A Comprehensive Evaluation of the Impact of Bovine Milk Containing Different Beta-Casein Profiles on Gut Health of Ageing Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milks
2.2. Animals and Diets
2.3. Lymphocytes Preparation from Mouse Small Intestine and Flow Cytometry Phenotypic Analysis
2.4. Cytokine Secretion in Intestinal Tissue
2.5. Enzymatic Activity
2.6. Histomorphological Evaluation
2.7. Immunohistochemical Staining
2.8. Serum Immunoglobulins G (IgG) Measurements
2.9. SCFA Analysis
2.10. DNA Extraction from Faecal Samples and Next Generation Sequencing (NGS) Analysis
2.11. Bioinformatics
2.12. Statistical Analyses
3. Results
3.1. Milk Components and Lipid Profile
3.2. Body Weight and Food Intake
3.3. Gut Immune Phenotyping and Cytokine Secretion
3.4. Gut Enzymatic Activities
3.5. Histomorphological Evaluation
3.6. Immunohistochemical Staining
3.7. Analysis of Serum IgG
3.8. Analysis of Faecal SCFAs
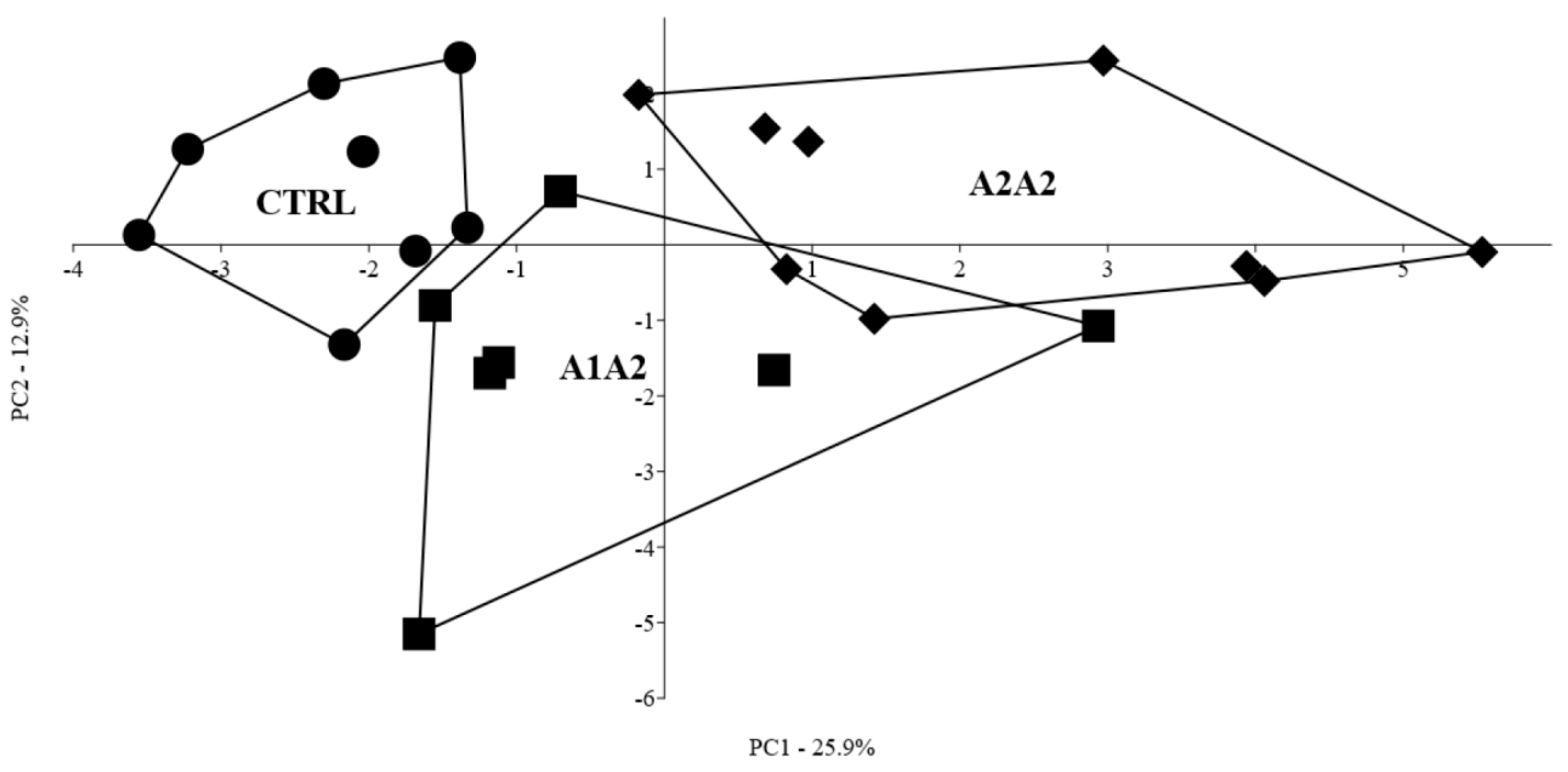
3.9. Multivariate Profiling of the Effect of the Different Diets
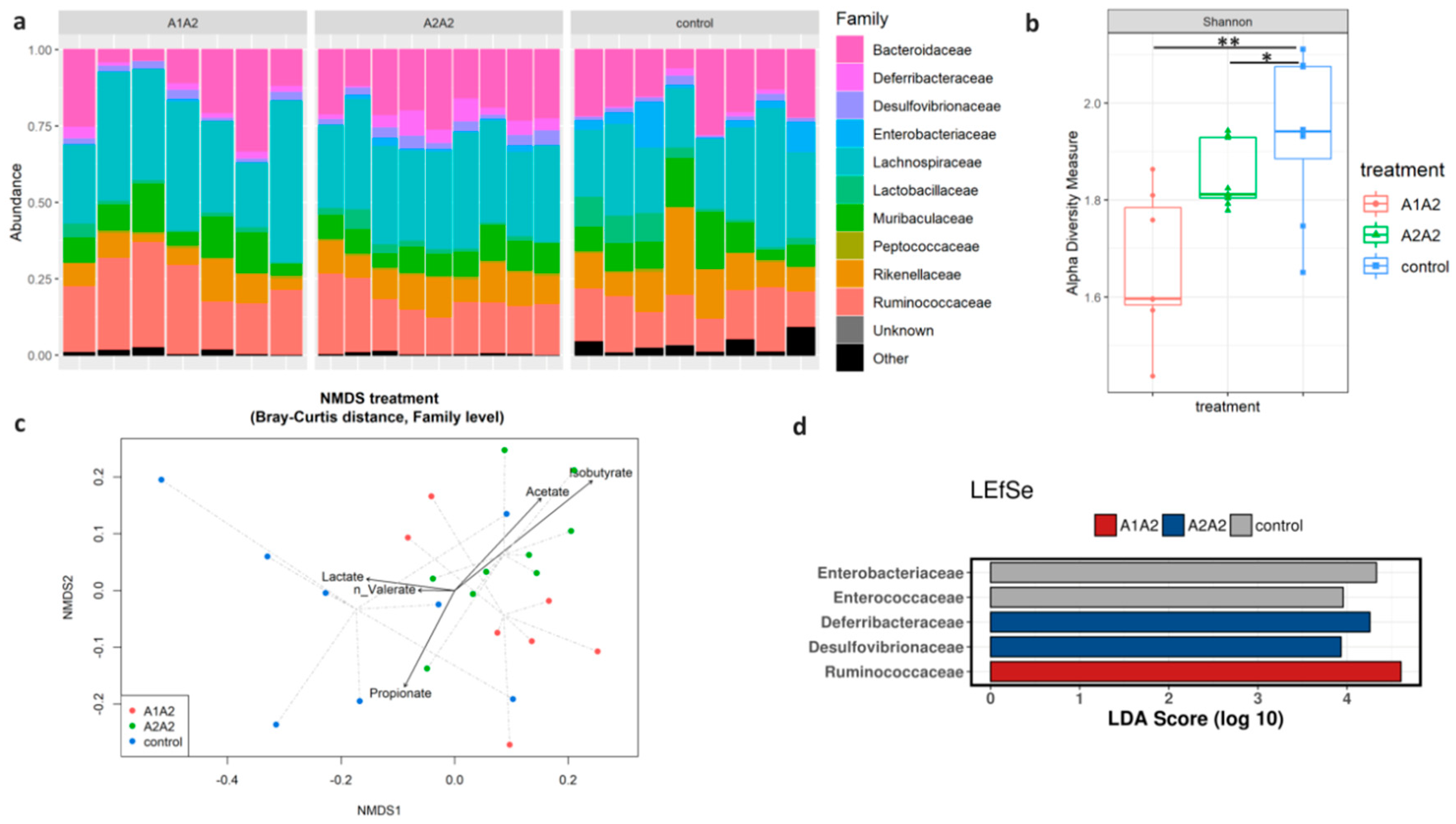
3.10. Analysis of Faecal Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muehlhoff, E.; Bennett, A.; McMahon, D. Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 beta-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, S.; Trompette, A.; Claustre, J.; El Homsi, M.; Garzon, J.; Jourdan, G.; Scoazec, J.Y.; Plaisancie, P. Beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1105–G1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, C.N. Beta-casein A1, ischaemic heart disease mortality, and other illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R.B.; Harris, D.P.; Hill, J.P.; Bibby, N.J.; Wasmuth, H.E. Type I (insulin-dependent) diabetes mellitus and cow milk: Casein variant consumption. Diabetologia 1999, 42, 292–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cade, R.; Privette, M.; Fregly, M.; Rowland, N.; Sun, Z.; Zele, V.; Wagemaker, H.; Edelstein, C. Autism and Schizophrenia: Intestinal Disorders. Nutr. Neurosci. 2000, 3, 57–72. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Report of EFSA prepared by a DATEX Working Group on the potential health impact of β-casomorphins and related peptides. EFSA Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Barnett, M.P.; McNabb, W.C.; Roy, N.C.; Woodford, K.B.; Clarke, A.J. Dietary A1 beta-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 beta-casein in Wistar rats. Int. J. Food Sci. Nutr. 2014, 65, 720–727. [Google Scholar] [CrossRef]
- Ul Haq, M.R.; Kapila, R.; Sharma, R.; Saliganti, V.; Kapila, S. Comparative evaluation of cow beta-casein variants (A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur. J. Nutr. 2014, 53, 1039–1049. [Google Scholar] [CrossRef]
- Milan, A.M.; Shrestha, A.; Karlstrom, H.J.; Martinsson, J.A.; Nilsson, N.J.; Perry, J.K.; Day, L.; Barnett, M.P.G.; Cameron-Smith, D. Comparison of the impact of bovine milk beta-casein variants on digestive comfort in females self-reporting dairy intolerance: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 149–160. [Google Scholar] [CrossRef]
- Sheng, X.; Li, Z.; Ni, J.; Yelland, G. Effects of Conventional Milk Versus Milk Containing Only A2 beta-Casein on Digestion in Chinese Children: A Randomized Study. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 375–382. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sun, J.; Jiang, Z.Q.; Yang, Y.X. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: A multicentre, randomised controlled study. Nutr. J. 2017, 16, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2016, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: A blinded randomised cross-over pilot study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef]
- Beard, J.R.; Alana, M.; Cassels, A. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; Available online: http://www.who.int/ageing/publications/world-report-2015/en/ (accessed on 19 October 2015).
