Boosting the Photoaged Skin: The Potential Role of Dietary Components
Abstract
:1. Introduction
2. Materials and Methods
3. Skin Architecture
4. UV-Induced Skin Photoaging
5. Mechanisms of Skin Photoaging
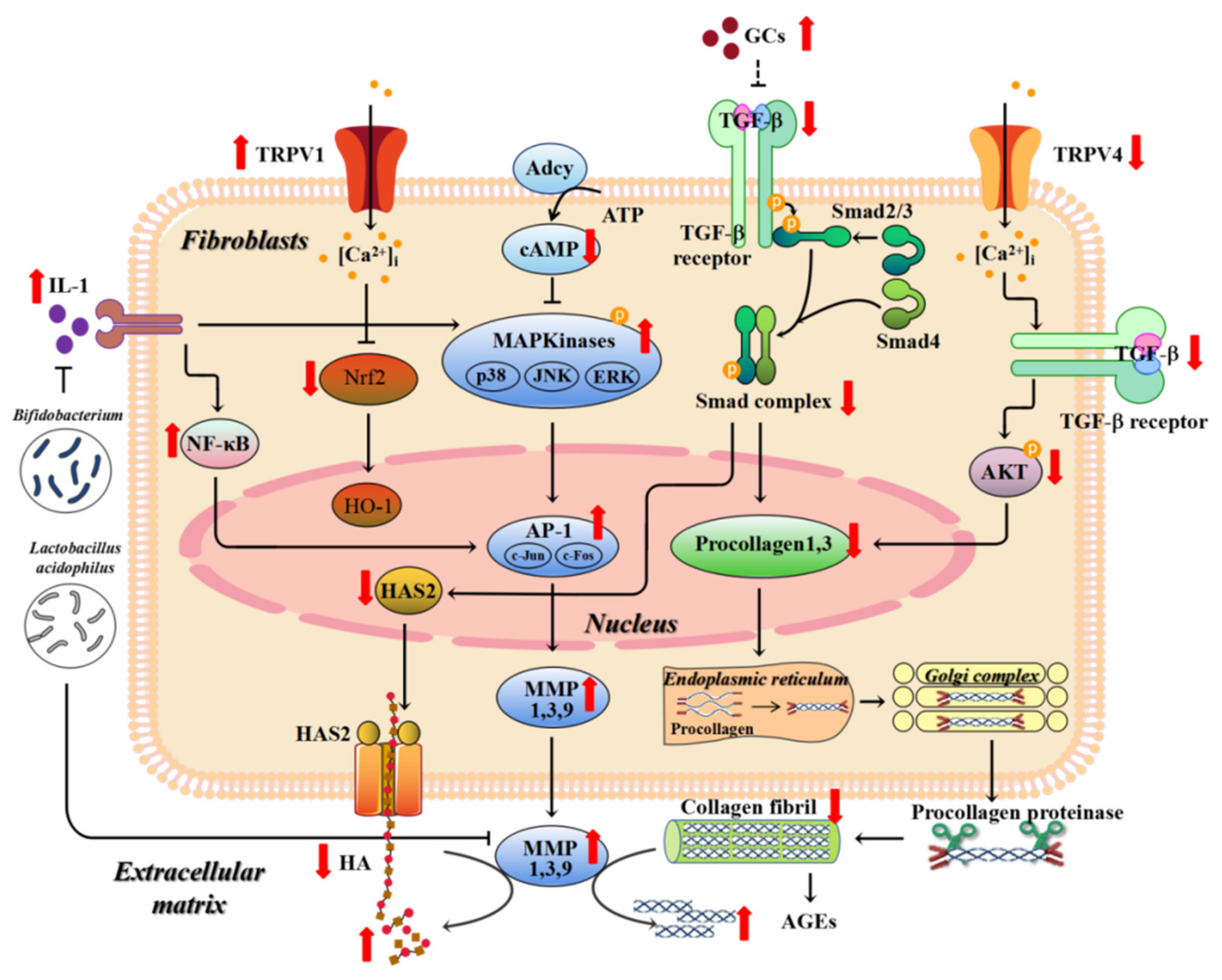
5.1. Matrix Metalloproteinases (MMPs)
5.2. TGF (Transforming Growth Factor)-β
5.3. Reduction of Skin Adipose Tissue
5.4. Inflammation and Immune Disorders
5.5. Oxidative Stress
5.6. Nuclear DNA and mtDNA Damage
5.7. Telomere Shortening
5.8. MicroRNA (miRNA)
5.9. Accumulation of Advanced Glycation End Products (AGEs)
5.10. Gut Microbes
5.11. Activation of Hypothalamic–Pituitary–Adrenal (HPA) Axis
5.12. Transient Receptor Potential Cation Channel V (TRPV)
6. Efficacy and Mechanisms of Dietary Components in Mitigating Skin Photoaging: Animal and Human Evidence
6.1. Phytochemicals
6.1.1. Carotenoids
6.1.2. Polyphenols
6.1.3. Plant Extracts and Fermentation
6.2. Proteins and Peptides, Carbohydrates, and Fattty Acids
6.2.1. Proteins and Peptides
6.2.2. Carbohydrates
6.2.3. Fatty Acids
6.2.4. Other Animal-Derived Active Substances
6.3. Probiotics
6.4. Vitamins and Minerals
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kang, S.M.; Chung, J.H. The role of TRPV1 channel in aged human skin. J. Dermatol. Sci. 2012, 65, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Dermato Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Huang, A.H.; Chien, A.L. Photoaging: A review of current literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. North Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Park, J.; Moon, Y.; Kang, W.; Park, T. Alpha-ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts. Molecules 2019, 24, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueres, J.T.; Bases, P.E. An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing. Nutr. Hosp. 2015, 32 (Suppl. S1), 62–66. [Google Scholar]
- Song, H.; Meng, M.; Cheng, X.; Li, B.; Wang, C. The effect of collagen hydrolysates from silver carp (Hypophthalmichthys molitrix) skin on UV-induced photoaging in mice: Molecular weight affects skin repair. Food Funct. 2017, 8, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Lingrong, W.; Qing, G.; Chung-wah, M.; Yazhong, G.; Lijun, Y.; Rui, H.L.; Xiong, F.; Dong, L. Effect of polysaccharides from Tremella fuciformis on UV-induced photoaging. J. Funct. Foods 2016, 20, 400–410. [Google Scholar]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Bruna, R.; Andréa, M. Olive oil reduces chronic psychological stress-induced skin aging in mice through the NF-κB and NRF2 pathways. J. Funct. Foods 2019, 54, 310–319. [Google Scholar]
- Kimoto-Nira, H. New lactic acid bacteria for skin health via oral intake of heat-killed or live cells. Anim. Sci. J. 2018, 89, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Xiao, J.Z. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benef. Microbes 2015, 6, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagatin, E.; Guadanhim, L.R.; Enokihara, M.M.; Sanudo, A.; Talarico, S.; Miot, H.A.; Gibson, L. Low-dose oral isotretinoin versus topical retinoic acid for photoaging: A randomized, comparative study. Int. J. Dermatol. 2014, 53, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, M.S.; Kwon, E.K.; Skupsky, H.; Kwon, S.Y.; Grant-Kels, J.M. Nutrition and the deleterious side effects of nutritional supplements. Clin. Dermatol. 2010, 28, 371–379. [Google Scholar] [CrossRef]
- Kim, P. Nutraceuticals and skin health: Key benefits and protective properties. J. Aesthetic Nurs. 2018, 7, 35–40. [Google Scholar]
- Richard, W.; Stefan, G.; Wolfgang, W.; Jean, C.G.; Jason, K.W. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar]
- Stalder, J.F.; Tennstedt, D.; Deleuran, M.; Fabbrocini, G.; de Lucas, R.; Haftek, M.; Taieb, C.; Coustou, D.; Mandeau, A.; Fabre, B.; et al. Fragility of epidermis and its consequence in dermatology. J. Eur. Acad. Dermatol. Venereol. 2014, 28 (Suppl. S4), 1–18. [Google Scholar] [CrossRef] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of dermis: Scarring and cells involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- William, D.L. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast. Surg. Clin. 2017, 25, 283–289. [Google Scholar]
- Varani, J. Fibroblast aging: Intrinsic and extrinsic factors. Drug Discov. Today Ther. Strateg. 2010, 7, 65–70. [Google Scholar] [CrossRef]
