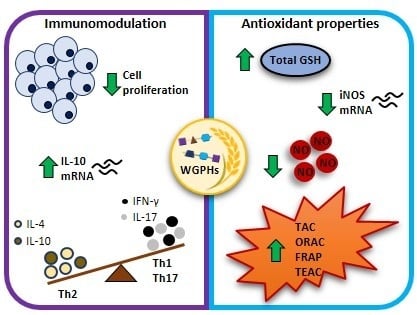
Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization and Preparation of Wheat Gluten Protein Hydrolysates
2.2. Cell Culture
2.3. Cell Proliferation and Viability Assays
2.4. Cytokine Determination
2.5. RNA Extraction, Reverse Transcription and Real-Time PCR
2.6. Enzymatic Activity Assays
2.7. Statistical Analysis
3. Results
3.1. WGPHs Reduce Cell Proliferation without Modifying Cell Viability in PBMCs
3.2. WGPHs Do not Trigger an Inflammatory Response
3.3. WGPHs Increase the PBMCs Antioxidant Capacity
3.4. WGPHs Reduce NO Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287. [Google Scholar] [CrossRef] [Green Version]
- Bhupathiraju, S.N.; Tucker, K.L. Coronary heart disease prevention: Nutrients, foods, and dietary patterns. Clin. Chim. Acta 2011, 412, 1493–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulston, A.M.; Boushey, C.J.; Ferruzzi, M.G.; Delahanty, L.M. Nutrition in the Prevention and Treatment of Disease, 4th ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Anand, S.S.; Hawkes, C.; De Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.; Weis, T.; Bernstein, A.M.; Krauss, R.M.; et al. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report From the Workshop Convened by the World Heart Federation. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, J.; Aiking, H. Strategies towards healthy and sustainable protein consumption: A transition framework at the levels of diets, dishes, and dish ingredients. Food Qual. Prefer. 2019, 73, 171–181. [Google Scholar] [CrossRef]
- Bruckner, D.W. Small-Scale Animal Agriculture. In The Routledge Handbook of Animal Ethics; Informa UK Limited: London, UK, 2019; pp. 198–210. [Google Scholar]
- Rosell, C.M. The science of doughs and bread quality. In Flour and Breads and their Fortification in Health and Disease Prevention; Elsevier: Cambridge, MA, USA, 2011; pp. 3–14. [Google Scholar]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-Like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Burnett, C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Hydrolyzed Wheat Protein and Hydrolyzed Wheat Gluten as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 55S–66S. [Google Scholar] [CrossRef]
- Merz, M.; Kettner, L.; Langolf, E.; Appel, D.; Blank, I.; Stressler, T.; Fischer, L. Production of wheat gluten hydrolysates with reduced antigenicity employing enzymatic hydrolysis combined with downstream unit operations. J. Sci. Food Agric. 2015, 96, 3358–3364. [Google Scholar] [CrossRef]
- Horiguchi, N.; Horiguchi, H.; Suzuki, Y. Effect of wheat gluten hydrolysate on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2005, 69, 2445–2449. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.A. Review: Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci. 2000, 30, 145–158. [Google Scholar] [PubMed]
- Santamaria, P. Cytokines and chemokines in autoimmune disease: An overview. Adv. Exp. Med. Biol. 2003, 520, 1–7. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Erben, U.; Kühl, A.A. The Role of T-Cell Subsets in Chronic Inflammation in Celiac Disease and Inflammatory Bowel Disease Patients: More Common Mechanisms or More Differences? Inflamm. Intest. Dis. 2016, 1, 52–62. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [Green Version]
- Ozben, T. Antioxidant supplementation on cancer risk and during cancer therapy: An update. Curr. Top. Med. Chem. 2015, 15, 170–178. [Google Scholar] [CrossRef]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018, 9. [Google Scholar] [CrossRef]