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Candela, M.; Turroni, S.; Garagnani, P.; Franceschi, C.; Brigidi, P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol. Res. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Nuccitelli, S.; Carnevali, P.; Brigidi, P.; Vitali, B.; Nobili, F.; Rami, R.; Garaguso, I.; Mengheri, E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel. Dis. 2009, 15, 1526–1536. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Brasili, E.; Rami, R.; Nobili, F.; Orsi, C.; Zambrini, A.V.; Mengheri, E. Beneficial effects of a selected probiotic mixture administered to high fat-fed mice before and after the development of obesity. J. Funct. Foods 2018, 45, 321–329. [Google Scholar] [CrossRef]
- Canali, R.; Vignolini, F.; Nobili, F.; Mengheri, E. Reduction of oxidative stress and cytokine-induced neutrophil chemoattractant (CINC) expression by red wine polyphenols in zinc deficiency induced intestinal damage of rat. Free Radic Biol. Med. 2000, 28, 1661–1670. [Google Scholar] [CrossRef]
- Thomazini, B.F.; Dolder, M.A. Dose dependent treatment with isotretinoin induces more changes in the ileum than in the duodenum and jejunum in Wistar rats. Tissue Cell 2017, 49, 203–208. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.V.; Azevedo, S.C.; Bazotte, R.B.; Peralta, R.M.; Buttow, N.C.; Pedrosa, M.M.; de Godoi, V.A.; Natali, M.R. Supplementation with L-Glutamine and L-Alanyl-L-Glutamine Changes Biochemical Parameters and Jejunum Morphophysiology in Type 1 Diabetic Wistar Rats. PLoS ONE 2015, 10, e0143005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanches, S.C.; Ramalho, L.N.; Augusto, M.J.; da Silva, D.M.; Ramalho, F.S. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. Biomed Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef] [PubMed]
- Canale, A.; Valente, M.E.; Ciotti, A. Determination of volatile carboxylic acids (C1–C5i) and lactic acid in aqueous acid extracts of silage by high performance liquid chromatography. J. Sci. Food Agric. 1984, 35, 1178–1182. [Google Scholar] [CrossRef]
- Zinno, P.; Motta, V.; Guantario, B.; Natella, F.; Roselli, M.; Bello, C.; Comitato, R.; Carminati, D.; Tidona, F.; Meucci, A.; et al. Supplementation with dairy matrices impacts on homocysteine levels and gut microbiota composition of hyperhomocysteinemic mice. Eur. J. Nutr. 2020, 59, 345–358. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. CRAN-Package Vegan. 2016. Available online: https://cran.r-project.org (accessed on 15 June 2020).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Zingone, F.; Bucci, C.; Iovino, P.; Ciacci, C. Consumption of milk and dairy products: Facts and figures. Nutrition 2017, 33, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Kroemer, G. Immunostimulatory gut bacteria. Science 2019, 366, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Stetter, K.O. Family I. Deferribacteraceae fam. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, R.W., Garrity, G.M., Eds.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Chung, Y.W.; Gwak, H.J.; Moon, S.; Rho, M.; Ryu, J.H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar] [CrossRef] [Green Version]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Fukunaga, M.; Suriki, K.; Kuda, T.; Shikano, A.; Toyama, A.; Takahashi, H.; Kimura, B. Typical indigenous bacteria in the cecum of ddY mice fed a casein-beef tallow diet or whole-egg diet. J. Food Biochem. 