- Bukhari, S.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu, K.N.; Czernuszka, J.; Rahman, M.T. Molecular mechanisms of Stress-Responsive changes in collagen and elastin networks in skin. Skin Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A. Hyaluronic acid in dermatology. Skinmed 2017, 15, 441–448. [Google Scholar]
- Rock, K.; Grandoch, M.; Majora, M.; Krutmann, J.; Fischer, J.W. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): New insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011, 286, 18268–18276. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Cannarozzo, G.; Fazia, G.; Bennardo, L.; Tamburi, F.; Amoruso, G.F.; Del, D.E.; Nistico, S.P. A new 675 nm laser device in the treatment of facial aging: A prospective observational study. Photobiomodulation Photomed. Laser Surg. 2021, 39, 118–122. [Google Scholar] [CrossRef]
- Nistico, S.P.; Silvestri, M.; Zingoni, T.; Tamburi, F.; Bennardo, L.; Cannarozzo, G. Combination of fractional CO2 laser and rhodamine-intense pulsed light in facial rejuvenation: A randomized controlled trial. Photobiomodulation Photomed. Laser Surg. 2021, 39, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.A.; Yoo, T.; Hwang, J.S.; Kang, E.S.; Paek, K.S.; Park, C.; Kim, J.H.; Do, J.T.; Seo, H.G. Peroxisome proliferator-activated receptor delta modulates MMP-2 secretion and elastin expression in human dermal fibroblasts exposed to ultraviolet B radiation. J. Dermatol. Sci. 2014, 76, 44–50. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Illman, S.A.; Keski-Oja, J.; Lohi, J. Promoter characterization of the human and mouse epilysin (MMP-28) genes. Gene 2001, 275, 185–194. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron inhibits UVB-Induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e159998. [Google Scholar] [CrossRef]
- Park, J.E.; Pyun, H.B.; Woo, S.W.; Jeong, J.H.; Hwang, J.K. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, T. Decanal Protects against UVB-Induced Photoaging in Human Dermal Fibroblasts via the cAMP Pathway. Nutrients 2020, 12, 1214. [Google Scholar] [CrossRef]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.; Fisher, G.J. Role of Age-Associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.K.; Kim, E.J.; Cheng, Y.; Shin, M.H.; Oh, J.H.; Lee, D.H.; Chung, J.H. Inhibition of DNA methylation in the COL1A2 promoter by anacardic acid prevents UV-Induced decrease of type i procollagen expression. J. Investig. Dermatol. 2017, 137, 1343–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, K.A.; Yi, B.R.; Choi, K.C. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, L.; Wang, W.; Su, X.; Huang, Y.; Su, L.; Liu, M.; Sun, Y.; Yang, B.; Zhou, H. The effect of cAMP-PKA activation on TGF-beta1-induced profibrotic signaling. Cell. Physiol. Biochem. 2015, 36, 1911–1927. [Google Scholar] [CrossRef]
- Porsch, H.; Bernert, B.; Mehic, M.; Theocharis, A.D.; Heldin, C.H.; Heldin, P. Efficient TGFbeta-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2. Oncogene 2013, 32, 4355–4365. [Google Scholar] [CrossRef]
- Chen, S.X.; Zhang, L.J.; Gallo, R.L. Dermal white adipose tissue: A newly recognized layer of skin innate defense. J. Investig. Dermatol. 2019, 139, 1002–1009. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, J.E.; Kim, S.; Kim, M.K.; Park, C.H.; Chung, J.H. UV modulation of subcutaneous fat metabolism. J. Investig. Dermatol. 2011, 131, 1720–1726. [Google Scholar] [CrossRef] [Green Version]
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes and hair cycling: Is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp. Dermatol. 2016, 25, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Martins, V.; Gonzalez, D.L.S.F.; Wu, Z.; Capelozzi, V.; Phan, S.H.; Liu, T. FIZZ1-induced myofibroblast transdifferentiation from adipocytes and its potential role in dermal fibrosis and lipoatrophy. Am. J. Pathol. 2015, 185, 2768–2776. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, J.; Jung, E.; Kim, Y.S.; Roh, K.; Jung, K.H.; Park, D. Ultraviolet a regulates adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via up-regulation of Kruppel-like factor 2. J. Biol. Chem. 2010, 285, 32647–32656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.H.; Pappas, A.; Zhang, L.; Ruvolo, E.; Cavender, D. IL-11, IL-1alpha, IL-6, and TNF-alpha are induced by solar radiation in vitro and may be involved in facial subcutaneous fat loss in vivo. J. Dermatol. Sci. 2013, 71, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef]
