Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits
Abstract
:1. Introduction
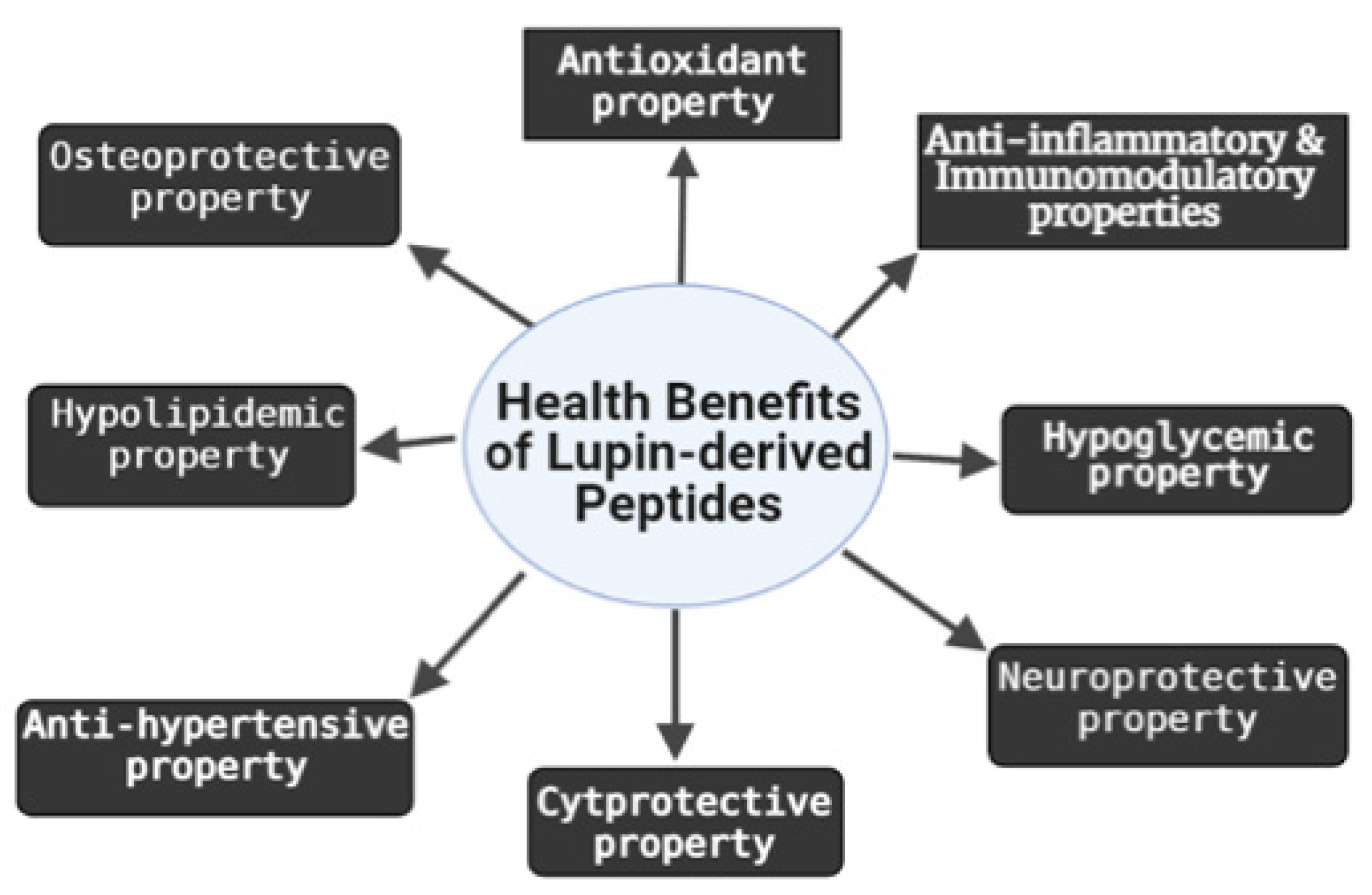
2. Biological Activities of LDPs
2.1. Antioxidant and Cytoprotective Effects
2.2. Anti-Inflammatory and Immunomodulatory Effects
2.3. Osteoprotective Effect
2.4. Neuroprotective Effect
2.5. Hypocholesterolemic and Hypoglycemic Effects
2.6. Anti-Hypertensive Effect
3. Structure-Activity Relationship of Bioactive LDPs
4. Transepithelial Transport, Biostability and Bioavailability of LDPs
5. Safety Concerns of LDPs
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan diet health benefits in metabolic syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Rigi, S.; Mousavi, S.M.; Benisi-Kohansal, S.; Esmaillzadeh, A.; Azadbakht, L. The association between plant-based dietary patterns and risk of breast cancer: A case–control study. Sci. Rep. 2021, 11, 3391. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. Optimization of the enzymatic hydrolysis of lupin (lupinus) proteins for producing ACE-inhibitory peptides. J. Agric. Food Chem. 2014, 62, 1846–1851. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Weisse, K.; Hirche, F.; Eder, K.; Stangl, G.I. Lupin protein influences the expression of hepatic genes involved in fatty acid synthesis and triacylglycerol hydrolysis of adult rats. Br. J. Nutr 2008, 99, 952–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viveros, A.; Centeno, C.; Ariia, I.; Brenes, A. Cholesterol-lowering effects of dietary lupin (Lupinus albus var Multolupa) in chicken diets. Poult. Sci. 2007, 86, 2631–2638. [Google Scholar] [CrossRef]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Batistuti, J.S.; da Cruz, R.J.; Sadiva, P.H.N.; Areas, J.A.G. Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate. Food Chem. 2012, 132, 1521–1526. [Google Scholar] [CrossRef] [Green Version]
- Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Parolari, A.; Magni, C.; Duranti, M. Lupin seed γ-conglutin lowers blood glucose in hyperglycaemic rats and increases glucose consumption of HepG2 cells. Br. J. Nutr. 2012, 107, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Comp. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Krämer, J.; Jahreis, G.; Kiehntopf, M. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Schutkowski, A.; Hirche, F.; Geissler, S.; Radtke, J.; Stangl, G.I. Additive effects of lupin protein and phytic acid on aortic calcification in ApoE deficient mice. J. Clin. Transl. Endocrinol. 2014, 2, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radtke, J.; Geissler, S.; Schutkowski, A.; Brandsch, C.; Kluge, H.; Duranti, M.M.; Keller, S.; Jahreis, G.; Hirche, F.; Stangl, G.I. Lupin protein isolate versus casein modifies cholesterol excretion and mRNA expression of intestinal sterol transporters in a pig model. Nutr. Metab. 2014, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Lima-Cabello, E.; Alché, J.D.; Morales-Santana, S.; Clemente, A.; Jimenez-Lopez, J.C. Narrow-leafed lupin (Lupinus angustifolius L.) seeds gamma-conglutin is an anti-inflammatory protein promoting insulin resistance improvement and oxidative stress amelioration in PANC-1 pancreatic cell-line. Antioxidants 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbandy, M.; Rho, J.R. New flavone-di-C-glycosides from the seeds of Egyptian lupin (Lupinus termis). Phytochem. Lett. 2014, 9, 127–131. [Google Scholar] [CrossRef]
- Ruiz-López, M.A.; Barrientos-Ramírez, L.; García-López, P.M.; Valdés-Miramontes, E.H.; Zamora-Natera, J.F.; Rodríguez-Macias, R.; Salcedo-Pérez, E.; Bañuelos-Pineda, J.; Vargas-Radillo, J.J. Nutritional and bioactive compounds in Mexican Lupin beans species: A mini-review. Nutrients 2019, 11, 1785. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Tech. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Hwang, I.M.; Lee, H.W.; Lee, H.M.; Yang, J.S.; Seo, H.Y.; Chung, Y.J.; Kim, S.H. Rapid and simultaneous quantification of five quinolizidine alkaloids in Lupinus angustifolius L. and its processed foods by UPLC−MS/MS. ACS Omega 2020, 5, 20825–20830. [Google Scholar] [CrossRef]
