The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Composition of the TPN Admixtures
2.3. Blood Collection and Sample Analysis
2.4. Statistical Analysis
3. Results
3.1. Group Characteristics
3.2. Macronutrient Intake and Plasma Glucose Control
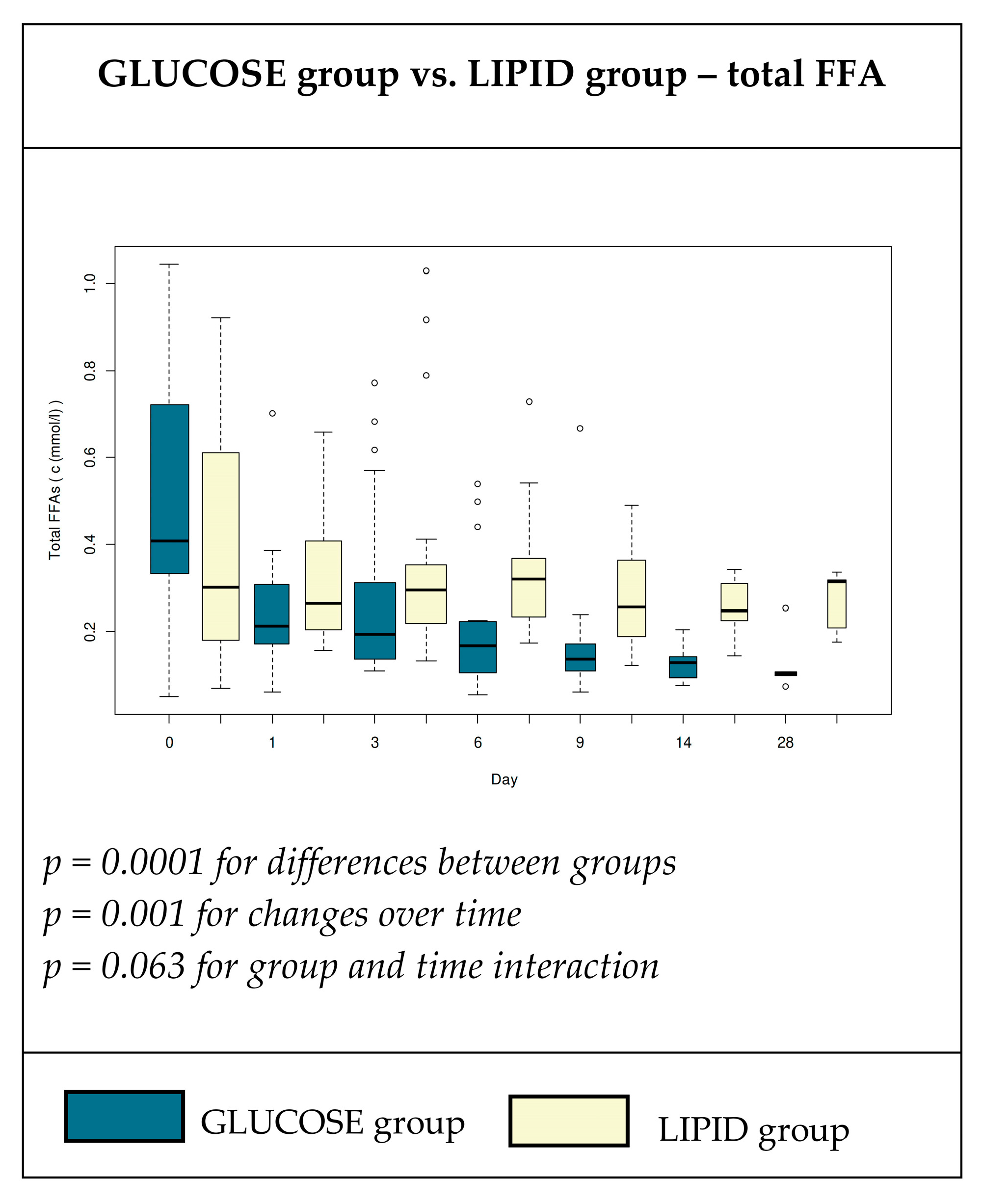
3.3. Plasma Concentrations of Total Free Fatty Acids
3.4. Antioxidant and Adipose Tissue Hormones
4. Discussion
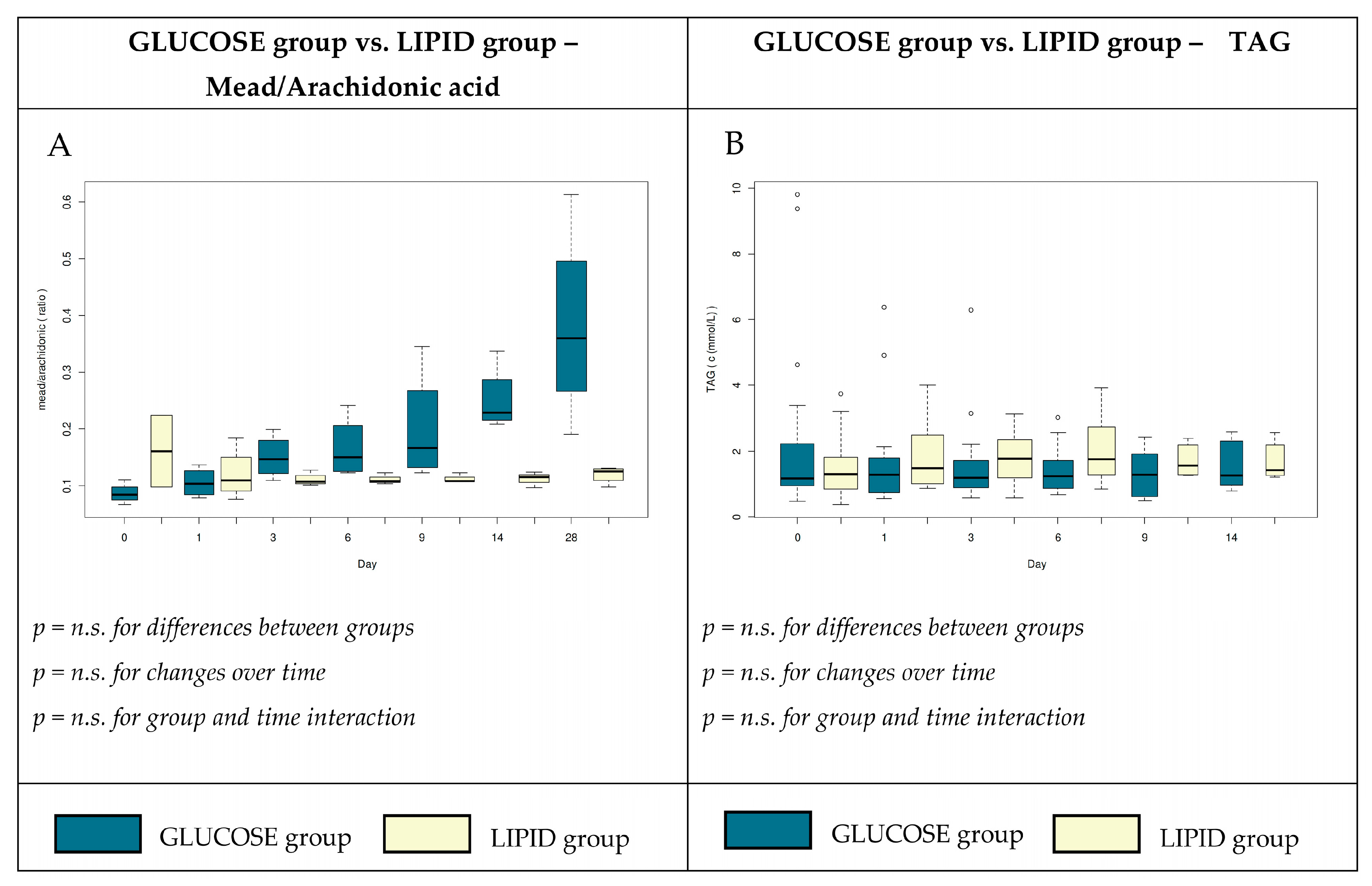
4.1. Plasma Total FFA and Individual FA
4.2. Adipose Tissue Derived Hormones and Glucose Homeostasis
4.3. Metabolic Alterations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| TPN | Total Parenteral Nutrition |
| FFA | Free Fatty Acids |
| FA | Fatty Acids |
| EFAD | Essential Fatty Acid Deficiency |
| IBW | Ideal Body Weight |
| PAI-1 | Plasminogen Activator Inhibitor |
References
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barazzoni, R.; Deutz, N.E.P.; Biolo, G.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cuerda, C.; Delzenne, N.; Leon Sanz, M.; Ljungqvist, O.; et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2017, 36, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C.; Adolph, M.; Deutz, N.E.; Grau, T.; Innes, J.K.; Klek, S.; Lev, S.; Mayer, K.; Michael-Titus, A.T.; Pradelli, L.; et al. Lipids in the intensive