- Wang, X.; Bi, Z.; Chu, W.; Wan, Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int. J. Mol. Med. 2005, 16, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.M.; Ullrich, S.E. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J. Immunol. 1992, 149, 3865–3871. [Google Scholar] [PubMed]
- Steven, Q.W.; Richard, S.; Marianne, B.; David, P.; Ashfaq, A.M.; Alfred, W.K.; Robert, S.B. Ultraviolet a and melanoma: A review. J. Am. Acad. Dermatol. 2001, 44, 837–846. [Google Scholar]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Park, W.H. Effects of antioxidants and MAPK inhibitors on cell death and reactive oxygen species levels in H2O2-treated human pulmonary fibroblasts. Oncol. Lett. 2013, 5, 1633–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazawa, M.; Iwasaki, K.; Sakamoto, K. Iron chelators may help prevent photoaging. J. Cosmet. Dermatol. 2006, 5, 210–217. [Google Scholar] [CrossRef]
- Bissett, D.L.; Chatterjee, R.; Hannon, D.P. Chronic ultraviolet radiation-induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem. Photobiol. 1991, 54, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Fowler, M.; Naftalin, R.J.; Siow, R. UVA irradiation increases ferrous iron release from human skin fibroblast and endothelial cell ferritin: Consequences for cell senescence and aging. Free Radic. Biol. Med. 2020, 155, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, K.; Brunk, U.T. Cellular injury induced by oxidative stress is mediated through lysosomal damage. Free Radic. Biol. Med. 1995, 19, 565–574. [Google Scholar] [CrossRef]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- Siametis, A.; Niotis, G.; Garinis, G.A. DNA damage and the aging epigenome. J. Investig. Dermatol. 2021, 141, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Medve-Konig, K.; Pfahlberg, A.; Gers-Barlag, H.; Gefeller, O.; Krutmann, J. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J. Investig. Dermatol. 2004, 122, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Sonntag, W.E.; de Cabo, R.; Baur, J.A.; Csiszar, A. Mitochondrial protection by resveratrol. Exerc. Sport Sci. Rev. 2011, 39, 128–132. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.; Mitchell, J.R. Telomerase in the human organism. Oncogene 2002, 21, 564–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckingham, E.M.; Klingelhutz, A.J. The role of telomeres in the ageing of human skin. Exp. Dermatol. 2011, 20, 297–302. [Google Scholar] [CrossRef]
- Miyata, Y.; Okada, K.; Fujimoto, A.; Hata, K.; Kagami, H.; Tomita, Y.; Ueda, M. The effect of the long-term cultivation on telomere length and morphology of cultured epidermis. J. Dermatol. Sci. 2004, 34, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, R.; Xiao, X.; Deng, Z.L.; Jian, D.; Xie, H.F.; Li, J. Microrna-217 modulates human skin fibroblast senescence by directly targeting DNA methyltransferase 1. Oncotarget 2017, 8, 33475–33486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, K.; Tigges, J.; Sass, S.; Schutze, A.; Florea, A.M.; Fender, A.C.; Theis, F.J.; Krutmann, J.; Boege, F.; Fritsche, E.; et al. MiR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: Possible implication for skin aging. J. Investig. Dermatol. 2015, 135, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Blackstone, B.N.; Wilgus, T.A.; Roy, S.; Wulff, B.C.; Powell, H.M. Skin biomechanics and miRNA expression following chronic UVB irradiation. Adv. Wound Care 2020, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D. Advanced glycation end products in skin ageing and photoageing: What are the implications for epidermal function? Exp. Dermatol. 2016, 25, 947–948. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Tuckey, R.C.; Paus, R. Differential expression of HPA axis homolog in the skin. Mol. Cell. Endocrinol. 2007, 265–266, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skobowiat, C.; Slominski, A.T. UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J. Investig. Dermatol. 2015, 135, 1638–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacke, H.; Docke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, J.; Ma, J.; Chang, S.; Yun, H.; Jin, H.; Sun, A.; Zou, Y.; Ge, J. Glucocorticoid ameliorates early cardiac dysfunction after coronary microembolization and suppresses TGF-beta1/Smad3 and CTGF expression. Int. J. Cardiol. 2013, 167, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Centrella, M.; McCarthy, T.L.; Canalis, E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol. Cell. Biol. 1991, 11, 4490–4496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutroneo, K.R. Relationship between glucocorticoid-mediated early decrease of protein synthesis and the steady state decreases of glucocorticoid receptor and TGF-beta activator protein. Int. J. Biochem. Cell Biol. 2002, 34, 194–203. [Google Scholar] [CrossRef]