- Johnson, S.K.; Chua, V.; Hall, S.R.; Baxter, L.A. Lupin kernel fibre foods improve bowel function and beneficially modify some putative faecal risk factors for colon cancer in men. Br. J. Nutr. 2006, 95, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Stapel, J.; Oppermann, C.; Richter, D.U.; Ruth, W.; Briese, V. Anti-carcinogenic effects of ethanolic extracts from root and shoot of Lupinus angustifolius on breast carcinoma cell lines MCF-7 and BT20. J. Med. Plants Res. 2015, 9, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.B.; Hamed, M.S.; Khiralla, G.M.; Mohamed, A.F. Cactus and lupin extracts as prospective anticancer agents compared with utoral drug. J. Food Biochem. 2020, e13299. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of β-galactosides. Food Chem. 2006, 98, 291–299. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Borowczyk, P.; Zaworska, A.; Nowak, W.; Frankiewicz, A.; Gulewicz, P. The effect of dry yeast fermentation on chemical composition and protein characteristics of blue lupin seeds. Food Technol. Biotechnol. 2016, 54, 360. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Chirinos, R.; Ranilla, L.G.; Pedreschi, R. Bioactive Potential of Andean Fruits, Seeds, and Tubers. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 84, pp. 287–343. [Google Scholar]
- Millan-Linares, M.C.; Lemus-Conejo, A.; Yust, M.M.; Pedroche, J.; Carrillo-Vico, A.; Millan, F.; Montserrat-de la Paz, S. GPETAFLR, a novel bioactive peptide from Lupinus angustifolius L. protein hydrolysate, reduces osteoclastogenesis. J. Funct. Foods 2018, 47, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Millan-Linares, M.C.; Toscano, R.; Lemus-Conejo, A.; Martin, M.E.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S. GPETAFLR, a biopeptide from Lupinus angustifolius L., protects against oxidative and inflammatory damage in retinal pigment epithelium cells. J. Food Biochem. 2019, 43, e12995. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Aiello, G. YDFYPSSTKDQQS (P3), a peptide from lupin protein, absorbed by Caco-2 cells, modulates cholesterol metabolism in HepG2 cells via SREBP-1 activation. J. Food Biochem 2018, 42, e12524. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranald, G.; Sambuy, Y.; Arnoldi, A. Soybean- and lupin-derived peptides inhibit DPP-IV activity on in situ human intestinal caco-2 cells and ex vivo human serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.Y.; Yao, Y. Peptides from extruded lupin (Lupinus albus L.) regulate inflammatory activity via the p38 MAPK signal transduction pathway in RAW 264.7 cells. J. Agric. Food Chem. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Bollati, C.; Li, J.; Bartolomei, M.; Ranaldi, G.; Ferruzza, S.; Fassi, E.M.A.; Grazioso, G.; Sambuy, Y.; et al. Trans-epithelial transport, metabolism, and biological activity assessment of the multi-target lupin peptide LILPKHSDAD (P5) and its metabolite LPKHSDAD (P5-Met). Nutrients 2021, 13, 863. [Google Scholar] [CrossRef]
- Kamran, F.; Phillips, M.; Reddy, N. Functional properties of Australian blue lupin (Lupinus angustifolius) protein and biological activities of protein hydrolysates. Legume Sci. 2021, 3, e65. [Google Scholar] [CrossRef]
- Guzmán, T.J.; Düfer, M.; Wiedemann, M.; Olguín-Alor, R.; Soldevila, G.; Gurrola-Díaz, C.M. Lupin γ-conglutin protects against cell death induced by oxidative stress and lipotoxicity, but transiently inhibits in vitro insulin secretion by increasing KATP channel currents. Int. J. Biol. Macromol. 2021, 187, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin peptides lower low-density lipoprotein (LDL) cholesterol through an up-regulation of the LDL receptor/sterol regulatory element binding protein 2 (SREBP2) pathway at HepG2 cell line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Ackland, T.R.; Beilin, L.J.; Hodgson, J.M. Effects of lupin kernel flour-enriched bread on blood pressure: A controlled intervention study. Am. J. Clin. Nutr. 2009, 89, 7663–7772. [Google Scholar] [CrossRef] [Green Version]
