Nutrients, Diet, and Other Factors in Prenatal Life and Bone Health in Young Adults: A Systematic Review of Longitudinal Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search
2.2. Study Selection
2.3. Data Collection
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
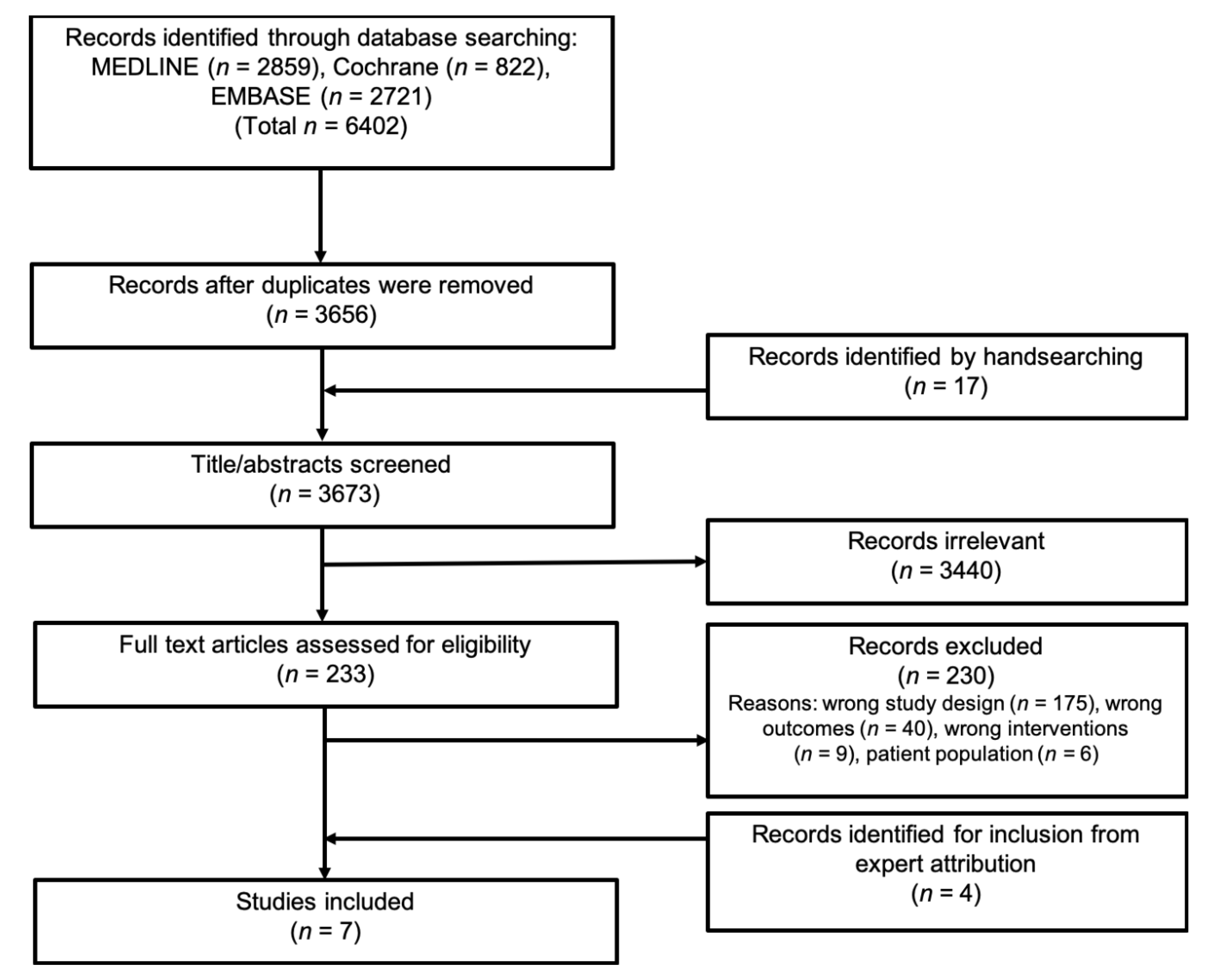
3.1. Literature Search
3.2. Cohort Study Characteristics
3.3. Risk of Bias within the Studies
3.4. Qualitative Syntheses of Results
3.5. Maternal Diet and Vitamin D
3.5.1. Total Body BMD
3.5.2. Femoral Neck BMD
3.5.3. Lumbar Spine BMD
3.6. Preeclampsia and Gestational Hypertension
3.6.1. Total Body BMD
3.6.2. Lumbar Spine BMD
3.6.3. Femoral Neck/Total Hip BMD
3.7. Maternal Age
3.7.1. Total Body aBMD
3.7.2. Lumbar Spine aBMD
3.7.3. Femoral Neck BMD
3.7.4. Subsample Analysis of Maternal Age
3.8. Maternal Cigarette Smoking
3.8.1. Total Body BMD
3.8.2. Femoral Neck BMD
3.8.3. Lumbar Spine
3.8.4. Fractures
4. Discussion
4.1. Directions for Future Research
4.2. Strength and Limitations of the Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. WHO Scientific Group on the Assessment of Osteoporosis At Primary Health Care Level; Summary Meeting Report; WHO: Brussels, Belgium, 1–13 May 2004. [Google Scholar]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K.L. Risk of fracture in women with type 2 diabetes: The women’s health initiative observational study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Westlake, S.; Harvey, N.; Dennison, E. Developmental origins of osteoporotic fracture. Adv. Exp. Med. Biol. 2009, 639, 217–236. [Google Scholar] [CrossRef]
- Hyde, N.K.; Brennan-Olsen, S.L.; Wark, J.D.; Hosking, S.M.; Pasco, J.A. Maternal Dietary Nutrient Intake During Pregnancy and Offspring Linear Growth and Bone: The Vitamin D in Pregnancy Cohort Study. Calcif. Tissue Int. 2017, 100, 47–54. [Google Scholar] [CrossRef]
- Jones, G.; Riley, M.D.; Dwyer, T. Maternal diet during pregnancy is associated with bone mineral density in children: A longitudinal study. Eur. J. Clin. Nutr. 2000, 54, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.; Rasmussen, M.; Olsen, S.; Vestergaard, P.; Mølgaard, C.; Halldorsson, T.; Strøm, M. Maternal Dietary Patterns during Pregnancy in Relation to Offspring Forearm Fractures: Prospective Study from the Danish National Birth Cohort. Nutrients 2015, 7, 2382–2400. [Google Scholar] [CrossRef]
- Javaid, M.K.; Crozier, S.R.; Harvey, N.C.; Gale, C.R.; Dennison, E.M.; Boucher, B.J.; Arden, N.K.; Godfrey, K.M.; Cooper, C.; Study group, T. princess A.H. Maternal vitamin D status during pregnancy and childhood bone bone mass at age 9 years: A longitudinal study. Lancet 2006, 367, 36–43. [Google Scholar] [CrossRef]
- Tobias, J.H.; Steer, C.D.; Emmett, P.M.; Tonkin, R.J.; Cooper, C.; Ness, A.R. Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos. Int. 2005, 16, 1731–1741. [Google Scholar] [CrossRef]
- Sayers, A.; Tobias, J.H. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J. Clin. Endocrinol. Metab. 2009, 94, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.A.; Wills, A.K.; Fraser, A.; Sayers, A.; Fraser, W.D.; Tobias, J.H. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: A prospective cohort study. Lancet 2013, 381, 2176–2183. [Google Scholar] [CrossRef] [Green Version]
