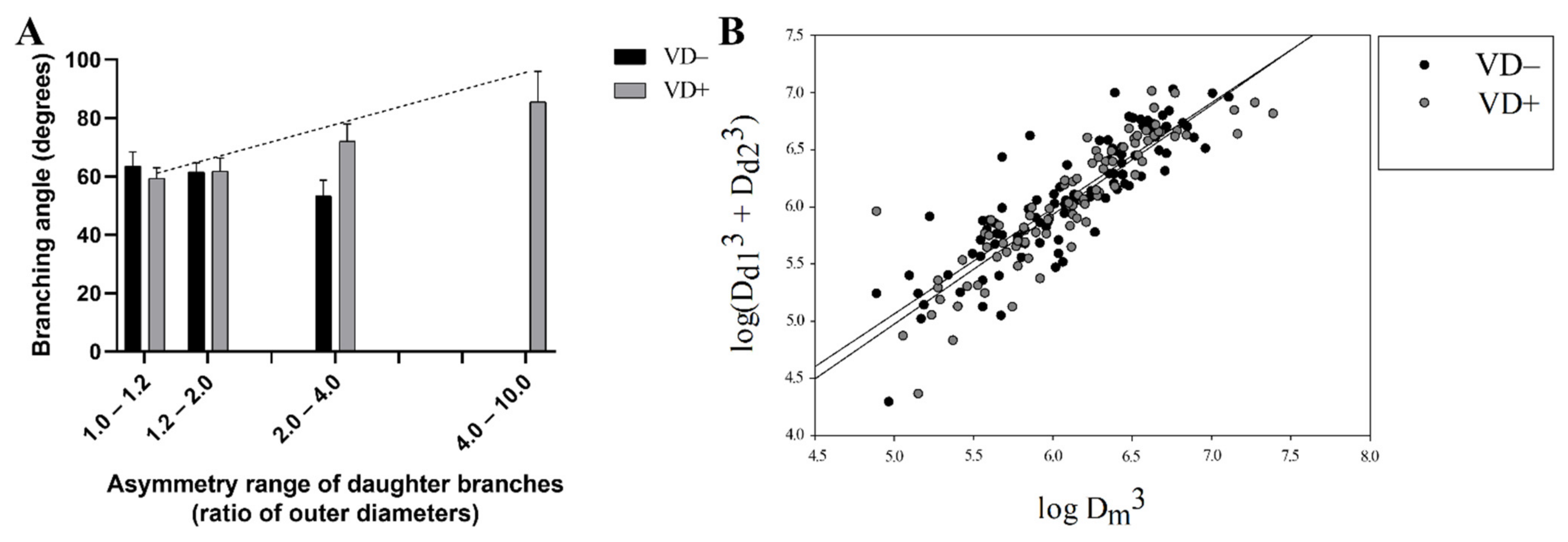
3.3. Branching Analysis
3.4. Abnormalities
3.5. Vascular Ring-Unit Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mccollum, E.V.; Pitz, W.; Simmonds, N.; Becker, J.E.; Shipley, P.G.; Bunting, R.W. Studies on experimental rickets: XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Lerner, P.P.; Sharony, L.; Miodownik, C. Association between mental disorders, cognitive disturbances and vitamin D serum level: Current state. Clin. Nutr. ESPEN 2018, 23, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.; Shin, E.-A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- von Websky, K.; Hasan, A.A.; Reichetzeder, C.; Tsuprykov, O.; Hocher, B. Impact of vitamin D on pregnancy-related disorders and on offspring outcome. J. Steroid Biochem. Mol. Biol. 2018, 180, 51–64. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef]
- Kilkkinen, A.; Knekt, P.; Aro, A.; Rissanen, H.; Marniemi, J.; Heliövaara, M.; Impivaara, O.; Reunanen, A. Vitamin D Status and the Risk of Cardiovascular Disease Death. Am. J. Epidemiol. 2009, 170, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Patel, R.S.; Tangpricha, V.; Quyyumi, A.A. Vitamin D and cardiovascular disease: Is the evidence solid? Eur. Heart J. 2013, 34, 3691–3698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef]
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef]
- Kunadian, V.; Ford, G.A.; Bawamia, B.; Qiu, W.; Manson, J.E. Vitamin D deficiency and coronary artery disease: A review of the evidence. Am. Heart J. 2013, 167, 283–291. [Google Scholar] [CrossRef]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Rai, V.; Agrawal, D.K. Role of Vitamin D in Cardiovascular Diseases. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1039–1059. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2021, 18, 96–110. [Google Scholar] [CrossRef]
- Manousaki, D.; Mokry, L.E.; Ross, S.; Goltzman, D.; Richards, J.B. Mendelian Randomization Studies Do not Support a Role for Vitamin D in Coronary Artery Disease. Circ. Cardiovasc. Genet. 2016, 9, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, S.; Brøndum-Jacobsen, P.; Bojesen, S.E.; Nordestgaard, B.G. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. Br. Med. J. 2014, 349, g6330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milazzo, V.; de Metrio, M.; Cosentino, N.; Marenzi, G.; Tremoli, E. Vitamin D and acute myocardial infarction. World J. Cardiol. 2017, 9, 14–20. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and Vitamin D Receptor: New Insights in the Treatment of Hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef]
- Chen, N.-C.; Hsu, C.-Y.; Mao, P.C.-M.; Dreyer, G.; Wu, F.-Z.; Chen, C.-L. The effects of correction of vitamin D deficiency on arterial stiffness: A systematic review and updated meta-analysis of randomized controlled trials. J. Steroid Biochem. Mol. Biol. 2019, 198, 105561. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, C.; de Nigris, F.; Williams-Ignarro, S.; Pignalosa, O.; Sica, V.; Ignarro, L.J. Nitric oxide and atherosclerosis: An update. Nitric Oxide 2006, 15, 265–279. [Google Scholar] [CrossRef]