- Huang, K.F.; Ma, K.H.; Jhap, T.Y.; Liu, P.S.; Chueh, S.H. Ultraviolet B irradiation induced Nrf2 degradation occurs via activation of TRPV1 channels in human dermal fibroblasts. Free Radic. Biol. Med. 2019, 141, 220–232. [Google Scholar] [CrossRef]
- Han, S.; Kang, S.M.; Oh, J.H.; Lee, D.H.; Chung, J.H. Src kinase mediates UV-induced TRPV1 trafficking into cell membrane in HaCaT keratinocytes. Photodermatol. Photoimmunol. Photomed. 2018, 34, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Um, J.Y.; Kang, S.Y.; Kim, H.J.; Chung, B.Y.; Park, C.W.; Kim, H.O. Transient receptor potential vanilloid-3 (TRPV3) channel induces dermal fibrosis via the TRPV3/TSLP/Smad2/3 pathways in dermal fibroblasts. J. Dermatol. Sci. 2020, 97, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.A.; Yeh, I.; et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E3225–E3234. [Google Scholar] [CrossRef] [Green Version]
- Kida, N.; Sokabe, T.; Kashio, M.; Haruna, K.; Mizuno, Y.; Suga, Y.; Nishikawa, K.; Kanamaru, A.; Hongo, M.; Oba, A.; et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflug. Arch. 2012, 463, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goswami, R.; Merth, M.; Cohen, J.; Lei, K.Y.; Zhang, D.X.; Rahaman, S.O. TRPV4 ion channel is a novel regulator of dermal myofibroblast differentiation. Am. J. Physiol. Cell Physiol. 2017, 312, C562–C572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Seki, S.; Ueda, F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people-a randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [Green Version]
- Ng, Q.X.; De Deyn, M.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of astaxanthin supplementation on skin health: A systematic review of clinical studies. J. Diet. Suppl. 2020, 18, 169–182. [Google Scholar] [CrossRef]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Marini, A.; Jaenicke, T.; Grether-Beck, S.; Le Floc’H, C.; Cheniti, A.; Piccardi, N.; Krutmann, J. Prevention of polymorphic light eruption by oral administration of a nutritional supplement containing lycopene, beta-carotene, and Lactobacillus johnsonii: Results from a randomized, placebo-controlled, double-blinded study. Photodermatol. Photoimmunol. Photomed. 2014, 30, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Del, G.R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [Green Version]
- Tamaru, E.; Watanabe, M.; Nomura, Y. Dietary immature Citrus unshiu alleviates UVB- induced photoaging by suppressing degradation of basement membrane in hairless mice. Heliyon 2020, 6, e4218. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.B.; Han, H.S.; Shin, J.S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Hydrangenol isolated from the leaves of hydrangea serrata attenuates wrinkle formation and repairs skin moisture in UVB-irradiated hairless mice. Nutrients 2019, 11, 2354. [Google Scholar] [CrossRef] [Green Version]
- Moon, N.R.; Kang, S.; Park, S. Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-beta1 and wnt signaling pathways. J. Photochem. Photobiol. B 2018, 178, 92–100. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn polyphenol extract inhibits UVB-induced skin photoaging by regulating MMP expression and type i procollagen production in mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Q.; Zou, J.; Zhao, Y.; Chang, X. Protective effects of hawthorn (Crataegus pinnatifida) polyphenol extract against UVB-induced skin damage by modulating the p53 mitochondrial pathway in vitro and in vivo. J. Food Biochem. 2019, 43, e12708. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Buonocore, D.; Lazzeretti, A.; Tocabens, P.; Nobile, V.; Cestone, E.; Santin, G.; Bottone, M.G.; Marzatico, F. Resveratrol-procyanidin blend: Nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin. Cosmet. Investig. Dermatol. 2012, 5, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Asada, K.; Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Effects of hot water extract of Curcuma longa on human epidermal keratinocytes in vitro and skin conditions in healthy participants: A randomized, double-blind, placebo-controlled trial. J. Cosmet. Dermatol. 2019, 18, 1866–1874. [Google Scholar] [CrossRef]