- Händel, M.N.; Frederiksen, P.; Cohen, A.; Cooper, C.; Heitmann, B.L.; Abrahamsen, B. Neonatal vitamin D status from archived dried blood spots and future risk of fractures in childhood: Results from the D-tect study, a population-based case-cohort study. Am. J. Clin. Nutr. 2017, 106, 155–161. [Google Scholar] [CrossRef]
- Händel, M.N.; Frederiksen, P.; Osmond, C.; Cooper, C.; Abrahamsen, B.; Heitmann, B.L. Prenatal exposure to Vitamin D from fortified margarine and risk of fractures in late childhood: Period and cohort results from 222 000 subjects in the D-tect observational study. Br. J. Nutr. 2017, 117, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.; Walker-Bone, K.; Robinson, S.; Taylor, P.; Shore, S.; Wheeler, T.; Cooper, C. Neonatal Bone Mass: Influence of Parental Birthweight, Maternal Smoking, Body Composition, and Activity During Pregnancy. J. Bone Miner. Res. 2001, 16, 1694–1703. [Google Scholar] [CrossRef]
- Parviainen, R.; Auvinen, J.; Pokka, T.; Serlo, W.; Sinikumpu, J.J. Maternal smoking during pregnancy is associated with childhood bone fractures in offspring – A birth-cohort study of 6718 children. Bone 2017, 101, 202–205. [Google Scholar] [CrossRef]
- Hallal, P.C.; Siqueira, F.V.; Menezes, A.M.B.; Araújo, C.L.P.; Norris, S.A.; Victora, C.G. The role of early life variables on the risk of fractures from birth to early adolescence: A prospective birth cohort study. Osteoporos. Int. 2009, 20, 1873–1879. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.J.; Cooper, C.; Dawson-Hughes, B.; Gordon, C.M.; Rizzoli, R. Life-course approach to nutrition. Osteoporos. Int. 2015, 26, 2723–2742. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, C.J.; Beaupré, G.S.; Carter, D.R. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos. Int. 2003, 14, 843–847. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev. Esp. Nutr. Hum. Y Diet. 2016, 20, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.H.; Riis, K.R.; Händel, M.N.; Abrahamsen, B. Bone health in young adulthood: A systematic review and meta-analysis on prognostic factors in pregnancy and offspring bone health. Protocol for a systematic review. PROSPERO 2019, 1–5. [Google Scholar]
- Matsuzaki, M.; Kuper, H.; Kulkarni, B.; Radhakrishna, K.V.; Viljakainen, H.; Taylor, A.E.; Sullivan, R.; Bowen, L.; Tobias, J.H.; Ploubidis, G.B.; et al. Life-course determinants of bone mass in young adults from a transitional rural community in India: The Andhra Pradesh Children and Parents Study (APCAPS). Am. J. Clin. Nutr. 2014, 99, 1450–1459. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [Green Version]
- Hannam, K.; Lawlor, D.A.; Tobias, J.H. Maternal Preeclampsia is associated with reduced adolescent offspring hip BMD in a UK population-based birth cohort. JBMR 2015, 30, 1684–1691. [Google Scholar] [CrossRef] [Green Version]
- Miettola, S.; Hovi, P.; Andersson, S.; Strang-Karlsson, S.; Pouta, A.; Laivuori, H.; Järvenpää, A.L.; Eriksson, J.G.; Mäkitie, O.; Kajantie, E. Maternal preeclampsia and bone mineral density of the adult offspring. Am. J. Obs. Gynecol. 2013, 209, e1–e443. [Google Scholar] [CrossRef]
