Effect of Cod Residual Protein Supplementation on Markers of Glucose Regulation in Lean Adults: A Randomized Double-Blind Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Ethics
2.2. Study Design, Intervention and Protocol
2.3. Production and Analyses of Intervention Capsules
2.4. Analyses of Biological Samples
2.5. Outcomes
2.6. Sample Size
2.7. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. The Contents of Trimethylamine N-oxide, Anserine, Microorganisms and Heavy Metals from the Capsules
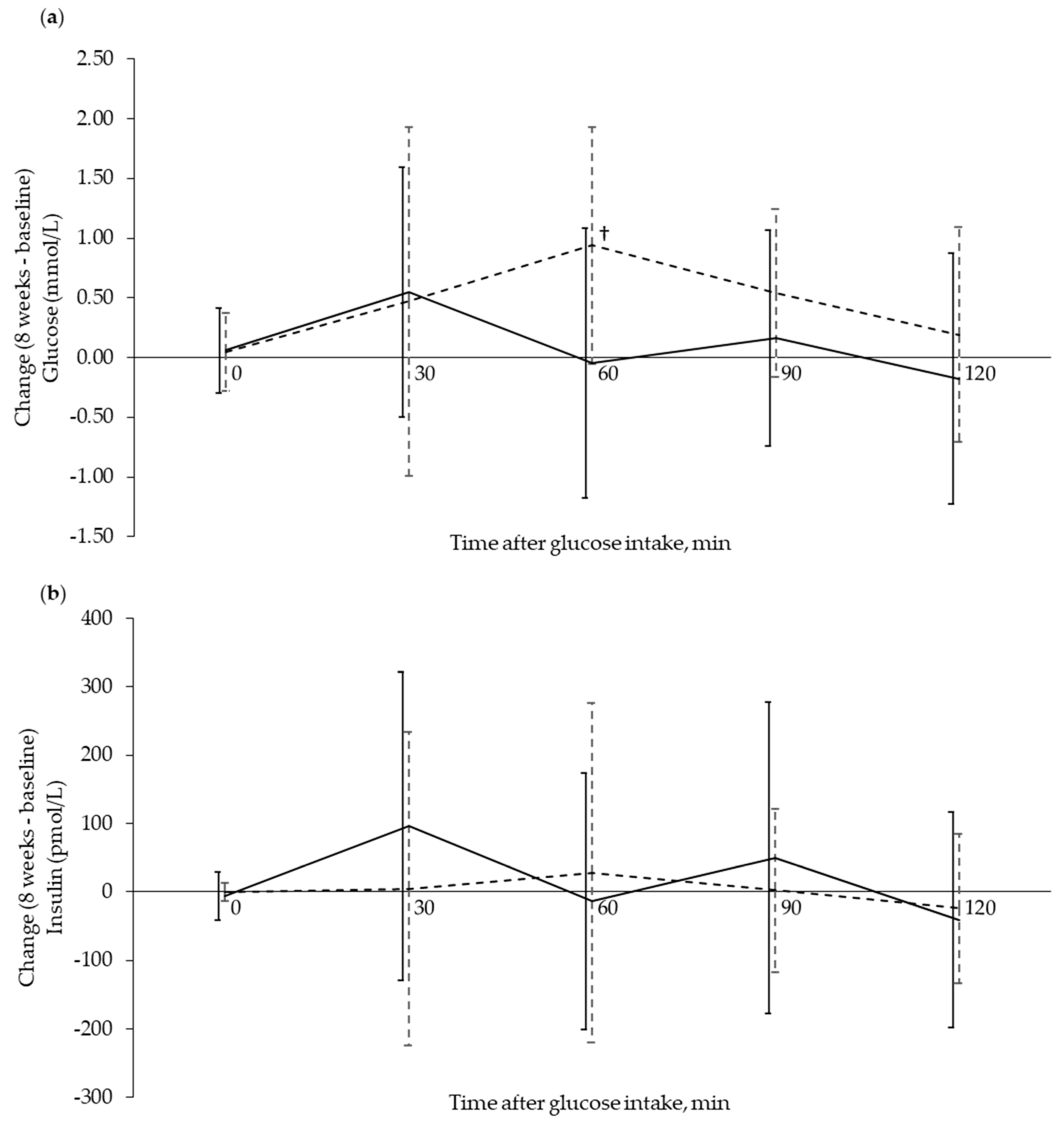
3.3. Circulating Markers Related to Glucose Regulation
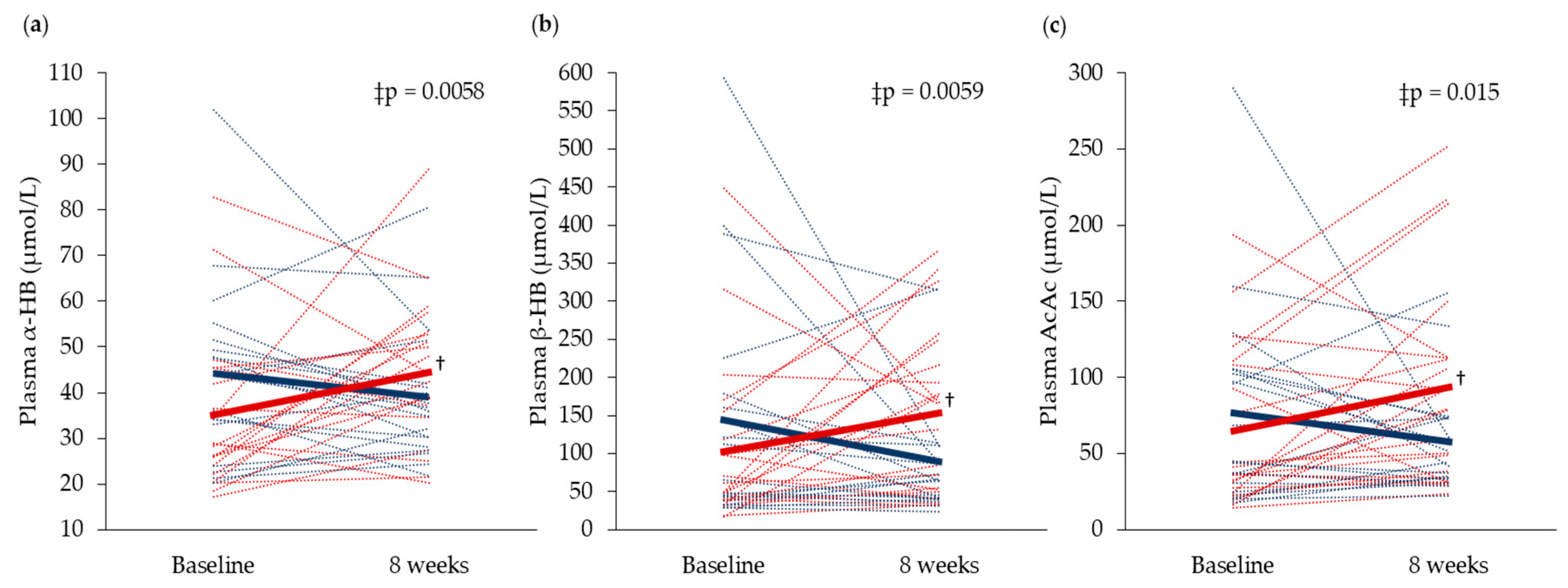
3.4. Plasma and Urine Biomarkers Related to Cod Residual Protein Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kromhout, D.; Bosschieter, E.B.; de Lezenne Coulander, C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A.; Receveur, O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: An ecological study. Diabetes Metab. 2003, 29, 635–642. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Mozaffarian, D.; Chiuve, S.E.; Rimm, E.B. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutr. 2008, 88, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish consumption and chd mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012, 15, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Tian, C.; Jia, C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: A meta-analysis of prospective studies. Br. J. Nutr. 2012, 108, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B.; et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. Bmj 2012, 345, e6698. [Google Scholar] [CrossRef] [Green Version]
- Alhassan, A.; Young, J.; Lean, M.E.J.; Lara, J. Consumption of fish and vascular risk factors: A systematic review and meta-analysis of intervention studies. Atherosclerosis 2017, 266, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Feskens, E.J.; Bowles, C.H.; Kromhout, D. Inverse association between fish intake and risk of glucose intolerance in normoglycemic elderly men and women. Diabetes Care 1991, 14, 935–941. [Google Scholar] [CrossRef]
- Lavigne, C.; Tremblay, F.; Asselin, G.; Jacques, H.; Marette, A. Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E62–E71. [Google Scholar] [CrossRef]
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: A randomized controlled trial. Diabetes Care 2007, 30, 2816–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikoren, L.A.; Nygard, O.K.; Lied, E.; Rostrup, E.; Gudbrandsen, O.A. A randomised study on the effects of fish protein supplement on glucose tolerance, lipids and body composition in overweight adults. Br. J. Nutr. 2013, 109, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Drotningsvik, A.; Mjos, S.A.; Hogoy, I.; Remman, T.; Gudbrandsen, O.A. A low dietary intake of cod protein is sufficient to