- Messner, D.J.; Surrago, C.; Fiordalisi, C.; Chung, W.Y.; Kowdley, K.V. Isolation and characterization of iron chelators from turmeric (Curcuma longa): Selective metal binding by curcuminoids. Biometals 2017, 30, 699–708. [Google Scholar] [CrossRef]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Moore, C.E.; De Spirt, S.; Tronnier, H.; Stahl, W. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-Garcia, O.; Perez-Sanchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Song, D.; Kim, J.; Choi, J.; Kim, J.R.; Yoon, H.S.; Bae, J.S.; Han, M.; Lee, S.; Hong, J.S.; et al. Oral supplementation with cocoa extract reduces UVB-Induced wrinkles in hairless mouse skin. J. Investig. Dermatol. 2016, 136, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Ying, R.; Zhang, Z.; Song, W.; Li, B.; Hou, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B 2019, 192, 26–33. [Google Scholar]
- Kim, H.K. Garlic supplementation ameliorates UV-Induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules 2016, 21, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Park, S.Y.; Hwang, E.; Park, B.; Seo, S.A.; Cho, J.G.; Zhang, M.; Yi, T.H. Dietary Foeniculum vulgare Mill extract attenuated UVB irradiation-induced skin photoaging by activating of Nrf2 and inhibiting MAPK pathways. Phytomedicine 2016, 23, 1273–1284. [Google Scholar] [CrossRef]
- Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat extract oil (WEO) attenuates UVB-induced photoaging via collagen synthesis in human keratinocytes and hairless mice. Nutrients 2020, 12, 300. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Jeong, D.H.; Kim, S.; Lee, S.W.; Sin, H.S.; Yu, K.Y.; Jeong, S.I.; Kim, S.Y. Fermentation of blackberry with l. Plantarum JBMI f5 enhance the protection effect on UVB-mediated photoaging in human foreskin fibroblast and hairless mice through regulation of MAPK/NF-kappaB signaling. Nutrients 2019, 11, 2429. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motwani, M.S.; Khan, K.; Pai, A.; Joshi, R. Efficacy of a collagen hydrolysate and antioxidants-containing nutraceutical on metrics of skin health in Indian women. J. Cosmet. Dermatol. 2020, 19, 3371–3382. [Google Scholar] [CrossRef]
- Hong, Y.H.; Chang, U.J.; Kim, Y.S.; Jung, E.Y.; Suh, H.J. Dietary galacto-oligosaccharides improve skin health: A randomized double blind clinical trial. Asia Pac. J. Clin. Nutr. 2017, 26, 613–618. [Google Scholar] [PubMed]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef]
- Gollner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an oral hyaluronan solution improves skin hydration, wrinkle reduction, elasticity, and skin roughness: Results of a clinical study. J. Evid. Based Complementary Altern. Med. 2017, 22, 816–823. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, J.; Jung, S.; Sung, K.H.; Son, Y.K.; Bae, J.M.; Kim, B.H. Alleviation of ultraviolet b-induced photoaging by 7-MEGA™500 in hairless mouse skin. Toxicol. Res. 2019, 35, 353–359. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, T. Dietary suberic acid protects against UVB-induced skin photoaging in hairless mice. Nutrients 2019, 11, 2948. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.C.; Ahrens, E.J.; Schreibman, P.H.; Crouse, J.R. Measurement of squalene in human tissues and plasma: Validation and application. J. Lipid Res. 1976, 17, 38–45. [Google Scholar] [CrossRef]
- Cho, S.; Choi, C.W.; Lee, D.H.; Won, C.H.; Kim, S.M.; Lee, S.; Lee, M.J.; Chung, J.H. High-dose squalene ingestion increases type I procollagen and decreases ultraviolet-induced DNA damage in human skin in vivo but is associated with transient adverse effects. Clin. Exp. Dermatol. 2009, 34, 500–508. [Google Scholar] [CrossRef]
- Orengo, I.F.; Black, H.S.; Wolf, J.J. Influence of fish oil supplementation on the minimal erythema dose in humans. Arch. Dermatol. Res. 1992, 284, 219–221. [Google Scholar] [CrossRef]