- Belski, R.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Woodman, R.J.; Ackland, T.R.; Beilin, L.J.; Dove, E.R.; Carlyon, N.B.; Jayaseena, V.; et al. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: A 12-month randomized controlled weight loss trial. Int. J. Obes. 2011, 35, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchoucha, R.; Fradj, M.K.B.; Bouchoucha, M.; Akrout, M.; Feki, M.; Kaabachi, N.; Raies, N.; Slimane, H. Anti-hyperglycemic and anti-hyperlipidemic effects of Lupinus albus in type 2 diabetic patients: A randomized double-blind, placebo-controlled clinical trial. Int. J. Pharmacol. 2016, 12, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Skalkos, S.; Moschonis, G.; Thomas, C.J.; McMillan, J.; Kouris-Blazos, A. Effect of lupin-enriched biscuits as substitute mid-meal snacks on post-prandial interstitial glucose excursions in post-surgical hospital patients with type 2 diabetes. Nutrients 2020, 12, 1239. [Google Scholar] [CrossRef]
- Ward, N.C.; Mori, T.A.; Beilin, L.J.; Johnson, S.; Williams, C.; Gan, S.K.; Puddey, I.B.; Woodman, R.; Phillips, M.; Connolly, E.; et al. The effect of regular consumption of lupin-containing foods on glycaemic control and blood pressure in people with type 2 diabetes mellitus. Food Funct. 2020, 11, 741–747. [Google Scholar] [CrossRef]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Lammi, C. The health benefits of sweet lupin seed flours and isolated proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Santos-Sánchez, G.; Pedroche, J.; Millán, F.; Carrera Sánchez, C.; Fernández-Pachón, M.S.; Millán-Linares, M.C.; Martínez-López, A.; et al. Safety and efficacy of a beverage containing lupine protein hydrolysates on the immune, oxidative and lipid status in healthy subjects: An intervention study (the Lupine-1 Trial). Mol. Nutr. Food Res. 2021, 65, e2100139. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef] [PubMed]
- Matemu, A.; Nakamura, S.; Katayama, S. Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two peptides from soy β-conglycinin induce a hypocholesterolemic effect in HepG2 Cells by a statin-like mechanism: Comparative in vitro and in silico modeling studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammi, C.; Zanoni, C.; Arnoldi, A. IAVPGEVA, IAVPTGVA, and LPYP, three peptides from soy glycinin, modulate cholesterol metabolism in HepG2 cells through the activation of the LDLR-SREBP2 pathway. J. Funct. Foods. 2015, 14, 469–478. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three peptides from soy glycinin modulate glucose metabolism in human hepatic HepG2 cells. Int. J. Mol. Sci. 2015, 16, 27362–27370. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Juillerat, M.A.; Lee, C.H. Identification of LDL-receptor transcription stimulating peptides from soybean hydrolysate in human hepatocytes. J. Agric. Food Chem. 2008, 56, 4372–4376. [Google Scholar] [CrossRef]
- Nagaoka, S.; Nakamura, A.; Shibata, H.; Kanamaru, Y. Soystatin (VAWWMY), a novel bile acid-binding peptide, decreased micellar solubility and inhibited cholesterol absorption in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1738–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Lunasin, a novel seed peptide for cancer prevention. Peptides 2009, 30, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Lee, J.R.; Jeong, J.B.; Park, J.H.; Cheong, Y.K.; de Lumen, B.O. The cancer preventive seed peptide lunasin from rye is bioavailable and bioactive. Nutr. Cancer 2009, 61, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.F.; Chen, N.; Macasieb, J.; de Lumen, B.O. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001, 61, 7473–7478. [Google Scholar] [PubMed]
- Galvez, A.F. Identification of lunasin as the active component in soy protein responsible for reducing LDL cholesterol and risk of cardiovascular disease. Circulation 2012, 126, 10693. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Y.; Xu, Y.; Tian, Q.; Lei, G.; Zhao, C.; Gao, Z.; Pan, Q.; Zhao, W.; Nong, L.; et al. Lunasin functionally enhances LDL uptake via inhibiting PCSK9 and enhancing LDLR expression in vitro and in vivo. Oncotarget 2017, 8, 80826–80840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dia, V.P.; Torres, S.; De Lumen, B.O.; Erdman, J.W., Jr.; De Mejia, E.G. Presence of lunasin in plasma of men after soy protein consumption. J. Agric. Food Chem. 2009, 57, 1260–1266. [Google Scholar] [CrossRef]