increase growth, improve serum and tissue fatty acid compositions, and lower serum postprandial glucose and fasting non-esterified fatty acid concentrations in obese zucker fa/fa rats. Eur. J. Nutr. 2015, 54, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Vildmyren, I.; Cao, H.J.V.; Haug, L.B.; Valand, I.U.; Eng, O.; Oterhals, A.; Austgulen, M.H.; Halstensen, A.; Mellgren, G.; Gudbrandsen, O.A. Daily intake of protein from cod residual material lowers serum concentrations of nonesterified fatty acids in overweight healthy adults: A randomized double-blind pilot study. Mar. Drugs 2018, 16, 197. [Google Scholar] [CrossRef] [Green Version]
- Lavigne, C.; Marette, A.; Jacques, H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E491–E500. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Natali, A.; Camastra, S.; Nannipieri, M.; Mari, A.; Adam, K.P.; Milburn, M.V.; Kastenmuller, G.; Adamski, J.; Tuomi, T.; et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013, 62, 1730–1737. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stancakova, A.; Pihlajamaki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef] [Green Version]
- Cobb, J.; Eckhart, A.; Motsinger-Reif, A.; Carr, B.; Groop, L.; Ferrannini, E. Alpha-hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care 2016, 39, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Richardsen, R.; Nystøyl, R.; Strandheim, G.; Marthinussen, A. Report: Analysis of marine residual raw material. SINTEF 2016. [Google Scholar]
- Vildmyren, I.; Halstensen, A.; Oterhals, A.; Gudbrandsen, O.A. Cod protein powder lowered serum nonesterified fatty acids and increased total bile acid concentrations in healthy, lean, physically active adults: A randomized double-blind study. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The open study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Drotningsvik, A.; Midttun, O.; McCann, A.; Ueland, P.M.; Hogoy, I.; Gudbrandsen, O.A. Dietary intake of cod protein beneficially affects concentrations of urinary markers of kidney function and results in lower urinary loss of amino acids in obese zucker fa/fa rats. Br. J. Nutr. 2018, 120, 740–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagen, I.V.; Helland, A.; Bratlie, M.; Midttun, O.; McCann, A.; Sveier, H.; Rosenlund, G.; Mellgren, G.; Ueland, P.M.; Gudbrandsen, O.A. Tmao, creatine and 1-methylhistidine in serum and urine are potential biomarkers of cod and salmon intake: A randomised clinical trial in adults with overweight or obesity. Eur. J. Nutr. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-n-oxide (tmao) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Schmedes, M.; Balderas, C.; Aadland, E.K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, O.; Holthe, A.; Mellgren, G.; Young, J.F.; et al. The effect of lean-seafood and non-seafood diets on fasting and postprandial serum metabolites and lipid species: Results from a randomized crossover intervention study in healthy adults. Nutrients 2018, 10, 598. [Google Scholar] [CrossRef] [Green Version]