2019, 43, e13064. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Jirsova, Z.; Heczkova, M.; Dankova, H.; Malinska, H.; Videnska, P.; Vespalcova, H.; Micenkova, L.; Bartonova, L.; Sticova, E.; Lodererova, A.; et al. The Effect of Butyrate-Supplemented Parenteral Nutrition on Intestinal Defence Mechanisms and the Parenteral Nutrition-Induced Shift in the Gut Microbiota in the Rat Model. Biomed Res. Int. 2019, 2019, 7084734. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.S.; Haynes, L. Aging of the CD4 T Cell Compartment. Open Longev. Sci. 2012, 6, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; Tarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–343. [Google Scholar] [CrossRef]
- Wada, Y.; Phinney, B.S.; Weber, D.; Lonnerdal, B. In vivo digestomics of milk proteins in human milk and infant formula using a suckling rat pup model. Peptides 2017, 88, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Sanchon, J.; Fernandez-Tome, S.; Miralles, B.; Hernandez-Ledesma, B.; Tome, D.; Gaudichon, C.; Recio, I. Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem. 2018, 239, 486–494. [Google Scholar] [CrossRef]
- Thomson, A.B. Small intestinal disorders in the elderly. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 861–874. [Google Scholar] [CrossRef]
- Johnson, M.D.; Witherden, D.A.; Havran, W.L. The Role of Tissue-resident T Cells in Stress Surveillance and Tissue Maintenance. Cells 2020, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Tiruppathi, C.; Miyamoto, Y.; Ganapathy, V.; Leibach, F.H. Genetic evidence for role of DPP IV in intestinal hydrolysis and assimilation of prolyl peptides. Am. J. Physiol. 1993, 265, G81–G89. [Google Scholar] [CrossRef] [PubMed]
- Chotikatum, S.; Naim, H.Y.; El-Najjar, N. Inflammation induced ER stress affects absorptive intestinal epithelial cells function and integrity. Int. Immunopharmacol. 2018, 55, 336–344. [Google Scholar] [CrossRef]

| Ingredient | Control Diet 1 | Milk-Supplemented Diets 2 |
|---|---|---|
| (g/Kg) | ||
| Maize starch | 467.5 | 455.5 |
| Amino acid mix | 140 | 110 |
| Lyophilised milk 2 | - | 120 |
| Maltodextrin | 155 | 155 |
| Sucrose | 100 | 55 |
| Soya oil | 40 | 7 |
| Cellulose | 50 | 50 |
| Mineral mix | 35 | 35 |
| Vitamin mix | 10 | 10 |
| Choline chloride | 2.5 | 2.5 |
| tert-Butylhydroquinone | 0.008 | 0.008 |
| CTRL | A1A2 | A2A2 | p | |
|---|---|---|---|---|
| Body weight (g) | ||||
| Initial | 29.1 ± 3.1 | 28.3 ± 4.1 | 28.4 ± 3.8 | 0.909 |
| Final | 30.0 ± 3.7 | 29.6 ± 4.7 | 31.3 ± 3.9 | 0.664 |
| Food intake (g/day) | 4.7 ± 0.4 | 4.6 ± 0.6 | 4.5 ± 0.4 | 0.526 |
| Analysis | Parameter | Localisation | CTRL | A1A2 | A2A2 | |
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | p-Value | |||
| Gut immune phenotyping and cytokine secretion | CD3CD4 | D/J IE | 5.13 ± 2.32 a | 5.13 ± 0.74 a | 12.3 ± 4.52 b | 0.001 § |
| CD3CD8 | D/J IE | 79.9 ± 5.04 a | 77.0 ± 3.36 a | 66.2 ± 8.82 b | 0.004 § | |
| CD3αβ | D/J IE | 56.3 ± 14.5 | 42.9 ± 7.13 | 48.6 ± 9.07 | 0.075 $ | |
| CD3γδ | D/J IE | 36.8 ± 11.3 a | 51.5 ± 8.49 b | 34.2 ± 7.15 a | 0.003 $ | |