- Li, H.; Aluko, R.E. Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. J. Agric. Food Chem. 2010, 58, 11471–11476. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Xia, J.; Song, H.; Huang, K.; Li, S.; Guan, X. Purification and characterization of antioxidant peptides from enzymatic hydrolysate of mungbean protein. J. Food Sci. 2020, 85, 1735–1741. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, H.Y.; Rappsilber, J.; Ishihama, Y. Isolation of acetylated and unmodified protein N-Terminal peptides by strong cation exchange chromatographic separation of TrypN-digested peptides. Mol. Cell Prot. MCP 2020, 20, 100003. [Google Scholar] [CrossRef] [PubMed]
- Ngashangva, N.; Mukherjee, P.; Sharma, K.C.; Kalita, M.C.; Indira, S. Analysis of antimicrobial peptide metabolome of bacterial endophyte isolated from traditionally used medicinal plant Millettia pachycarpa Benth. Front. Microbiol. 2021, 12, 656896. [Google Scholar] [CrossRef]
- Alves, T.O.; D’Almeida, C.T.S.; Scherf, K.A.; Ferreira, M.S.L. Modern approaches in the identification and quantification of immunogenic peptides in cereals by LC-MS/MS. Front. Plant. Sci. 2019, 10, 1470. [Google Scholar] [CrossRef]
- Arroume, N.; Froidevaux, R.; Kapel, R.; Cudennec, B.; Ravallec, R.; Flahaut, C.; Bazinet, L.; Jacques, P.; Dhulster, P. Food peptides: Purification, identification and role in the metabolism. Curr. Opin. Food Sci. 2016, 7, 101–107. [Google Scholar] [CrossRef]
- Barati, M.; Javanmardi, F.; Jazayeri, S.M.H.M.; Jabbari, M.; Rahmani, J.; Barati, F.; Nickho, H.; Davoodi, S.H.; Roshanravan, N.; Khaneghah, A.M. Techniques, perspectives, and challenges of bioactive peptide generation: A comprehensive systematic review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1488–1520. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.W.F.S.; Cunha, N.B.; da Carneiro, J.A.; da Costa, R.A.; de Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine organisms as a rich source of biologically active peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.C.; Mora, L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The impact of oxidative stress on blood-retinal barrier physiology in age-related macular degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Toma, C.; De Cillà, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and nitrosative stress in age-related macular degeneration: A review of their role in different stages of disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid. Med. Cell Longev. 2020, 1675957. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. BioMed Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef] [Green Version]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Dei Più, L.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Gu, L.; Zhao, M.; Li, W.; You, L.; Wang, J.; Wang, H.; Ren, J. Chemical and cellular antioxidant activity of two novel peptides designed based on glutathione structure. Food Chem. Toxicol. 2012, 50, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wei, H.; Yu, H.; Xing, Q.; Zou, Y.; Zhou, Y.; Peng, J. Fish skin gelatin hydrolysate production by ginger powder induces glutathione synthesis to prevent hydrogen peroxide induced intestinal oxidative stress via the Pept1-p62-Nrf2 Cascade. J. Agric. Food Chem. 2018, 66, 11601–11611. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M.; et al. Modulation of Keap1/Nrf2/ARE signaling pathway by curcuma- and garlic-derived hybrids. Front. Pharmacol. 2020, 10, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, X.; Li, J.; Zhou, T. Targeting Nrf2-mediated oxidative stress response signaling pathways as new therapeutic strategy for pituitary adenomas. Front. Pharmacol. 2021, 12, 565748. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Mo, Z. Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases. J. Tissue Eng. Regen. Med. 2020, 14, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Cabral de Guimaraes, T.A.; Daich-Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2021, 2021, 318452. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Exp. Rev. Clin. Pharmacol 2021, 14, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Hadziahmetovic, M.; Malek, G. Age-related macular degeneration revisited: From pathology and cellular stress to potential therapies. Front. Cell Dev. Biol. 2021, 8, 612812. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Arnoldi, A.; Lammi, C. Nanostructure, self-assembly, mechanical properties, and antioxidant activity of a lupin-derived peptide hydrogel. Biomedicines 2021, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J.F. Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients 2019, 11, 2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruz, P.; Juillerat, P.; Kullak-Ublick, G.; Schoepfer, A.M.; Mantzaris, G.J.; Rogler, G. Management of the elderly inflammatory bowel disease patient. Digestion 2020, 101, 105–119. [Google Scholar] [CrossRef]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of inflammatory reaction in health and disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Fukuda, D.; Sata, M. Frontiers of inflammatory disease research: Inflammation in cardiovascular–cerebral diseases. Inflamm. Regen. 2021, 41, 10. [Google Scholar] [CrossRef]
- Juarranz, Y. Molecular and cellular basis of autoimmune diseases. Cells 2021, 10, 474. [Google Scholar] [CrossRef]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in depression and the potential for anti-inflammatory treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—A review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Alvarez-Sanchez, N.; Millan-Linares, M.C.; del Mar Yust, M.; Pedroche, J.; Millan, F.; Lardone, P.J.; Carrera-Sanchez, C.; Guerrero, J.M.; Carrillo-Vico, A. Lupine protein hydrolysates decrease the inflammatory response and improve the oxidative status in human peripheral lymphocytes. Food Res. Int. 2019, 126, 108585. [Google Scholar] [CrossRef]
- Millán-Linares, M.C.; Bermúdez, B.; del Mar Yust, M.; Millán, F.; Pedroche, J. Anti-inflammatory activity of lupine (Lupinus angustifolius L.) protein hydrolysates in THP-1-derived macrophages. J. Funct. Foods 2014, 8, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.W.; Gao, X.M.; Wang, H.; Zhu, Y.; Zhang, J.; Hu, L.M.; Su, Y.F.; Kang, L.Y.; Zhang, B.L. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J. Steroid Biochem. Mol. Biol. 2009, 133, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Spiller, F.; Oliveira Formiga, R.; Fernandes da Silva Coimbra, J.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide 2019, 89, 32–40. [Google Scholar] [CrossRef]
- Misko, T.P.; Trotter, J.L.; Cross, A.H. Mediation of inflammation by encephalitogenic cells: Interferon gamma induction of nitric oxide synthase and cyclooxygenase 2. J. Neuroimmunol. 1995, 61, 195–204. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Bozkurt, S.B.; Tuncer Gokdag, I.; Hakki, S.S. Porphyromonas gingivalis-Lipopolysaccharide induces cytokines and enzymes of the mouse cementoblasts. Cytokine 2021, 138, 155380. [Google Scholar] [CrossRef]
- Fasolo, J.M.M.A.; Vizuete, A.F.K.; Rico, E.P.; Rambo, R.B.S.; Toson, N.S.B.; Santos, E.; de Oliveira, D.L.; Gonçalves, C.A.S.; Schapoval, E.E.S.; Heriques, A.T. Anti-inflammatory effect of rosmarinic acid isolated from Blechnum brasiliense in adult zebrafish brain. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108874. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The effect of inflammation on bone. Front. Physiol. 2021, 11, 511799. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, I.H.; Iimura, T.; Kong, S.W. Two macrophages, osteoclasts and microglia: From development to pleiotropy. Bone Res. 2021, 9, 11. [Google Scholar] [CrossRef]
- Cawley, K.M.; Bustamante-Gomez, N.C.; Guha, A.G.; MacLeod, R.S.; Xiong, J.; Gubrij, I.; Liu, Y.; Mulkey, R.; Palmieri, M.; Thostenson, J.D.; et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep. 2020, 32, 108052. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, M.; Kitaura, H.; Fujimura, Y.; Kohara, H.; Morita, Y.; Yoshida, N. IL-12 inhibits lipopolysaccharide stimulated osteoclastogenesis in mice. J. Immunol. Res. 2015, 2015, 214878. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Pan, M.; Gao, Y.; Zhang, L.; Ge, W.; Tang, P. Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration. Cell Biosci. 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, H.; Ha, I.J.; Yang, W.M. Zanthoxylum piperitum alleviates the bone loss in osteoporosis via inhibition of RANKL-induced c-fos/NFATc1/NF-κB pathway. Phytomedicine 2021, 80, 153397. [Google Scholar] [CrossRef]