- Bennett, A.L.; Lavie, C.J. Vitamin D Metabolism and the Implications for Atherosclerosis. In Ultraviolet Light in Human Health, Diseases and Environment; Springer: Cham, Switzerland, 2017; Volume 996, pp. 185–192. [Google Scholar] [CrossRef]
- Sziva, R.; Fontányi, Z.; Pál, É.; Hadjadj, L.; Monori-Kiss, A.; Horváth, E.; Benkő, R.; Magyar, A.; Heinzlmann, A.; Benyó, Z.; et al. Vitamin D Deficiency Induces Elevated Oxidative and Biomechanical Damage in Coronary Arterioles in Male Rats. Antioxidants 2020, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Fontányi, Z.; Sziva, R.; Pál, É.; Hadjadj, L.; Monori-Kiss, A.; Horváth, E.; Benkő, R.; Magyar, A.; Heinzlmann, A.; Benyó, Z.; et al. Vitamin D Deficiency Reduces Vascular Reactivity of Coronary Arterioles in Male Rats. Curr. Issues Mol. Biol. 2021, 43, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, L.; Monori-Kiss, A.; Horváth, E.M.; Heinzlmann, A.; Magyar, A.; Sziva, R.E.; Miklós, Z.; Pál, É.; Gál, J.; Szabó, I.; et al. Geometric, elastic and contractile-relaxation changes in coronary arterioles induced by Vitamin D deficiency in normal and hyperandrogenic female rats. Microvasc. Res. 2018, 122, 78–84. [Google Scholar] [CrossRef]
- Pál, É.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Mezei, Z.; Lippai, N.; Magyar, A.; Heinzlmann, A.; Karvaly, G.; Monos, E.; et al. Vitamin D deficiency causes inward hypertrophic remodeling and alters vascular reactivity of rat cerebral arterioles. PLoS ONE 2018, 13, e0192480. [Google Scholar] [CrossRef] [PubMed]
- Pál, É.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Lippai, N.; Horváth, E.M.; Magyar, A.; Monos, E.; Nádasy, G.L.; Benyó, Z.; et al. Gender, hyperandrogenism and vitamin D deficiency related functional and morphological alterations of rat cerebral arteries. PLoS ONE 2019, 14, e0216951. [Google Scholar] [CrossRef]
- Sipos, M.; Péterffy, B.; Sziva, R.; Magyar, P.; Hadjadj, L.; Bányai, B.; Süli, A.; Soltész-Katona, E.; Gerszi, D.; Kiss, J.; et al. Vitamin D Deficiency Cause Gender Specific Alterations of Renal Arterial Function in a Rodent Model. Nutrients 2021, 13, 704. [Google Scholar] [CrossRef]
- Sipos, M.; Gerszi, D.; Dalloul, H.; Bányai, B.; Sziva, R.; Kollarics, R.; Magyar, P.; Török, M.; Ács, N.; Szekeres, M.; et al. Vitamin D Deficiency and Gender Alter Vasoconstrictor and Vasodilator Reactivity in Rat Carotid Artery. Int. J. Mol. Sci. 2021, 22, 8029. [Google Scholar] [CrossRef]