- Jones, G.; Hynes, K.L.; Dwyer, T. The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: A 16-year longitudinal study. Osteoporos. Int. 2013, 24, 1605–1611. [Google Scholar] [CrossRef]
- Martínez-Mesa, J.; Menezes, A.M.B.; Howe, L.D.; Wehrmeister, F.C.; Muniz, L.C.; González-Chica, D.A.; Assunção, M.C.; Gonçalves, H.; Barros, F.C. Lifecourse relationship between maternal smoking during pregnancy, birth weight, contemporaneous anthropometric measurements and bone mass at 18 years old. The 1993 Pelotas Birth Cohort. Early Hum. Dev. 2014, 90, 901–906. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Dwyer, T.; Riley, M.; Cochrane, J.; Jones, G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur. J. Clin. Nutr. 2010, 64, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Whitehouse, A.J.; Hart, P.H.; Kusel, M.; Mountain, J.; Lye, S.; Pennell, C.; Walsh, J.P. Maternal Vitamin D Status During Pregnancy and Bone Mass in Offspring at 20 years of age: A prospective Cohort Study. J. Bone Miner. Res. 2014, 29, 1088–1095. [Google Scholar] [CrossRef]
- Rudäng, R.; Mellström, D.; Clark, E.; Ohlsson, C.; Lorentzon, M. Advancing maternal age is associated with lower bone mineral density in young adult male offspring. Osteoporos. Int. 2012, 23, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Riley, M.; Dwyer, T. Maternal Smoking During Pregnancy, Growth, and Bone Mass in Prepubertal Children. J. Bone Miner. Res. 1999, 14, 146–151. [Google Scholar] [CrossRef]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Harvey, N.C.; Javaid, M.K.; Arden, N.K.; Poole, J.R.; Crozier, S.R.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal predictors of neonatal bone size and geometry: The Southampton Women’s Survey. J. Dev. Orig. Health Dis. 2010, 1, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Jones, G. Early life nutrition and bone development in children. Nestle Nutr. Workshop Ser. Pediatr. Program. 2011, 68, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Ganpule, A.; Yajnik, C.S.; Fall, C.H.D.; Rao, S.; Fisher, D.J.; Kanade, A.; Cooper, C.; Naik, S.; Joshi, N.; Lubree, H.; et al. Bone mass in Indian children—Relationships to maternal nutritional status and diet during pregnancy: The Pune maternal nutrition study. J. Clin. Endocrinol. Metab. 2006, 91, 2994–3001. [Google Scholar] [CrossRef] [Green Version]
- Händel, M.N.; Moon, R.J.; Titcombe, P.; Abrahamsen, B.; Heitmann, B.L.; Calder, P.C.; Dennison, E.M.; Robinson, S.M.; Godfrey, K.M.; Inskip, H.M.; et al. Maternal serum retinol and β-carotene concentrations and neonatal bone mineralization: Results from the Southampton Women’s Survey cohort. Am. J. Clin. Nutr. 2016, 104, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Cole, Z.A.; Gale, C.R.; Kassim Javaid, M.; Robinson, S.M.; Law, C.; Boucher, B.J.; Crozier, S.R.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal dietary patterns during pregnancy and childhood bone mass: A longitudinal study. J. Bone Miner. Res. 2009, 24, 663–668. [Google Scholar] [CrossRef]
- Namgung, R.; Tsang, R.C. Bone in the pregnant mother and newborn at birth. Clin. Chim. Acta. 2003, 333, 1–11. [Google Scholar] [CrossRef]