care unit: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [Green Version]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient. JPEN J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.; Bozzetti, F.; Cynober, L.; Elia, M.; Shenkin, A.; Sobotka, L. Meta-analysis is not enough: The critical role of pathophysiology in determining optimal care in clinical nutrition. Clin. Nutr. 2016, 35, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Arabi, Y.M.; Tamimi, W.; Jones, G.; Jawdat, D.; Tamim, H.; Al-Dorzi, H.M.; Sadat, M.; Afesh, L.; Sakhija, M.; Al-Dawood, A. Free Fatty Acids’ Level and Nutrition in Critically Ill Patients and Association with Outcomes: A Prospective Sub-Study of PermiT Trial. Nutrients 2019, 11, 384. [Google Scholar] [CrossRef] [Green Version]
- Krogh-Madsen, R.; Plomgaard, P.; Akerstrom, T.; Moller, K.; Schmitz, O.; Pedersen, B.K. Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E371–E379. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, G.; Pelletier, P.; Albacker, T.; Lachapelle, K.; Joanisse, D.R.; Hatzakorzian, R.; Lattermann, R.; Sato, H.; Marette, A.; Schricker, T. Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. J. Clin. Endocrinol. Metab. 2011, 96, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Kalupahana, N.S.; Voy, B.H.; Saxton, A.M.; Moustaid-Moussa, N. Energy-restricted high-fat diets only partially improve markers of systemic and adipose tissue inflammation. Obesity 2011, 19, 245–254. [Google Scholar] [CrossRef]
- Langouche, L.; Perre, S.V.; Thiessen, S.; Gunst, J.; Hermans, G.; D’Hoore, A.; Kola, B.; Korbonits, M.; Van den Berghe, G. Alterations in adipose tissue during critical illness: An adaptive and protective response? Am. J. Respir. Crit. Care Med. 2010, 182, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Devine, B.J. Gentamicin therapy. Drug Intell. Clin. Pharm. 1974, 8, 650–655. [Google Scholar] [CrossRef]
- Saluk, J.; Hoppensteadt, D.; Syed, D.; Liles, J.; Abro, S.; Walborn, A.; Bansal, V.; Fareed, J. Biomarker profiling of plasma samples utilizing RANDOX biochip array technology. Int. Angiol. 2017, 36, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Solichova, D.; Blaha, M.; Aufartova, J.; Krcmova, L.K.; Plisek, J.; Honegrova, B.; Kasalova, E.; Lanska, M.; Urbanek, L.; Sobotka, L. The Effect of LDL-Apheresis and Rheohaemapheresis Treatment on Vitamin E. J. Nutr. Sci. Vitaminol. 2015, 61, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. St. Softw. 2012, 50, 23. [Google Scholar] [CrossRef] [Green Version]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 2 April 2020).