- Rowicka, G.; Czaja-Bulsa, G.; Chelchowska, M.; Riahi, A.; Strucińska, M.; Weker, H.; Ambroszkiewicz, J. Oxidative and Antioxidative Status of Children with Celiac Disease Treated with a Gluten Free-Diet. Oxidative Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxidative Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar] [PubMed]
- Barilli, A.; Gaiani, F.; Prandi, B.; Cirlini, M.; Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; De’Angelis, N.; Sforza, S.; Angelis, G.D.; et al. Gluten peptides drive healthy and celiac monocytes toward an M2-like polarization. J. Nutr. Biochem. 2018, 54, 11–17. [Google Scholar] [CrossRef]
- Serena, G.; Huynh, D.; Lima, R.S.; Vise, L.M.; Freire, R.; Ingano, L.; Leonard, M.M.; Senger, S.; Fasano, A. Intestinal Epithelium Modulates Macrophage Response to Gliadin in Celiac Disease. Front. Nutr. 2019, 6, 167. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Millán-Linares, M.D.C.; Yust, M.D.M.; Pedroche, J.; Millán, F.; Lardone, P.J.; Carrera-Sánchez, C.; Guerrero, J.M.; Carrillo-Vico, A. Lupine protein hydrolysates decrease the inflammatory response and improve the oxidative status in human peripheral lymphocytes. Food Res. Int. 2019, 126, 108585. [Google Scholar] [CrossRef]
- Escudero-López, B.; Cerrillo, I.; Gil-Izquierdo, Á.; Hornero-Méndez, D.; Martin, G.H.; Berná, G.; Medina, S.; Ferreres, F.; Martin, F.; Fernández-Pachón, M.-S. Effect of thermal processing on the profile of bioactive compounds and antioxidant capacity of fermented orange juice. Int. J. Food Sci. Nutr. 2016, 67, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zhou, H.; Qian, H. Enzymatic preparation and functional properties of wheat gluten hydrolysates. Food Chem. 2007, 101, 615–620. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Bermudez, B.; Yust, M.D.M.; Millan, F.; Pedroche, J. Anti-inflammatory activity of lupine (Lupinus angustifolius L.) protein hydrolysates in THP-1-derived macrophages. J. Funct. Foods 2014, 8, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Millan-Linares, M.C.; Yust, M.D.M.; Alcaide-Hidalgo, J.M.; Millán, F.; Pedroche, J. Lupine protein hydrolysates inhibit enzymes involved in the inflammatory pathway. Food Chem. 2014, 151, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millan-Linares, M.C.; Millan, F.; Pedroche, J.; Yust, M.D.M. GPETAFLR: A new anti-inflammatory peptide from Lupinus angustifolius L. protein hydrolysate. J. Funct. Foods 2015, 18, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Tsuruki, T.; Kishi, K.; Takahashi, M.; Tanaka, M.; Matsukawa, T.; Yoshikawa, M. Soymetide, an immunostimulating peptide derived from soybean β-conglycinin, is an fMLP agonist. FEBS Lett. 2003, 540, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Chalamaiah, M.; Ren, X.; Ma, H.; Wu, J. Identification of New Anti-inflammatory Peptides from Zein Hydrolysate after Simulated Gastrointestinal Digestion and Transport in Caco-2 Cells. J. Agric. Food Chem. 2018, 66, 1114–1120. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; De Mejia, E.G.; Dia, V.P.; Loarca-Piña, G. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem. 2014, 157, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.H.; Cho, S.-Y.; Kim, M.-J.; Kim, S.-J.; Lee, S.-H.; Lee, M.-Y.; Lee, Y.M. Anti-Inflammatory effects of Salvia plebeia R. Br extract in vitro and in ovalbumin-induced mouse model. Biol. Res. 2016, 49, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 1–21. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Elmalimadi, M.B.; Stefanović, A.B.; Šekuljica, N.Ž.; Žuža, M.G.; Luković, N.D.; Jovanovic, J.; Jugovic, Z.K. The synergistic effect of heat treatment on alcalase-assisted hydrolysis of wheat gluten proteins: Functional and antioxidant properties. J. Food Process. Preserv. 