- Sjolin, J.; Hjort, G.; Friman, G.; Hambraeus, L. Urinary excretion of 1-methylhistidine: A qualitative indicator of exogenous 3-methylhistidine and intake of meats from various sources. Metabolism 1987, 36, 1175–1184. [Google Scholar] [CrossRef]
- Drotningsvik, A.; Pampanin, D.M.; Slizyte, R.; Carvajal, A.; Hogoy, I.; Remman, T.; Gudbrandsen, O.A. Hydrolyzed proteins from herring and salmon rest raw material contain peptide motifs with angiotensin-i converting enzyme inhibitors and resulted in lower urine concentrations of protein, cystatin c and glucose when fed to obese zucker fa/fa rats. Nutr. Res. 2018, 52, 14–21. [Google Scholar] [CrossRef]
- Lenz, E.M.; Bright, J.; Wilson, I.D.; Hughes, A.; Morrisson, J.; Lindberg, H.; Lockton, A. Metabonomics, dietary influences and cultural differences: A 1h nmr-based study of urine samples obtained from healthy british and swedish subjects. J. Pharm. Biomed. Anal. 2004, 36, 841–849. [Google Scholar] [CrossRef]
- Dumas, M.E.; Maibaum, E.C.; Teague, C.; Ueshima, H.; Zhou, B.; Lindon, J.C.; Nicholson, J.K.; Stamler, J.; Elliott, P.; Chan, Q.; et al. Assessment of analytical reproducibility of 1h nmr spectroscopy based metabonomics for large-scale epidemiological research: The intermap study. Anal. Chem. 2006, 78, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.J. An absorption apparatus for the micro-determination of certain volatile substances: The determination of urea and ammonia in body fluids. Biochem. J. 1933, 27, 430–434. [Google Scholar] [PubMed]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L.; Frost, B. A new, rapid, high-sensitivity analysis of amino acids in food type samples. J. Assoc. Off. Anal. Chem. 1987, 70, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; McCann, A.; Aarseth, O.; Krokeide, M.; Kvalheim, G.; Meyer, K.; Ueland, P.M. Combined measurement of 6 fat-soluble vitamins and 26 water-soluble functional vitamin markers and amino acids in 50 mul of serum or plasma by high-throughput mass spectrometry. Anal. Chem. 2016, 88, 10427–10436. [Google Scholar] [CrossRef]
- Midttun, O.; Kvalheim, G.; Ueland, P.M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using hplc-ms/ms. Anal. Bioanal. Chem. 2013, 405, 2009–2017. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F. Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Chain, E.P.o.C.i.t.F. Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- Hovland, I.H.; Leikanger, I.S.; Stokkeland, O.; Waage, K.H.; Mjos, S.A.; Brokstad, K.A.; McCann, A.; Ueland, P.M.; Slizyte, R.; Carvajal, A.; et al. Effects of low doses of fish and milk proteins on glucose regulation and markers of insulin sensitivity in overweight adults: A randomised, double blind study. Eur. J. Nutr. 2019, 1–17. [Google Scholar] [CrossRef]