- Levinson, M.; Groeger, J.; Jeevanandam, M.; Brennan, M.F. Free fatty acid turnover and lipolysis in septic mechanically ventilated cancer-bearing humans. Metabolism 1988, 37, 618–625. [Google Scholar] [CrossRef]
- Shaw, J.H.; Wolfe, R.R. Response to glucose and lipid infusions in sepsis: A kinetic analysis. Metabolism 1985, 34, 442–449. [Google Scholar] [CrossRef]
- Wolfe, R.R. Sepsis as a modulator of adaptation to low and high carbohydrate and low and high fat intakes. Eur. J. Clin. Nutr. 1999, 53, S136–S142. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R.; Herndon, D.N.; Peters, E.J.; Jahoor, F.; Desai, M.H.; Holland, O.B. Regulation of lipolysis in severely burned children. Ann. Surg. 1987, 206, 214–221. [Google Scholar] [CrossRef]
- Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006, 53, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.B.; Langouche, L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit. Care Med. 2013, 41, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.C.; Frisch, F.; Cyr, D.; Genereux, P.; Patterson, B.W.; Giguere, R.; Baillargeon, J.P. On the suppression of plasma nonesterified fatty acids by insulin during enhanced intravascular lipolysis in humans. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E849–E856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegmayr, B.; Olivecrona, T.; Olivecrona, G. Lipoprotein lipase disturbances induced by uremia and hemodialysis. Semin. Dial. 2009, 22, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr. Metab. 2007, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, R.D.; Wolfe, R.R. Effect of total parenteral nutrition on free fatty acid metabolism in burned patients. JPEN J. Parenter. Enteral Nutr. 1984, 8, 357–360. [Google Scholar] [CrossRef]
- Shaw, J.H.; Wolfe, R.R. An integrated analysis of glucose, fat, and protein metabolism in severely traumatized patients. Studies in the basal state and the response to total parenteral nutrition. Ann. Surg. 1989, 209, 63–72. [Google Scholar] [CrossRef]
- Langouche, L.; Vander Perre, S.; Wouters, P.J.; D’Hoore, A.; Hansen, T.K.; Van den Berghe, G. Effect of intensive insulin therapy on insulin sensitivity in the critically ill. J. Clin. Endocrinol. Metab. 2007, 92, 3890–3897. [Google Scholar] [CrossRef] [Green Version]
- Soeters, M.R.; Soeters, P.B. The evolutionary benefit of insulin resistance. Clin. Nutr. 2012, 31, 1002–1007. [Google Scholar] [CrossRef]
- Dai, Y.J.; Sun, L.L.; Li, M.Y.; Ding, C.L.; Su, Y.C.; Sun, L.J.; Xue, S.H.; Yan, F.; Zhao, C.H.; Wang, W. Comparison of Formulas Based on Lipid Emulsions of Olive Oil, Soybean Oil, or Several Oils for Parenteral Nutrition: A Systematic Review and Meta-Analysis. Adv. Nutr. 2016, 7, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.Y.; Yang, J.; Xia, Y.; Tong, D.N.; Zaloga, G.P.; Qin, H.L. Safety and efficacy of an olive oil-based triple-chamber bag for parenteral nutrition: A prospective, randomized, multi-center clinical trial in China. Nutr. J. 2015, 14, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umpierrez, G.E.; Spiegelman, R.; Zhao, V.; Smiley, D.D.; Pinzon, I.; Griffith, D.P.; Peng, L.; Morris, T.; Luo, M.; Garcia, H.; et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit. Care Med. 2012, 40, 1792–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, P.