- Rhie, S.J.; Jung, E.Y.; Shim, I. The role of neuroinflammation on pathogenesis of affective disorders. J. Exerc. Rehab. 2020, 16, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Sakrajda, K.; Szczepankiewicz, A. Inflammation-related changes in mood disorders and the immunomodulatory role of lithium. Int. J. Mol. Sci. 2021, 22, 1532. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative stress parameters and antioxidants in patients with bipolar disorder: Results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disord. 2021, 23, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M. The impact of nutrients on mental health and well-being: Insights from the literature. Front. Nutr. 2021, 8, 656290. [Google Scholar] [CrossRef]
- Lemus-Conejo, A.; Millan-Linares, M.C.; Toscano, R.; Millan, F.; Pedroche, J.; Muriana, F.J.G.; Montserrat-de la Paz, S. GPETAFLR, a peptide from Lupinus angustifolius L. prevents inflammation in microglial cells and confers neuroprotection in brain. Nutr. Neurosci. 2020, 1–13. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Lemus-Conejo, A.; Toscano, R.; Pedroche, J.; Millán Rodríguez, F.; Millán-Linares, M.D.C. GPETAFLR, an octapeptide isolated from Lupinus angustifolius L. protein hydrolysate, promotes the skewing to M2 phenotype in human primary monocytes. Food Funct. 2019, 10, 3303–3311. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, M.B.; Nordestgaard, B.G. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: A contemporary primary prevention cohort. Lancet 2020, 396, 1644–1652. [Google Scholar] [CrossRef]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Gregoire, J.; Grover, S.A.; et al. Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Stępień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. 2011, 63, 859–866. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Janssen, L.; Allard, N.A.E.; Saris, C.G.J.; Keijer, J.; Hopman, M.T.E.; Timmers, S. Muscle toxicity of drugs: When drugs turn physiology into pathophysiology. Physiol. Rev. 2020, 100, 633–672. [Google Scholar] [CrossRef] [PubMed]
- Jayatilaka, S.; Desai, K.; Rijal, S.; Zimmerman, D. Statin-induced autoimmune necrotizing myopathy. J. Prim. Care Community Health 2021, 12, 21501327211028714. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Hypocholesterolaemic activity of lupin peptides: Investigation on the crosstalk between human enterocytes and hepatocytes using a co-culture system including Caco-2 and HepG2 cells. Nutrients 2016, 8, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammi, C.; Zanoni, C.; Aiello, G.; Arnoldi, A.; Grazioso, G. Lupin peptides modulate the protein-protein interaction of PCSK9 with the low density lipoprotein receptor in HepG2 cells. Sci. Rep. 2016, 6, 29931. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Investigations on the hypocholesterolaemic activity of LILPKHSDAD and LTFPGSAED, two peptides from lupin beta-conglutin: Focus on LDLR and PCSK9 pathways. J. Funct. Foods 2017, 32, 1–8. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Calabresi, L.; Arnoldi, A. Lupin protein exerts cholesterol-lowering effects targeting PCSK9: From clinical evidences to elucidation of the in vitro molecular mechanism using HepG2 cells. J. Funct. Foods 2016, 23, 230–240. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Álvarez-Ríos, A.I.; Fernández-Santos, J.M.; Vázquez-Román, M.V.; Rodríguez-Ortiz, B.; Álvarez-Sánchez, N.; Álvarez-López, A.I.; Millán-Linares, M.D.C.; Millán, F.; et al. Lupinus angustifolius protein hydrolysates reduce abdominal adiposity and ameliorate metabolic associated fatty liver disease (MAFLD) in Western diet fed-ApoE−/− mice. Antioxidants 2021, 10, 1222. [Google Scholar] [CrossRef]
- Grazioso, G.; Bollati, C.; Sgrignani, J.; Arnoldi, A.; Lammi, C. The first food-derived peptide inhibitor of the protein-protein interaction between gain-of-function PCSK9D374Yand the LDL receptor. J. Agric. Food Chem. 2018, 66, 10552–10557. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Lecca, D.; Pia Abbracchio, M.; Arnoldi, A. Lupin peptide T9 (GQEQSHQDEGVIVR) modulates the mutant PCSK9D374Y Pathway: In vitro characterization of its dual hypocholesterolemic behavior. Nutrients 2019, 11, 1665. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-derived bioactive peptides: A treatment to cure diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef]