| CD45CD19 | D/J IE | 4.95 ± 4.05 a | 11.6 ± 2.49 ab | 21.6 ± 8.33 b | 0.001 § | |
| CD45NK | D/J IE | 1.81 ± 0.53 a | 3.13 ± 0.81 b | 3.65 ± 1.17 b | 0.001 $ | |
| TNF-α | Colon | 5.47 ± 2.85 | 4.71 ± 2.76 | 5.44 ± 5.03 | 0.648 § | |
| IL-6 | Colon | 6.31 ± 4.36 | 5.79 ± 3.14 | 5.47 ± 3.53 | 0.833 § | |
| Gut enzymatic activities | DPP-4 | Jejunum | 326 ± 102 | 267 ± 83.5 | 292 ± 103 | 0.513 $ |
| MPO | Jejunum | 29.4 ± 18.0 | n.d. | n.d. | ||
| Histomorphological evaluation | Vh | Duodenum | 10.0 ± 1.76 a | 8.33 ± 0.58 b | 9.57 ± 0.63 a | 0.034 § |
| Cd | Duodenum | 1.18 ± 0.15 a | 1.09 ± 0.11 ab | 0.99 ± 0.14 b | 0.030 $ | |
| Vh/Cd | Duodenum | 8.51 ± 1.20 ab | 7.72 ± 1.09 a | 9.86 ± 1.71 b | 0.018 $ | |
| Vh | Ileum | 3.40 ± 0.42 | 3.21 ± 0.35 | 3.33 ± 0.31 | 0.578 $ | |
| Cd | Ileum | 0.89 ± 0.09 | 0.79 ± 0.13 | 0.84 ± 0.08 | 0.166 $ | |
| Vh/Cd | Ileum | 3.81 ± 0.39 | 4.13 ± 0.78 | 3.98 ± 0.51 | 0.558 $ | |
| Immunohistochemical staining | CD8+ IHC | Duodenum | 1.79 ± 0.79 | 2.07 ± 1.48 | 3.11 ± 1.47 | 0.082 § |
| CD19+ IHC | Duodenum | 1.97 ± 0.77 | 1.72 ± 1.01 | 1.45 ± 0.69 | 0.437 $ | |
| CD45+ IHC | Duodenum | 3.05 ± 1.12 | 3.20 ± 1.11 | 4.11 ± 0.53 | 0.059 $ | |
| Immunoglobulin | IgGs | Serum | 2.72 ± 1.01 | 2.24 ± 1.29 | 2.65 ± 0.41 | 0.571 $ |
| Short-Chain Fatty Acids | Acetate | Faecal | 6.70 ± 6.76 | 9.85 ± 13.6 | 14.8 ± 9.14 | 0.202 § |
| isobutyrate | Faecal | 25.1 ± 10.5 a | 77.4 ± 62.0 ab | 80.4 ± 32.6 b | 0.012 § | |
| n-valerate | Faecal | 30.4 ± 17.4 | 39.8 ± 10.5 | 35.7 ± 8.09 | 0.618 § | |
| SCFAs | Faecal | 60.1 ± 20.9 a | 127 ± 79.2 b | 131 ± 42.6 b | 0.008 § |
| Organ | Diet | p | ||
|---|---|---|---|---|
| CTRL | A1A2 | A2A2 | ||
| Gut, median (IR) | 0.50 (0.0–1.0) a | 1.00 (1.0–2.0) b | 1.00 (1.0–2.0) b | 0.002 |
| Spleen | Absence of alterations | |||
| Liver, vacuolar degeneration (H&E), median (IR) | 1.00 (0.0–2.7) a | 2.00 (1.0–4.0) ab | 3.0 (2.5–3.5) b | 0.04 |
| Liver, lymphoplasmacytic infiltration, median (IR) | 0.5 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.63 |
| Liver, steatosis (Sudan Black), median (IR) | 0.00 (0.0–1.0) | 0.00 (0.0–2.0) | 0.0 (0.0–1.00) | 0.729 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guantario, B.; Giribaldi, M.; Devirgiliis, C.; Finamore, A.; Colombino, E.; Capucchio, M.T.; Evangelista, R.; Motta, V.; Zinno, P.; Cirrincione, S.; et al. A Comprehensive Evaluation of the Impact of Bovine Milk Containing Different Beta-Casein Profiles on Gut Health of Ageing Mice. Nutrients 2020, 12, 2147. https://doi.org/10.3390/nu12072147
Guantario B, Giribaldi M, Devirgiliis C, Finamore A, Colombino E, Capucchio MT, Evangelista R, Motta V, Zinno P, Cirrincione S, et al. A Comprehensive Evaluation of the Impact of Bovine Milk Containing Different Beta-Casein Profiles on Gut Health of Ageing Mice. Nutrients. 2020; 12(7):2147. https://doi.org/10.3390/nu12072147
Chicago/Turabian StyleGuantario, Barbara, Marzia Giribaldi, Chiara Devirgiliis, Alberto Finamore, Elena Colombino, Maria Teresa Capucchio, Rocchina Evangelista, Vincenzo Motta, Paola Zinno, Simona Cirrincione, and et al. 2020. "A Comprehensive Evaluation of the Impact of Bovine Milk Containing Different Beta-Casein Profiles on Gut Health of Ageing Mice" Nutrients 12, no. 7: 2147. https://doi.org/10.3390/nu12072147