- Händel, M.N.; Heitmann, B.L.; Abrahamsen, B. Nutrient and food intakes in early life and risk of childhood fractures: A systematic review and meta-analysis. Am. J. Clin. Nutr 2015, 102, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Shrier, I.; Platt, R.W. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 2008, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Aagaard-Tillery, K.M.; Porter, T.F.; Lane, R.H.; Varner, M.W.; Lacoursiere, D.Y. In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am. J. Obstet. Gynecol. 2008, 198, 66.e1–66.e6. [Google Scholar] [CrossRef]
- Wang, X.; Tager, I.B.; Van Vunakis, H.; Speizer, F.E.; Hanrahan, J.P. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int. J. Epidemiol. 1997, 26, 978–988. [Google Scholar] [CrossRef] [PubMed]
- De Steenwinkel, F.D.O.; Hokken-Koelega, A.C.S.; Hazes, J.M.W.; Dolhain, R.J.E.M. Does medication use or disease activity during pregnancy in patients with rheumatoid arthritis affect bone density in their prepubertal offspring? Arthritis Rheumatol. 2014, 66, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, X.; Li, Y.; Zhang, R.; Nie, Z.; Cheng, X.; Zhang, X.; Wang, H. Course-, dose-, and stage-dependent toxic effects of prenatal dexamethasone exposure on long bone development in fetal mice. Toxicol. Appl. Pharmacol. 2018, 351, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shang-Guan, Y.; Ma, J.; Hu, H.; Wang, L.; Magdalou, J.; Chen, L.; Wang, H. Mitogen-inducible gene-6 partly mediates the inhibitory effects of prenatal dexamethasone exposure on endochondral ossification in long bones of fetal rats. Br. J. Pharmacol. 2016, 173, 2250–2262. [Google Scholar] [CrossRef] [PubMed]
- Swolin-Eide, D.; Dahlgren, J.; Nilsson, C.; Albertsson Wikland, K.; Holmäng, A.; Ohlsson, C. Affected skeletal growth but normal bone mineralization in rat offspring after prenatal dexamethasone exposure. J. Endocrinol. 2002, 174, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubnov-Raz, G.; Hemilä, H.; Vurembrand, Y.; Kuint, J.; Maayan-Metzger, A. Maternal use of selective serotonin reuptake inhibitors during pregnancy and neonatal bone density. Early Hum. Dev. 2012, 88, 191–194. [Google Scholar] [CrossRef]
- Lundberg, R.; Lyche, J.L.; Ropstad, E.; Aleksandersen, M.; Rönn, M.; Skaare, J.U.; Larsson, S.; Örberg, J.; Lind, P.M. Perinatal exposure to PCB 153, but not PCB 126, alters bone tissue composition in female goat offspring. Toxicology 2006, 228, 33–40. [Google Scholar] [CrossRef]
- Cocchi, D.; Tulipano, G.; Colciago, A.; Sibilia, V.; Pagani, F.; Viganò, D.; Rubino, T.; Parolaro, D.; Bonfanti, P.; Colombo, A.; et al. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat. Part 1: Effects on somatic growth, growth hormone-axis activity and bone mass in the offspring. Toxicol. Appl. Pharmacol. 2009, 237, 127–136. [Google Scholar] [CrossRef]
- Romero, A.N.; Herlin, M.; Finnilä, M.; Korkalainen, M.; Håkansson, H.; Viluksela, M.; Sholts, S.B. Skeletal and dental effects on rats following in utero/lactational exposure to the non-dioxin-like polychlorinated biphenyl PCB 180. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Koskela, A.; Finnilä, M.A.; Korkalainen, M.; Spulber, S.; Koponen, J.; Håkansson, H.; Tuukkanen, J.; Viluksela, M. Effects of developmental exposure to perfluorooctanoic acid (PFOA) on long bone morphology and bone cell differentiation. Toxicol. Appl. Pharmacol. 2016, 301, 14–21. [Google Scholar] [CrossRef]