- Dove, E.R.; Mori, T.A.; Chew, G.T.; Barden, A.E.; Woodman, R.J.; Puddey, I.B.; Sipsas, S.; Hodgson, J.M. Lupin and soya reduce glycaemia acutely in type 2 diabetes. Br. J. Nutr. 2011, 106, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Terruzzi, I.; Senesi, P.; Magni, C.; Montesano, C.; Scarafoni, A.; Luzi, L.; Duranti, M. Insulin-mimetic action of conglutin-γ, a lupin seed protein, in mouse myoblasts. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Durango, N.; Fuentes, C.A.; Castillo, A.E.; González-Gómez, L.M.; Vecchiola, A.; Fardella, C.E.; Kalergis, A.M. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: Molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int. J. Mol. Sci. 2016, 17, 797. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Kobori, H. Independent regulation of renin–angiotensin–aldosterone system in the kidney. Clin. Exp. Nephrol. 2018, 22, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.M.A.; West, C.A.; de Abreu, A.R.R.; Pai, A.V.; Mesquita, L.B.T.; Ji, H.; Chianca, D., Jr.; de Menezes, R.C.A.; Sandberg, K. Role of the renin angiotensin system in blood pressure allostasis-induced by severe food restriction in female Fischer rats. Sci. Rep. 2018, 8, 10327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Tain, Y.L. Targeting the renin–angiotensin–aldosterone system to prevent hypertension and kidney disease of developmental origins. Int. J. Mol. Sci. 2021, 22, 2298. [Google Scholar] [CrossRef]
- Nardo, A.E.; Sua’rez, S.; Quiroga, A.V.; Año’n, M.C. Amaranth as a source of antihypertensive peptides. Front. Plant Sci. 2020, 11, 578631. [Google Scholar] [CrossRef]
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme–inhibitory peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187. [Google Scholar] [CrossRef] [PubMed]
- Ghatage, T.; Goyal, S.G.; Dhar, A.; Bhat, A. Novel therapeutics for the treatment of hypertension and its associated complications: Peptide- and nonpeptide-based strategies. Hypertens. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Escudero, E.; Sentandreu, M.A.; Arihara, K.; Toldra, F. Angiotensin I-converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J. Agric. Food Chem. 2010, 58, 2895–2901. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Zhao, Y.; Zhu, X.; Yu, R.; Dong, S.; Wu, H. Novel natural angiotensin converting enzyme (ACE)-inhibitory peptides derived from sea cucumber-modified hydrolysates by adding exogenous proline and a study of their structure–activity relationship. Mar. Drugs 2018, 16, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aluko, R.E.; Girgih, A.T.; He, R.; Malomo, S.; Li, H.; Offengenden, M.; Wu, J.P. Structural and functional characterization of yellow field pea seed (Pisum sativum L.) protein-derived antihypertensive peptides. Food Res. Int. 2015, 77, 10–16. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.S.; Kasymova, T.D.; Kwon, D.Y. Isolation and identification of peptides from soy 11s-globulin with hypocholesterolemic activity. Chem Nat. Compd. 2005, 41, 710–714. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a highly potent inhibitory peptide acting as a competitive inhibitor of HMG-CoA reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Aiello, G.; Dellafiora, L.; Bollati, C.; Boschin, G.; Ranaldi, G.; Ferruzza, S.; Sambuy, Y.; Galaverna, G.; Arnoldi, A. Assessment of the multifunctional behavior of lupin peptide P7 and its metabolite using an integrated strategy. J. Agric. Food Chem. 2020, 68, 13179–13188. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides derived from soy and lupin protein as dipeptidyl-peptidase IV inhibitors: In vitro biochemical screening and in silico molecular modeling study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieter Boots, J.-W. Protein Hydrolysate Enriched in Peptides Inhibiting DPP-IV and Their Use. WO Patent 2006/068480 200, 2006. Available online: https://patents.google.com/patent/US20090075904A1/en (accessed on 10 September 2021).