- Lajtai, K.; Tarszabó, R.; Bányai, B.; Péterffy, B.; Gerszi, D.; Ruisanchez, É.; Sziva, R.E.; Korsós-Novák, Á.; Benkő, R.; Hadjadj, L.; et al. Effect of Vitamin D Status on Vascular Function of the Aorta in a Rat Model of PCOS. Oxidative Med. Cell. Longev. 2021, 2021, 8865979. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.D. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc. Natl. Acad. Sci. USA 1926, 12, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, C.D. The Physiological Principle of Minimum Work Applied to the Angle of Branching of Arteries. J. Gen. Physiol. 1926, 9, 835–841. [Google Scholar] [CrossRef]
- Wappler, E.A.; Antal, P.; Varbiro, S.; Székács, B.; Simon, A.; Nagy, Z.; Monos, E.; Nádasy, G.L. Network remodeling of intramural coronary resistance arteries in the aged rat: A statistical analysis of geometry. Mech. Ageing Dev. 2013, 134, 307–313. [Google Scholar] [CrossRef]
- Nádasy, G.L.; Szekeres, M.; Dézsi, L.; Varbiro, S.; Székács, B.; Monosa, E. Preparation of Intramural Small Coronary Artery and Arteriole Segments and Resistance Artery Networks from the Rat Heart for Microarteriography and for in Situ Perfusion Video Mapping. Microvasc. Res. 2001, 61, 282–286. [Google Scholar] [CrossRef]
- Török, M.; Merkely, P.; Monori-Kiss, A.; Horváth, E.M.; Sziva, R.E.; Péterffy, B.; Jósvai, A.; Sayour, A.A.; Oláh, A.; Radovits, T.; et al. Network analysis of the left anterior descending coronary arteries in swim-trained rats by an in situ video microscopic technique. Biol. Sex Differ. 2021, 12, 37. [Google Scholar] [CrossRef]
- Monori-Kiss, A.; Antal, P.; Szekeres, M.; Varbiro, S.; Fees, A.; Szekacs, B.; Nadasy, G.L. Morphological remodeling of the intramural coronary resistance artery network geometry in chronically Angiotensin II infused hypertensive female rats. Heliyon 2020, 6, e03807. [Google Scholar] [CrossRef]
- Hadjadj, L.; Várbíró, S.; Horváth, E.M.; Monori-Kiss, A.; Pál, E.; Karvaly, G.B.; Heinzlmann, A.; Magyar, A.; Szabo, I.; Sziva, R.E.; et al. Insulin resistance in an animal model of polycystic ovary disease is aggravated by vitamin D deficiency: Vascular consequences. Diabetes Vasc. Dis. Res. 2018, 15, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muldowney, S.; Kiely, M. Vitamin D and cardiometabolic health: A review of the evidence. Nutr. Res. Rev. 2010, 24, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Burgess, S.; Munroe, P.B.; Khan, H. Vitamin D and high blood pressure: Causal association or epiphenomenon? Eur. J. Epidemiol. 2014, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Park, K.M.; Lee, M.J.; Yang, D.H.; Kim, S.H.; Lee, S.Y. Vitamin D and Hypertension. Electrolytes Blood Press. 2017, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Alemzadeh, R.; Kichler, J.; Babar, G.; Calhoun, M. Hypovitaminosis D in obese children and adolescents: Relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008, 57, 183–191. [Google Scholar] [CrossRef]
- Veronese, N.; Trevisan, C.; Carraro, S.; Sarti, S.; Zanforlini, B.M.; de Rui, M.; Coin, A.; Manzato, E.; Sergi, G. Hypovitaminosis D and fat mass in healthy older people. Eur. J. Clin. Nutr. 2016, 70, 1080–1082. [Google Scholar] [CrossRef]