-R.; Andersson, C.; Strijbosch, R.; Lee, S.; Silvestri, A.; Gura, K.M.; Puder, M.; Bistrian, B.R. Effects of glucose or fat calories in total parenteral nutrition on fat metabolism and systemic inflammation in rats. Metabolism 2011, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Blake, W.L.; Salati, L.M.; Clarke, S.D. Potency of polyunsaturated and saturated fats as short-term inhibitors of hepatic lipogenesis in rats. J. Nutr. 1990, 120, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Teran-Garcia, M.; Adamson, A.W.; Yu, G.; Rufo, C.; Suchankova, G.; Dreesen, T.D.; Tekle, M.; Clarke, S.D.; Gettys, T.W. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): Evidence for dietary modulation of NF-Y binding to the Fasn promoter by SREBP-1c. Biochem. J. 2007, 402, 591–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, K.; Gokorsch, S.; Fegbeutel, C.; Hattar, K.; Rosseau, S.; Walmrath, D.; Seeger, W.; Grimminger, F. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am. J. Respir. Crit. Care Med. 2003, 167, 1321–1328. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. n-3 fatty acids, γ-linolenic acid, and antioxidants in sepsis. Crit. Care 2013, 17, 312. [Google Scholar] [CrossRef] [Green Version]
- Hajri, T.; Gharib, M.; Kaul, S.; Karpeh, M.S., Jr. Association between adipokines and critical illness outcomes. J Trauma Acute Care Surg. 2017, 83, 507–519. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Mastronardi, C.; Wong, M.-L.; Licinio, J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J. Endocrinol. Metab. 2012, 16, S549–S555. [Google Scholar] [CrossRef]
- Koch, A.; Gressner, O.A.; Sanson, E.; Tacke, F.; Trautwein, C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit. Care 2009, 13, R95. [Google Scholar] [CrossRef] [Green Version]
- McCowen, K.C.; Ling, P.R.; Friel, C.; Sternberg, J.; Forse, R.A.; Burke, P.A.; Bistrian, B.R. Patterns of plasma leptin and insulin concentrations in hospitalized patients after the initiation of total parenteral nutrition. Am. J. Clin. Nutr. 2002, 75, 931–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, L.H.; Dunn, G.D.; Brennan, M.F. Essential fatty acid deficiency during total parenteral nutrition. Ann. Surg. 1981, 193, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, L.; Meddings, L.; Alberda, C.; Wichansawakun, S.; Robbins, S.; Driscoll, D.; Bistrian, B. Essential Fatty Acid Deficiency in 2015: The Impact of Novel Intravenous Lipid Emulsions. JPEN J. Parenter. Enteral Nutr. 2015, 39, 61s–66s. [Google Scholar] [CrossRef] [PubMed]
- Llop, J.; Sabin, P.; Garau, M.; Burgos, R.; Perez, M.; Masso, J.; Cardona, D.; Sanchez Segura, J.M.; Garriga, R.; Redondo, S.; et al. The importance of clinical factors in parenteral nutrition-associated hypertriglyceridemia. Clin. Nutr. 2003, 22, 577–583. [Google Scholar] [CrossRef]