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Gallego, M.; Mora, L.; Toldrá, F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Aiello, G.; Vistoli, G.; Zanoni, C.; Arnoldi, A.; Sambuy, Y.; Ferruzza, S.; Ranaldi, G. A multidisciplinary investigation on the bioavailability and activity of peptides from lupin protein. J. Funct. Foods 2016, 24, 297–306. [Google Scholar] [CrossRef]
- Chai, T.T.; Ee, K.Y.; Kumar, D.T.; Manan, F.A.; Wong, F.C. Plant bioactive peptides: Current status and prospects towards use on human health. Protein Pept. Lett. 2021, 28, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, N.; Li, B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review. J. Food Biochem. 2018, 43, e12571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Udenigwe, C.; Abioye, R.; Okagu, I.U.; Obeme-Nmom, J. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Mafra, I. Lupine allergens: Clinical relevance, molecular characterization, cross-reactivity, and detection strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3886–3915. [Google Scholar] [CrossRef] [PubMed]
- Lima-Cabello, E.; Alché, J.D.; Jimenez-Lopez, J.C. Narrow-leafed lupin main allergen β-conglutin (Lup an 1) detection and quantification assessment in natural and processed foods. Foods 2019, 8, 513. [Google Scholar] [CrossRef] [Green Version]
- Czubinski, J.; Montowska, M.; Springer, E.; Pospiech, E.; Lampart-Szczapa, E. Immunoreactivity changes during lupin seed storage proteins digestion. Eur. Food Res. Technol. 2017, 243, 2095–2103. [Google Scholar] [CrossRef] [Green Version]
| Lupin Hydrolysates and Peptides | Biological Activities | References |
|---|---|---|
| Non-purified low molecular weight peptides | ACE-inhibitory and hypoglycemic effects | [4,5,33] |
| LTFPGSAED | Hypoglycemic and insulin-mimetic properties | [15,28] |
| GPETAFLR | Anti-osteoclastogenic effect in LPS-induced human monocyte-derived osteoclasts. The peptide also improved GSH synthesis and suppress intracellular ROS generation | [28] |
| IQDKEGIPPDQQR | Suppressed the expression and protein levels of pro-inflammatory cytokines and their receptors | [31] |
| LTFPGSAED, TFPGSAED and LTFPG | DPPIV, HMGCoAR and ACE-inhibitory activities | [32] |
| Pancreatin hydrolysates | ACE inhibitory and antibacterial (Bacillus cereus and Staphylococcus aureus) activities | [33] |
| FVPY | Antioxidant and lipid peroxidation properties | [75] |
| Unspecified low molecular weight peptides | Radical scavenging and ferric reducing antioxidant properties via Keap-1/Nrf2 signaling pathways | [80] |
| Hydrolysates | Anti-inflammatory and immunomodulatory effects | [93] |
| Izyme AL and Alcalase 2.4 L-generated hydrolysates | Increased the production of anti-inflammatory cytokines while inhibiting the production of NO and pro-inflammatory cytokines in LPS-induced mouse RAW 264.7 macrophages | [94] |
| GPETAFLR | Suppressed the expression and protein levels of pro-inflammatory cytokines and their receptors in LPS-induced retinal pigment epithelium cells | [94] |
| GPETAFLR | Promoted the differentiation of M2 phenotype of peripheral macrophage system that enhances anti-inflammatory processes while inhibiting M1 phenotype of peripheral macrophage system that enhance pro-inflammatory processes | [114,115] |
| LILPKHSDAD | Suppressed cholesterol synthesis by inhibiting HMGCoAR via AMPK pathway, and increased LDLR level via upregulation of SREBP-2 expression | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients 2021, 13, 3266. https://doi.org/10.3390/nu13093266
Okagu IU, Ndefo JC, Aham EC, Obeme-Nmom JI, Agboinghale PE, Aguchem RN, Nechi RN, Lammi C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients. 2021; 13(9):3266. https://doi.org/10.3390/nu13093266
Chicago/Turabian StyleOkagu, Innocent U., Joseph C. Ndefo, Emmanuel C. Aham, Joy I. Obeme-Nmom, Precious E. Agboinghale, Rita N. Aguchem, Regina N. Nechi, and Carmen Lammi. 2021. "Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits" Nutrients 13, no. 9: 3266. https://doi.org/10.3390/nu13093266