- Fry, C.M.; Sanders, T.A.B. Vitamin D and risk of CVD: A review of the evidence. Proc. Nutr. Soc. 2015, 74, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Sacerdote, A.; Dave, P.; Lokshin, V.; Bahtiyar, G. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr. Diabetes Rep. 2019, 19, 101. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.; McAllister, R.; Morgan, A.; Rai, T.; McGilligan, V.; Ennis, M.; Page, C.; Kelly, C.; Peace, A.; Corfe, B.; et al. The Interdependency and Co-Regulation of the Vitamin D and Cholesterol Metabolism. Cells 2021, 10, 2007. [Google Scholar] [CrossRef] [PubMed]
- Trechsel, U.; Taylor, C.M.; Eisman, J.A.; Bonjour, J.P.; Fleisch, H. Plasma levels of vitamin D metabolites in diphosphonate-treated rats. Clin. Sci. 1981, 61, 471–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirhosseini, N.Z.; Knaus, S.J.; Bohaychuk, K.; Singh, J.; Vatanparast, H.A.; Weber, L.P. Both high and low plasma levels of 25-hydroxy vitamin D increase blood pressure in a normal rat model. Br. J. Nutr. 2016, 116, 1889–1900. [Google Scholar] [CrossRef]
- Wilson, H.D.; Horst, R.L.; Schedl, H.P. Calcium intake regulates 1,25-dihydroxy-vitamin D formation in the diabetic rat. Diabetes 1982, 31 Pt 1, 401–405. [Google Scholar] [CrossRef]
- Takács, I.; Dank, M.; Majnik, J.; Nagy, G.; Szabó, A.; Szabó, B.; Szekanecz, Z.; Sziller, I.; Toldy, E.; Tislér, A.; et al. Magyarországi konszenzusajánlás a D-vitamin szerepéről a betegségek megelőzésében és kezelésében. Orv. Hetil. 2022, 163, 575–584. [Google Scholar]
- Halloran, B.P.; Barthell, E.N.; DeLuca, H.F. Vitamin D metabolism during pregnancy and lactation in the rat. Proc. Natl. Acad. Sci. USA 1979, 76, 5549–5553. [Google Scholar] [CrossRef] [Green Version]
- Weishaar, R.E.; Simpson, R.U. Vitamin D3 and cardiovascular function in rats. J. Clin. Investig. 1987, 79, 1706–1712. [Google Scholar] [CrossRef]
- Xiang, W.; Kong, J.; Chen, S.; Cao, L.-P.; Qiao, G.; Zheng, W.; Liu, W.; Li, X.; Gardner, D.G.; Li, Y.C. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am. J. Physiol. Metab. 2005, 288, E125–E132. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Berry, J.E.; Jarvis, A.K.; Somerman, M.J.; Simpson, R.U. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 1997, 272, H1751–H1758. [Google Scholar] [CrossRef]
- Kim, I.M.; Norris, K.C.; Artaza, J.N. Vitamin D and Cardiac Differentiation. Vitam. Horm. 2016, 100, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar]
- Kassab, G.S.; Rider, C.A.; Tang, N.J.; Fung, Y.C. Morphometry of pig coronary arterial trees. Am. J. Physiol. Circ. Physiol. 1993, 265, H350–H365. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, R.J.; Palmer, P.J.; Peiffer, G.L.; Schreiber, K.L.; Eastham, C.L.; Marcus, M.L. Morphometry of canine coronary arteries, arterioles, and capillaries during hypertension and left ventricular hypertrophy. Circ. Res. 1986, 58, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Rakusan, K.; Wicker, P. Morphometry of the small arteries and arterioles in the rat heart: Effects of chronic hypertension and exercise. Cardiovasc. Res. 1990, 24, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zamir, M.; Phipps, S.; Langille, B.L.; Wonnacott, T.H. Branching characteristics of coronary arteries in rats. Can. J. Physiol. Pharmacol. 