- Hagen, I.V.; Helland, A.; Bratlie, M.; Brokstad, K.A.; Rosenlund, G.; Sveier, H.; Mellgren, G.; Gudbrandsen, O.A. High intake of fatty fish, but not of lean fish, affects serum concentrations of tag and hdl-cholesterol in healthy, normal-weight adults: A randomised trial. Br. J. Nutr. 2016, 116, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Helland, A.; Bratlie, M.; Hagen, I.V.; Mjos, S.A.; Sornes, S.; Halstensen, A.I.; Brokstad, K.A.; Sveier, H.; Rosenlund, G.; Mellgren, G.; et al. High intake of fatty fish, but not of lean fish, improved postprandial glucose regulation and increased the n-3 pufa content in the leucocyte membrane in healthy overweight adults: A randomised trial. Br. J. Nutr. 2017, 117, 1368–1378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Hulver, M.W.; McMillan, R.P.; Cline, M.A.; Gilbert, E.R. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr. Metab. 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Inskeep, K.; Bowker-Kinley, M.M.; Popov, K.M.; Harris, R.A. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 1999, 48, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary precursors of trimethylamine in man: A pilot study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine n-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Myint, T.; Fraser, G.E.; Lindsted, K.D.; Knutsen, S.F.; Hubbard, R.W.; Bennett, H.W. Urinary 1-methylhistidine is a marker of meat consumption in black and in white california seventh-day adventists. Am. J. Epidemiol. 2000, 152, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010, 84, 301–307. [Google Scholar] [CrossRef]
- Cross, A.J.; Major, J.M.; Sinha, R. Urinary biomarkers of meat consumption. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1107–1111. [Google Scholar] [CrossRef] [Green Version]
- Altorf-van der Kuil, W.; Brink, E.J.; Boetje, M.; Siebelink, E.; Bijlsma, S.; Engberink, M.F.; van ‘t Veer, P.; Tome, D.; Bakker, S.J.; van Baak, M.A.; et al. Identification of biomarkers for intake of protein from meat, dairy products and grains: A controlled dietary intervention study. Br. J. Nutr. 2013, 110, 810–822. [Google Scholar] [CrossRef] [Green Version]

| Per Capsule | Cod-RP | Control |
|---|---|---|
| Cod residual powder * (mg) | 474 | 0 |
| Microcrystalline cellulose (mg) | 42 | 454 |
| Magnesium stearate (mg) | 5.3 | 4.6 |
| Silica (mg) | 5.3 | 4.6 |
| Total capsule weight (mg) | 527 | 463 |
| Energy † (kcal) | 1.59 | 0.02 |
| Cod-RP (n = 19) | Control (n = 21) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Women/Men | 7/12 | 11/10 | 0.36 |
| Age (years) | 28.0 ± 6.9 | 30.5 ± 7.2 | 0.28 |
| Body weight (kg) | 77.0 ± 16.0 | 73.1 ± 11.5 | 0.39 |