- Flöter, V.L.; Galateanu, G.; Fürst, R.W.; Seidlová-Wuttke, D.; Wuttke, W.; Möstl, E.; Hildebrandt, T.B.; Ulbrich, S.E. Sex-specific effects of low-dose gestational estradiol-17β exposure on bone development in porcine offspring. Toxicology 2016, 366–367, 60–67. [Google Scholar] [CrossRef]
- Connelly, K.J.; Larson, E.A.; Marks, D.L.; Klein, R.F. Neonatal estrogen exposure results in biphasic age-dependent effects on the skeletal development of male mice. Endocrinology 2015, 156, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimouni, F.; Steichen, J.J.; Tsang, R.C.; Hertzberg, V.; Miodovnik, M. Decreased Bone Mineral Content in Infants of Diabetic Mothers. Am. J. Perinatol. 1988, 5, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Weiler, H.A. Long-term effects of gestational diabetes on offspring health are more pronounced in skeletal growth than body composition and glucose tolerance. Br. J. Nutr. 2010, 104, 1641–1649. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [Green Version]
- Avdagić, S.C.; Barić, I.C.; Keser, I.; Cecić, I.; Šatalić, Z.; Bobić, J.; Gomzi, M. Differences in peak bone density between male and female students. Arh. Hig. Rada Toksikol. 2009, 60, 79–86. [Google Scholar] [CrossRef] [Green Version]
| 1st Author, Year, Country | Study Design | Sample Size (n) | Age, Offspring (Mean ± SD) | Maternal Age, at Birth (years) | Exposure, Cointervention | Outcome |
|---|---|---|---|---|---|---|
| Hannam [24], 2015, England | Prospective cohort | 3088 | 1 PE: 17.9 ± 0.5 GH: 17.8 ± 0.4 No HDP: 17.8 ± 0.4 | 15–19 y: n = 36 20–24 y: n = 367 25–29 y: n = 1165 30–34 y: n = 1101 >40 y: n = 51 | Preeclampsia and gestational hypertension. No co-intervention. | BMD |
| Jones [26], 2013, Tasmania | Prospective cohort | 415 | 2 Never breastfeed: 16.3 ± 0.5 Ever breastfeed: 16.3 ± 0.4 | (Mean ±SD) Offspring never breastfeed: 24.1 ± 5.0 Offspring ever breastfed: 26.2 ± 4.6 | 3 Maternal smoking during pregnancy. No cointervention | BMD, fractures |
| Martínez-Mesa [27], 2014, Southern Brazil | Prospective cohort | 3075 | 18 years | (Mean (s.e)) Offspring male: 26.5 (0.17) Offspring female: 26.5 (0.15) | 4 Maternal smoking during pregnancy. No co-intervention | BMD, BMC |
| Miettola [25], 2013, Finland | Prospective cohort | 5283 | 5 VLBW: 6 PE 22.5 ± 2.0 No PE 22.6 ± 2.2 5 Term: PE 21.5 ± 1.7 No PE 22.7 ± 2.2 | - | Preeclampsia no co-intervention | BMD, BMAD, BMC |
| Rudäng [30], 2012, Sweden | Prospective cohort | 1068 | 18.9 ± 0.6 | (Mean ±SD) 29.5 ± 4.8 | Maternal age. No co-intervention | aBMD, BMC, BA, CSA, endosteal and periosteal circumference, trabecular and cortical vBMD |
| Yin [28], 2010, Tasmania | Prospective cohort | 216 | 16.2 ± 0.4 | (Mean ±SD) 26.7 ± 4.7 | 7 Maternal dietary intake during the third trimester. No co-intervention | BMD, BMC |
| Zhu [29], 2014, Australia | Prospective cohort | 341 | Male: 20.2 ± 0.5 Female: 20.1 ± 0.5 | (Mean ±SD) 29.0 ± 5.6 | Serum 25-hydroxyvitamin D during pregnancy. No co-intervention | BMD, BMC, BA |
| 1st Author | Bias Due to Confound-ding | Bias Due to Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Departures from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of Reported Results | Overall Judgment |