1984, 62, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Zamir, M. Optimality principles in arterial branching. J. Theor. Biol. 1976, 62, 227–251. [Google Scholar] [CrossRef]
- Grundmann, M.; Haidar, M.; Placzko, S.; Niendorf, R.; Darashchonak, N.; Hubel, C.A.; von Versen-Höynck, F. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. Physiol. 2012, 303, C954–C962. [Google Scholar] [CrossRef]
- Jamali, N.; Song, Y.; Sorenson, C.M.; Sheibani, N. 1,25(OH)2D3 regulates the proangiogenic activity of pericyte through VDR-mediated modulation of VEGF production and signaling of VEGF and PDGF receptors. FASEB BioAdv. 2019, 1, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.Y.L.; Zheng, Y.; Hsu, W.; Lee, M.L.; Lau, Q.P.; Mitchell, P.; Wang, J.J.; Klein, R.; Wong, T.Y. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology 2011, 118, 812–818. [Google Scholar] [CrossRef]
- Kahe, F.; Sharfaei, S.; Pitliya, A.; Jafarizade, M.; Seifirad, S.; Habibi, S.; Chi, G. Coronary artery tortuosity: A narrative review. Coron. Artery Dis. 2020, 31, 187–192. [Google Scholar] [CrossRef] [PubMed]


| Morphologic Deformity | VD− | VD+ | Chi2 (χ2) Probe Significance Level |
|---|---|---|---|
| Parallel running | 2 | 3 | 0.65 |
| Broken course | 7 | 6 | 0.74 |
| Multiple branching | 11 | 8 | 0.49 |
| Tortuosity > 8 | 7 | 5 | 0.56 |
| Sum of all deformities | 27 | 22 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalloul, H.; Hainzl, T.; Monori-Kiss, A.; Hadjadj, L.; Nádasy, G.L.; Török, M.; Várbíró, S. Vitamin-D Deficiency and Supplementation Altered the Network of the Coronary Arteries in a Rodent Model—In Situ Video Microscopic Technique. Nutrients 2022, 14, 2041. https://doi.org/10.3390/nu14102041
Dalloul H, Hainzl T, Monori-Kiss A, Hadjadj L, Nádasy GL, Török M, Várbíró S. Vitamin-D Deficiency and Supplementation Altered the Network of the Coronary Arteries in a Rodent Model—In Situ Video Microscopic Technique. Nutrients. 2022; 14(10):2041. https://doi.org/10.3390/nu14102041
Chicago/Turabian StyleDalloul, Hicham, Tobias Hainzl, Anna Monori-Kiss, Leila Hadjadj, György L. Nádasy, Marianna Török, and Szabolcs Várbíró. 2022. "Vitamin-D Deficiency and Supplementation Altered the Network of the Coronary Arteries in a Rodent Model—In Situ Video Microscopic Technique" Nutrients 14, no. 10: 2041. https://doi.org/10.3390/nu14102041
APA StyleDalloul, H., Hainzl, T., Monori-Kiss, A., Hadjadj, L., Nádasy, G. L., Török, M., & Várbíró, S. (2022). Vitamin-D Deficiency and Supplementation Altered the Network of the Coronary Arteries in a Rodent Model—In Situ Video Microscopic Technique. Nutrients, 14(10), 2041. https://doi.org/10.3390/nu14102041