- Rhodes, L.E.; O’Farrell, S.; Jackson, M.J.; Friedmann, P.S. Dietary fish-oil supplementation in humans reduces UVB-erythemal sensitivity but increases epidermal lipid peroxidation. J. Investig. Dermatol. 1994, 103, 151–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucetta, K.Q.; Charrouf, Z.; Aguenaou, H.; Derouiche, A.; Bensouda, Y. The effect of dietary and/or cosmetic argan oil on postmenopausal skin elasticity. Clin. Interv. Aging 2015, 10, 339–349. [Google Scholar]
- Tanaka, M.; Yamamoto, Y.; Misawa, E.; Nabeshima, K.; Saito, M.; Yamauchi, K.; Abe, F.; Furukawa, F. Aloe sterol supplementation improves skin elasticity in Japanese men with sunlight-exposed skin: A 12-week double-blind, randomized controlled trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 435–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Yamamoto, Y.; Misawa, E.; Nabeshima, K.; Saito, M.; Yamauchi, K.; Abe, F.; Furukawa, F. Effects of aloe sterol supplementation on skin elasticity, hydration, and collagen score: A 12-week double-blind, randomized, controlled trial. Skin Pharmacol. Physiol. 2016, 29, 309–317. [Google Scholar] [CrossRef]
- Im, A.R.; Ji, K.Y.; Park, I.; Lee, J.Y.; Kim, K.M.; Na, M.; Chae, S. Anti-Photoaging effects of four insect extracts by downregulating matrix metalloproteinase expression via Mitogen-Activated protein kinase-dependent signaling. Nutrients 2019, 11, 1159. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.; Reid, G. Distant site effects of ingested prebiotics. Nutrients 2016, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Verma, N.K.; Thanabalu, T. Prebiotics in atopic dermatitis prevention and management. J. Funct. Foods 2021, 78, 104352. [Google Scholar] [CrossRef]
- Im, A.R.; Kim, H.S.; Hyun, J.W.; Chae, S. Potential for tyndalized Lactobacillus acidophilus as an effective component in moisturizing skin and anti-wrinkle products. Exp. Ther. Med. 2016, 12, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Gueniche, A.; Philippe, D.; Bastien, P.; Reuteler, G.; Blum, S.; Castiel-Higounenc, I.; Breton, L.; Benyacoub, J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes 2014, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Hinek, A.; Kim, H.J.; Wang, Y.; Wang, A.; Mitts, T.F. Sodium L-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin. J. Dermatol. Sci. 2014, 75, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar]
- Kishimoto, Y.; Saito, N.; Kurita, K.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 579–584. [Google Scholar] [CrossRef]
- Boyce, S.T.; Supp, A.P.; Swope, V.B.; Warden, G.D. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J. Investig. Dermatol. 2002, 118, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberlein-Konig, B.; Placzek, M.; Przybilla, B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J. Am. Acad. Dermatol. 1998, 38, 45–48. [Google Scholar] [CrossRef]
- Fuchs, J.; Kern, H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: A clinical study using solar simulated radiation. Free Radic. Biol. Med. 1998, 25, 1006–1012. [Google Scholar] [CrossRef]
- Bendich, A.; Langseth, L. The health effects of vitamin C supplementation: A review. J. Am. Coll. Nutr. 1995, 14, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Kappus, H.; Diplock, A.T. Tolerance and safety of vitamin E: A toxicological position report. Free Radic. Biol. Med. 1992, 13, 55–74. [Google Scholar] [CrossRef]
- Meydani, M. Vitamin E. Lancet 1995, 345, 170–175. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and skin disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, M.; Pierzak, M.; Kr, C.B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
| Ingredients | Model | Dose | Duration | Main Results | Reference |
|---|---|---|---|---|---|
| Phytochemicals | |||||
| Immature Citrus unshiu | HR-1 hairless mice, male, 6 weeks old | 200 mg/kg body weight/day | 7 weeks | skin hydration ↑ transepidermal water loss ↓ overgrowth of epidermal cell ↓ epidermal cell mortality ↓ basement membrane destruction ↓ | [104] |
| Hydrangenol | HR-1 hairless mice, male, 5 weeks old | 5, 10, 20, 40 mg/kg/day | 7 weeks | collagen, HA ↑ Nrf2, HO-1 ↑ wrinkles, dorsal thickness, dehydration ↓ HAS-1/-2 ↓ MAPKs, MMP-1/-3 ↓ COX-2 ↓ IL-6 ↓ | [105] |
| Dihydromyricetin and ellagic acid | ICR mice, male, 6 weeks old | 0.7% cellulose; 0.7% ellagic acid; 0.7% dihydromyricetin or 0.35% ellagic acid and 0.35% dihydromyricetin | 3 weeks | TGF-β1 ↑ wnt ↑ | [106] |