|---|---|---|---|---|---|---|---|---|
| Hannam [24] | Moderate | Low | Moderate | Low | Serious | Low | Moderate | Serious |
| Jones [26] | Serious | Serious | Moderate | Low | Serious | Moderate | Moderate | Serious |
| Martínez-Mesa [27] | Serious | Low | Low | Low | Serious | Low | Moderate | Serious |
| Miettola [25] | Serious | Low | Low | Low | Serious | Low | Serious | Serious |
| Rudäng [30] | Serious | Low | Low | Low | Serious | Low | Moderate | Serious |
| Yin [28] | Serious | Serious | Moderate | No information | Serious | Moderate | Moderate | Serious |
| Zhu [29] | Moderate | Low | Moderate | No information | Serious | Moderate | Moderate | Serious |
| Outcome | Total Body BMD | Lumbar Spine/Spine BMD | Femoral/Total Hip BMD | |
|---|---|---|---|---|
| 1st Author | ||||
| Yin [28] | Food groups | |||
| BMD not affected by maternal meat density 1 | BMD not affected by maternal meat density 1 | BMD not affected by maternal meat density 1 | ||
| BMD not affected by maternal fish density 1 | BMD not affected by maternal fish density 1 | BMD not affected by maternal fish density 1 | ||
| BMD not affected by maternal milk density 1 | BMD increases with increasing maternal milk density 1 (mL/kJ) 2β: +0.41 (r2 0.213) (p < 0.05) | BMD not affected by maternal milk density 1 | ||
| BMD not affected by maternal vegetable density 1 | BMD not affected by maternal vegetable density 1 | BMD not affected by maternal vegetable density 1 | ||
| BMD not affected by maternal fruit density 1 | BMD not affected by maternal fruit density 1 | BMD not affected by maternal fruit density 1 | ||
| Macronutrients | ||||
| BMD not affected by maternal protein density 1 | BMD not affected by maternal protein density 1 | BMD not affected by maternal protein density 1 | ||
| BMD not affected by maternal fat density 1 | BMD declines with increased maternal fat density 1 (g/kJ) 2β: −10.3 (r2 0.217) (p < 0.05) | BMD declines with increased maternal fat density 1 (g/kJ) 2β: −11.3 (r2 0.366) (p < 0.05) | ||
| BMD not affected by maternal carbohydrate density 1 | BMD not affected by maternal carbohydrate density 1 | BMD not affected by maternal carbohydrate density 1 | ||
| Micronutrients | ||||
| BMD not affected by maternal calcium density 1 | BMD increases with increasing maternal calcium density 1 (mg/kJ) 2 β: +0.36 (r2 0.216) (p < 0.05) | BMD not affected by maternal calcium density 1 | ||
| BMD not affected by maternal magnesium density 1 | BMD increases with increasing maternal magnesium density (mg/kJ) 2 β: +2.9 (r2 0.217) (p < 0.05) | Some of the analysis indicated increasing BMD with increasing maternal magnesium intake | ||
| BMD not affected by maternal phosphorus density 1 | BMD not affected by maternal phosphorus density 1 | BMD not affected by maternal phosphorus density 1 | ||
| Zhu [29] | BMD increases with higher maternal serum 25OHD concentration 3 Mean, 95% CI: 4.6 [0.1;9.1] mg/cm2 | - | - | |
| BMD declines with maternal vitamin D deficiency 4 Mean ± SD: 1053 ± 7 versus 1071 ± 5 mg/cm2 (p = 0.043) | - | - | ||
| Outcome | Total Body BMD | Lumbar Spine BMD | Femoral/Total Hip BMD | |
|---|---|---|---|---|