| BMI (kg/m2) | 24.8 ± 2.8 | 23.8 ± 2.3 | 0.25 |
| Body fat (%) | 19.7 ± 6.8 | 19.4 ± 6.7 | 0.89 |
| Body muscle (%) | 45.4 ± 4.7 | 45.4 ± 4.4 | 0.95 |
| Whole blood HbA1c (mmol/mol) | 32.4 ± 2.2 | 31.5 ± 2.0 | 0.23 |
| Plasma creatinine (µmol/L) | 80.1 ± 13.1 | 77.2 ± 11.1 | 0.46 |
| Urine albumin (mg/mmol creatinine) | 0.7 ± 0.5 | 1.0 ± 0.9 | 0.23 |
| Serum glucose (mmol/L) | 5.1 ± 0.4 | 4.9 ± 0.3 | 0.21 |
| Serum insulin (pmol/L) | 68.1 ± 32.8 | 56.3 ± 25.9 | 0.55 |
| Plasma α-HB (µmol/L) | 44.2 ± 19.3 | 35.1 ± 17.0 | 0.80 |
| Plasma β-HB (µmol/L) | 143 ± 156 | 102 ± 108 | 0.34 |
| Plasma AcAc (µmol/L) | 76.5 ± 67.0 | 65.1 ± 52.1 | 0.86 |
| Cigarette/snus * (n) | 1 | 2 | 1.00 |
| Baseline | 8 Weeks | p† | p‡ | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Plasma (µmol/L) | ||||
| TMAO | 0.048 | |||
| Cod-RP group | 4.3 ± 2.2 | 5.8 ± 3.3 | 0.032 | |
| Control group | 5.2 ± 6.2 | 4.6 ± 6.3 | 0.36 | |
| Creatine | 0.11 | |||
| Cod-RP group | 24.9 ± 15.3 | 26.8 ± 15.4 | 0.36 | |
| Control group | 32.2 ± 18.2 | 30.5 ± 21.8 | 0.17 | |
| 1-MeHis | 0.17 | |||
| Cod-RP group | 13.0 ± 11.5 | 7.8 ± 7.9 | 0.16 | |
| Control group | 9.2 ± 7.2 | 10.7 ± 9.1 | 0.68 | |
| 3-MeHis | 0.24 | |||
| Cod-RP group | 5.1 ± 1.3 | 4.9 ± 0.9 | 0.29 | |
| Control group | 4.5 ± 1.0 | 4.7 ± 1.1 | 0.29 | |
| Urine (µmol/mmol creatinine) | ||||
| TMAO | 0.026 | |||
| Cod-RP group | 44.3 ± 22.6 | 63.2 ± 31.5 | 0.016 | |
| Control group | 53.3 ± 68.3 | 42.0 ± 43.5 | 0.30 | |
| Creatine | 0.18 | |||
| Cod-RP group | 2.7 ± 4.5 | 6.6 ± 14.9 | 0.29 | |
| Control group | 7.0 ± 11.8 | 5.6 ± 11.1 | 0.37 | |
| 1-MeHis | 0.49 | |||
| Cod-RP group | 90.9 ± 81.4 | 54.1 ± 54.0 | 0.16 | |
| Control group | 69.0 ± 62.7 | 72.1 ± 57.9 | 0.72 | |
| 3-MeHis | 0.16 | |||
| Cod-RP group | 29.5 ± 9.4 | 28.2 ± 6.5 | 0.77 | |
| Control group | 25.1 ± 5.6 | 26.4 ± 6.7 | 0.41 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vildmyren, I.; Halstensen, A.; McCann, A.; Midttun, Ø.; Ueland, P.M.; Oterhals, Å.; Gudbrandsen, O.A. Effect of Cod Residual Protein Supplementation on Markers of Glucose Regulation in Lean Adults: A Randomized Double-Blind Study. Nutrients 2020, 12, 1445. https://doi.org/10.3390/nu12051445
Vildmyren I, Halstensen A, McCann A, Midttun Ø, Ueland PM, Oterhals Å, Gudbrandsen OA. Effect of Cod Residual Protein Supplementation on Markers of Glucose Regulation in Lean Adults: A Randomized Double-Blind Study. Nutrients. 2020; 12(5):1445. https://doi.org/10.3390/nu12051445
Chicago/Turabian StyleVildmyren, Iselin, Alfred Halstensen, Adrian McCann, Øivind Midttun, Per Magne Ueland, Åge Oterhals, and Oddrun Anita Gudbrandsen. 2020. "Effect of Cod Residual Protein Supplementation on Markers of Glucose Regulation in Lean Adults: A Randomized Double-Blind Study" Nutrients 12, no. 5: 1445. https://doi.org/10.3390/nu12051445