| Hawthorn polyphenol | BALB/c mice, female, 5–6 weeks old | 100, 300 mg/kg/day | 12 weeks | antioxidant enzyme activity ↑ type I procollagen ↑ epidermal thickening, dermal damage ↓ ROS ↓ MAPKs ↓ NF-kB ↓ | [107] |
| Hawthorn polyphenol | BALB/c mice, female, 5–6 weeks old | 100, 300 mg/kg/day | 12 weeks | ROS ↑ DNA damage ↓ p53 activation ↓ caspase activation ↓ | [108] |
| Cocoa extract | SKH-1 hairless mice, female, 6 weeks old | 39.1, 156.3, 625 mg/kg | 8 weeks | MAPK, MMP-1 ↓ cathepsin G ↓ wrinkles ↓ | [116] |
| Mycosporine-like amino acids extracted from Porphyra tenera | ICR mice, male | 5, 10, 20 mg/mL | 30 days | NF-kB ↑ hydroxyproline ↑ collagen ↑ MMP-1, MMP-3 ↓ TNF-α ↓ | [117] |
| Garlic supplementation | SKH-1 hairless mice, female, 6 weeks old | 1%, 2% | 8 weeks | dorsal skin, epidermal thickness ↑ procollagen ↑ SOD, CAT ↑ wrinkles ↓ ROS ↓ MDA ↓ MMP-1, MMP-2 ↓ | [118] |
| Foeniculum vulgare mill extract | HR-1 mice, male, 7 weeks old | 0.1%, 1% | 10 weeks | collagen ↑ Nrf2 ↑ elastin ↑ TGF-β 1 ↑ MAPK, MMPs ↓ | [119] |
| Wheat extract oil | SKH-1 hairless mice, 6 weeks old | 30, 60, 120 mg/kg | 12 weeks | moisture, skin elasticity ↑ procollagen type I, HA ↑ ceramide ↑ | [120] |
| Fermentation of blackberry with L. plantarum JBMI F5 | SKH-1 hairless mice, female, 6 weeks old | 158 mg/kg of blackberry and 1 × 1010 CFU of L. plantarum JBMI F5 | 4 weeks | type-1 procollagen ↑ antioxidant enzyme ↑ ECM density ↑ MAPK/NF-κ B signaling ↓ wrinkles ↓ epidermal thickening ↓ | [121] |
| Functional proteins and peptides, sugars, or oils | |||||
| Collagen hydrolysates from silver carp (Hypophthalmichthys molitrix) skin | Kunming mice, female, 5 weeks old | 50, 100 and 200 mg per kg body weight collagen hydrolysates | 6 weeks | hydroxyproline ↑ HA ↑ moisture contents ↑ antioxidative enzyme activities ↑ | [10] |
| Tremella fuciformis polysaccharides | SD rats, female, 6/7 weeks old | 100, 200, 300 mg/kg/day | 4 weeks | moisture contents ↑ collagen ↑ stability of type I/III collagen ratio ↑ glycosaminoglycans ↑ SOD, CAT ↑ | [11] |
| Sargassum fusiforme polysaccharide | Kunming mice, female, 7 weeks old | 200, 400, 600 mg/kg/day | 9 weeks | SOD, CAT ↑ MMP-1, MMP-9 ↓ ROS, MDA ↓ oxidative stress ↓ | [12] |
| 7 mega™500 | HR-1 hairless mice, male, 5 weeks old | 50, 100, 200 mg/kg | 12 weeks | wrinkles ↓ MMP-3 ↓ c-Jun ↓ | [128] |
| Dietary suberic acid | SKH-1 hairless mice, female, 6 weeks old | 0.05%, 0.1%, 0.2% suberic acid | 10 weeks | TGF-β/smad pathway ↑ COL1A1, COL1A2, COL3A1 ↑ HAS1, HAS2, HAS3 ↑ skin dryness ↓ wrinkles ↓ epidermal thickness ↓ MAPK/AP-1 pathway ↓ MMP-1a, MMP-1b, MMP-3, MMP-9 ↓ | [129] |
| Olive oil | Swiss mice, male, 8–12 weeks old | 1.5 g/kg per day, contained 74.7 g of oleic acid cis 9 (C18:1) per 100 g of oil and 0.104 mg/mL of total polyphenols | 4 weeks | collagen ↑ ROS ↑ lipid peroxidation ↓ protein carbonylation ↓ MMP-8 ↓ | [13] |
| Insect extracts | HR-1 hairless mice, male | 0.1 mL extracts containing 100 mg/kg body weight | 12 weeks | Collagen, HA ↑ TGF-β ↑ winkles ↓ epidermal thickness ↓ barrier dysfunction ↓ loss of transepidermal water ↓ MAPK, MMPs ↓ pro-inflammatory cytokines ↓ | [137] |
| Probiotics | |||||
| Bifidobacterium breve B-3 | HR-1 hairless mice, male, 6 weeks old | 2 × 109 cfu/mouse /day | 7 weeks | tight junction structure, basement membrane ↑ transepidermal water loss ↓ epidermal thickness ↓ IL-1β ↓ | [15] |
| Tyndalized Lactobacillus acidophilus | HR-1 hairless mice, male, 6 weeks old | 100 mg tyndalized Lactobacillus acidophilus/kg body weight/day | 12 weeks | skin hydration ↑ transepidermal water loss ↓ MMP-1, MMP-9 ↓ wrinkles ↓ | [141] |
| Ingredients | Country | Sample Subjects and Size | Study Design | Dose | Duration | Main Results | Reference |
|---|---|---|---|---|---|---|---|
| Phytochemicals | |||||||
| Astaxanthin | Japan | Human, 23 | a randomized, double-blind, placebo-controlled trial | 4 mg | 10 weeks | skin moisture ↑ skin texture ↑ | [97] |
| Lycopene | UK | Women, 20, mean age 33 years | a randomized controlled study | 55 g tomato paste (16 mg lycopene) | 12 weeks | MED ↑ MMP-1 ↓ mtDNA ↓ | [99] |
| Lycopene and lutein | Germany | Human, 65 | a double-blinded, placebo-controlled, crossover study | 5 mg lycopene and 10 mg lutein | 12 weeks | HO-1 ↓ intercellular adhesion molecule 1 ↓ MMP-1 ↓ | [99] |