2017, 41, e13207. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process. Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Koo, S.H.; Bae, I.Y.; Lee, S.; Lee, D.-H.; Hur, B.-S.; Lee, H.G. Evaluation of wheat gluten hydrolysates as taste-active compounds with antioxidant activity. J. Food Sci. Technol. 2011, 51, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Guo, X.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Protective effects of wheat germ protein isolate hydrolysates (WGPIH) against hydrogen peroxide-induced oxidative stress in PC12 cells. Food Res. Int. 2013, 53, 297–303. [Google Scholar] [CrossRef]
- Yin, H.; Pan, X.-C.; Wang, S.; Yang, L.-G.; Sun, G. Protective Effect of Wheat Peptides Against Small Intestinal Damage Induced by Non-Steroidal Anti-Inflammatory Drugs in Rats. J. Integr. Agric. 2014, 13, 2019–2027. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademiṙ, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-Neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-Radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Du, Y.; Esfandi, R.; Willmore, W.; Tsopmo, A. Antioxidant Activity of Oat Proteins Derived Peptides in Stressed Hepatic HepG2 Cells. Antioxidants 2016, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Huang, L.-H.; Sun, Q.; Jiang, Z.; Wu, X. Isolation, identification and synthesis of four novel antioxidant peptides from rice residue protein hydrolyzed by multiple proteases. Food Chem. 2015, 179, 290–295. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Food Res. Int. 2018, 105, 836–844. [Google Scholar] [CrossRef]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Più, L.D.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta (BBA)—Bioenerg. 1999, 1411, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar]
- Oishi, M.; Kiyono, T.; Sato, K.; Tokuhara, K.; Tanaka, Y.; Miki, H.; Nakatake, R.; Kaibori, M.; Nishizawa, M.; Okumura, T.; et al. pyroGlu-Leu inhibits the induction of inducible nitric oxide synthase in interleukin-1β-stimulated primary cultured rat hepatocytes. Nitric Oxide 2015, 44, 81–87. [Google Scholar] [CrossRef]
- Vernaza, M.G.; Dia, V.P.; De Mejia, E.G.; Kil Chang, Y. Antioxidant and antiinflammatory properties of germinated and hydrolysed Brazilian soybean flours. Food Chem. 2012, 134, 2217–2225. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; de Mejia, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-Induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef]





| % | WGPHs |
|---|---|
| Protein | 73.54 ± 0.44 |
| Moisture | 7.97 ± 0.15 |
| Ash | 4.60 ± 0.41 |
| Fiber | 0.00 ± 0.00 |
| Other a | 13.89 |
| Digestibility | 100.00 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Santos-Sánchez, G.; Pedroche, J.; Fernández-Pachón, M.-S.; Millán, F.; Millán-Linares, M.C.; Lardone, P.J.; Bejarano, I.; Guerrero, J.M.; et al. Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells. Nutrients 2020, 12, 1673. https://doi.org/10.3390/nu12061673
Cruz-Chamorro I, Álvarez-Sánchez N, Santos-Sánchez G, Pedroche J, Fernández-Pachón M-S, Millán F, Millán-Linares MC, Lardone PJ, Bejarano I, Guerrero JM, et al. Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells. Nutrients. 2020; 12(6):1673. https://doi.org/10.3390/nu12061673
Chicago/Turabian StyleCruz-Chamorro, Ivan, Nuria Álvarez-Sánchez, Guillermo Santos-Sánchez, Justo Pedroche, María-Soledad Fernández-Pachón, Francisco Millán, María Carmen Millán-Linares, Patricia Judith Lardone, Ignacio Bejarano, Juan Miguel Guerrero, and et al. 2020. "Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells" Nutrients 12, no. 6: 1673. https://doi.org/10.3390/nu12061673