| 1st author | ||||
| Hannam [24] | No association between BMD and PE | No association between BMD and PE | BMD was inversely associated with PE 1 Mean difference (95%CI): −0.30 (−0.50 to −0.10), p = 0.004 | |
| No association between BMD and GH in the fully adjusted data | No association between BMD and GH | No association between BMD and GH in the fully adjusted data | ||
| Miettola [25] | Preeclampsia had a direct association with BMD in VLBW offspring. 2 Mean difference(95%CI): 0.46(0.15–0.76) p = 0.003 | Preeclampsia had a direct association with BMD in VLBW offspring 2 Mean difference(95%CI): 0.42(0.08–0.76), p = 0.016 | Preeclampsia had a direct association with BMD in VLBW offspring 2 Mean difference(95%CI): 0.37(0.06–0.68), p = 0.020 | |
| Preeclampsia had a direct association with BMD in Term offspring 2 Mean difference (95%CI): 0.87(0.28–1.46), p = 0.004 | There was a direct association between BMD and PE in Term offspring in the fully adjusted model, but not in the first 3 models 3. 2 Mean difference(95%CI): 0.70(0.13–1.27), p = 0.017 | Preeclampsia had a direct association with BMD in Term offspring 2 Mean difference (95%CI): 0.70 (0.17–1.23), p = 0.010 | ||
| No association between GH in Term offspring and BMD. No analysis was performed for VLBW offspring. | ||||
| Outcome | Total Body aBMD (g/cm2) | Lumbar Spine aBMD (g/cm2) | Femoral Neck aBMD (g/cm2) | |
|---|---|---|---|---|
| 1st author | ||||
| Rudäng [30] | aBMD declines with increasing maternal age in a bivariate correlation but become non-significant in the stepwise linear regression model β: −0.031 (NS) r value: −0.070 (p < 0.03) | aBMD declines with increasing maternal age: β: −0.091(p < 0.01) r value: −0.092 (p < 0.01) | Femoral aBMD was not affected by maternal age. | |
| Outcome | BMD | |
|---|---|---|
| 1st author | ||
| 1Jones [26] | Total body BMD, lumbar spine BMD, and hip BMD Not affected by maternal smoking during pregnancy. | |
| 2Martínez-Mesa [27] | Total body BMD not affected by maternal smoking in an overall association | |
| Total body BMD not affected by maternal smoking after accounting for mediation by birth weight and concurrent height | ||
| Total body BMD not affected by maternal smoking after accounting for mediation by birth weight and concurrent weight | ||
| Total body BMD not affected by maternal smoking after accounting for mediation by birth weight and concurrent BMI | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, K.H.; Riis, K.R.; Abrahamsen, B.; Händel, M.N. Nutrients, Diet, and Other Factors in Prenatal Life and Bone Health in Young Adults: A Systematic Review of Longitudinal Studies. Nutrients 2020, 12, 2866. https://doi.org/10.3390/nu12092866
Jensen KH, Riis KR, Abrahamsen B, Händel MN. Nutrients, Diet, and Other Factors in Prenatal Life and Bone Health in Young Adults: A Systematic Review of Longitudinal Studies. Nutrients. 2020; 12(9):2866. https://doi.org/10.3390/nu12092866
Chicago/Turabian StyleJensen, Karina H., Kamilla R. Riis, Bo Abrahamsen, and Mina N. Händel. 2020. "Nutrients, Diet, and Other Factors in Prenatal Life and Bone Health in Young Adults: A Systematic Review of Longitudinal Studies" Nutrients 12, no. 9: 2866. https://doi.org/10.3390/nu12092866