| Lycopene, β-carotene and Lactobacillus johnsonii | France | PLE patients, 17 males and 43 females, 60 | a randomized, placebo-controlled, double-blinded study | nutritional supplement containing 2.5 mg lycopene, 4.7 mg of β-carotene and 5 × 108 cfu of the probiotic Lactobacillus johnsonii | 12 weeks | ICAM1 ↑ PLE score ↓ | [101] |
| Resveratrol– procyanidin blend | Italy | Men and women, 50, aged 35–65 years | a placebo-controlled, double-blind study | 8 mg transresveratrol and 14.63 mg procyanidin | 60 days | skin moisturization, elasticity ↑ values for systemic oxidative stress, plasmatic antioxidant capacity, skin antioxidant power ↑ skin roughness, wrinkles ↓ | [110] |
| Green tea polyphenols | Germany | Women, 60, aged 40–65 years | a double-blind, placebo-controlled study | 1402 mg green tea polyphenols | 12 weeks | skin elasticity, roughness ↓ water homeostasis ↑ blood flow and oxygen delivery to skin ↑ erythema ↓ | [114] |
| Curcumin | Japan | Human, 47 | a randomized, double-blind, placebo-controlled trial | 30 mg curcumin | 8 weeks | water content ↑ TNF-α ↓ IL-1 ↓ | [111] |
| Rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols | Spain | Women, 90 | a randomized, parallel-group study | Long-term: 250 mg/day; Short-term: 100, 250 mg/day | Long-term: 2 weeks; Short-term: 24, 48 h | skin redness ↓ wrinkles ↓ skin elasticity ↑ | [115] |
| Functional proteins and peptides, sugars, or oils | |||||||
| Bioactive collagen peptide VERISOL® | Brazil | Women, 114, aged 45–65 years | a double-blind, placebo-controlled study | 2.5 g | 8 weeks | procollagen I, elastin, fibrillin ↑ wrinkles ↓ | [122] |
| collagen peptide | Japan | Women, 33, aged 40–59 years | an ex vivo model and randomized, placebo-controlled clinical trial | 10 g specific mixture of collagen peptides of fish origin (Peptan®F) or porcineorigin (Peptan®P) | 12 weeks | skin hydration ↑ collagen density ↑ glycosaminoglycan ↑ | [123] |
| A collagen hydrolysate and antioxidant- containing nutraceutical | India | Women, 34, mean age 39.5 years | - | Marine collagen peptides (5 g of fish collagen peptides) and antioxidant blend (natural tomato extract, grape seed extract, green tea extract, vitamin C and vitamin E) | 30 days | skin hydration, firmness, elasticity, barrier function ↑ wrinkle width, open pores, skin roughness, the color of hyperpigmented blemishes ↓ | [124] |
| Galacto-oligosaccharides | Korea | Women | a randomized, double-blind clinical trial | 1 g twice a day | 12 weeks | TEWL ↓ wrinkles ↓ | [125] |
| Squalene | Korea | Women, 40, >50 years | - | 13.5, 27 g/day | 90 days | procollagen ↑ MED ↑ facial erythema ↓ keratinocytic apoptosis ↓ thymine dimer level ↓ wrinkles ↓ | [131] |
| Fish oil | US | Human, 10 | - | 10 capsules per day of fish oil containing each 280 mg EPA and 120 mg DHA | 4 weeks | MED ↑ | [132] |
| Argan oil | Morocco | Postmenopausal women, 60 | - | 25 mL/day | 60 days | gross elasticity of skin, net elasticity of skin, biological elasticity ↑ resonance running time ↓ | [134] |
| Aloe sterol | Japan | Men, 48 | a randomized, double-blind, placebo-controlled study | 40 mg/d | 12 weeks | skin elasticity ↑ | [135] |
| Aloe sterol | Japan | Women, 64 | a randomized, double-blind, placebo-controlled study | aloe sterol yogurt contained 40 µg of aloe sterol per 100 g | 12 weeks | skin elasticity ↑ skin hydration ↑ collagen ↑ | [135] |
| Probiotics | |||||||
| Lactococcus H61 | Japan | Women, 30 | a double-blind, placebo-controlled trial | 4 × 1010 cfu | 8 weeks | balance of intestinal microbes and intestinal health ↑ antioxidant activity ↑ skin immune response ↑ | [14] |
| Lactobacillus paracasei NCC 2461 | France | Women, 64, aged 18–40 years | a randomized, double-blind, placebo-controlled study | 1 × 1010 cfu | 4 weeks | barrier function ↑ skin sensitivity ↓ | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. https://doi.org/10.3390/nu13051691
Geng R, Kang S-G, Huang K, Tong T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients. 2021; 13(5):1691. https://doi.org/10.3390/nu13051691
Chicago/Turabian StyleGeng, Ruixuan, Seong-Gook Kang, Kunlun Huang, and Tao Tong. 2021. "Boosting the Photoaged Skin: The Potential Role of Dietary Components" Nutrients 13, no. 5: 1691. https://doi.org/10.3390/nu13051691
APA StyleGeng, R., Kang, S.-G., Huang, K., & Tong, T. (2021). Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients, 13(5), 1691. https://doi.org/10.3390/nu13051691