- Altomare, D.F.; Semeraro, N.; Colucci, M. Reduction of the plasma levels of tissue plasminogen activator after infusion of a lipid emulsion in humans. JPEN J. Parenter. Enteral Nutr. 1993, 17, 274–276. [Google Scholar] [CrossRef]
- Van der Poll, T.; Levi, M.; Braxton, C.C.; Coyle, S.M.; Roth, M.; ten Cate, J.W.; Lowry, S.F. Parenteral Nutrition Facilitates Activation of Coagulation but Not of Fibrinolysis during Human Endotoxemia. Int. J. Infect. Dis. 1998, 177, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.K. Vitamin E requirements in parenteral nutrition. Gastroenterology 2009, 137, S92–S104. [Google Scholar] [CrossRef]
- Steephen, A.C.; Traber, M.G.; Ito, Y.; Lewis, L.H.; Kayden, H.J.; Shike, M. Vitamin E status of patients receiving long-term parenteral nutrition: Is vitamin E supplementation adequate? JPEN J. Parenter. Enteral Nutr. 1991, 15, 647–652. [Google Scholar] [CrossRef]

| Diagnosis | Number of Patients |
|---|---|
| Acute pancreatitis | 22 |
| Gastroinstestinal bleeding | 8 |
| Bowel perforation, enteric fistulas | 5 |
| Bowel obstruction and ileus | 4 |
| Ischemic bowel disease | 3 |
| Crohn’s disease | 3 |
| Vasculitis with ileus | 2 |
| Chemical burns of the oesophagus and stomach | 1 |
| Variable | Group G n = 25 | Group L n = 23 | p-Value |
|---|---|---|---|
| Age (years) | 65 ± 9 | 65 ± 13 | 0.609 |
| Male/Female | 20/5 (M/F) | 17/6 | 0.528 |
| Weight (kg) | 82.4 ± 19.0 | 82.2 ± 16.0 | 0.115 |
| BMI (kg∙m−2) | 28.3 ± 4.9 | 26.2 ± 3.8 | 0.105 |
| NRS-2002 | 4.2 ± 0.7 | 4.1 ± 0.9 | 0.404 |
| APACHE II score | 20.8 ± 5.9 | 18.4 ± 4.0 | 0.144 |
| Diabetic patients, # (% of total) | 9 (36) | 10 (43) | 0.365 |
| Serum albumin (g∙L−1) | 29.8 ± 8.2 | 29.3 ± 7.4 | 0.185 |
| Serum transthyretin (mmol∙L−1) | 0.13 ± 0.06 | 0.16 ± 0.06 | 0.185 |
| Serum triacylglycerols (mmol∙L−1) | 1.5 ± 0.9 | 1.6 ± 0.9 | 0.704 |
| Serum total cholesterol (mmol∙L−1) | 3.1 ± 1.3 | 3.1 ± 1.2 | 0.378 |
| Serum HDL cholesterol (mmol∙L−1) | 0.7 ± 0.5 | 0.8 ± 0.5 | 0.905 |
| Leukocyte count (cells∙109∙L−1) | 12.6 ± 6.9 | 13.4 ± 7.0 | 0.840 |
| C-reactive protein [mg∙L-1) | 174.0 ± 163.3 | 91.0 ± 79.8 | 0.311 |
| Serum ALT(µkat∙ L−1) | 1.9 ± 2.3 | 1.0 ± 1.2 | 0.285 |
| Serum AST (µkat∙ L−1) | 3.1 ± 4.7 | 1.1 ± 1.9 | 0.111 |
| Serum GMT(µkat∙L−1) | 4.7 ± 5.3 | 1.3 ± 2.6 | 0.178 |
| Serum ALP (µkat∙L−1) | 2.0 ± 1.4 | 1.8 ± 1.4 | 0.401 |
| Variable | Group G n = 25 | Group L n = 23 | p-Value |
|---|---|---|---|
| Duration of TPN (days) | 14 ± 11 | 11 ± 8 | 0.704 |
| Starting TPN from the time of admission (days) | 2.3 ± 0.8 | 2.1 ± 0.7 | 0.680 |
| Energy (kcal∙kg−1 IBW∙day−1) | 30.5 ± 1.8 | 30.4 ± 1.3 | 0.764 |
| Non-protein energy (kcal∙kg−1 IBW∙day−1) | 23.2 ± 1.6 | 23.1 ± 1.0 | 0.788 |
| Amino acids (g∙kg−1 IBW∙day−1) | 1.8 ± 0.1 | 1.8 ± 0.1 | 0.775 |
| Glucose (g∙kg−1 IBW∙day−1) | 5.8 ± 0.4 | 2.9 ± 0.2 | <0.001 |
| Lipids (g∙kg−1 IBW∙day−1) | 0 | 1.2 ± 0.1 | <0.001 |
| Oleic acid received (g∙day−1) | 0 | 21.7 ± 2.4 | <0.001 |
| Linoleic acid received (g∙day−1) | 0 | 14.6 ± 1.6 | <0.001 |
| Blood glucose (mmol∙L−1) | 8.9 ± 1.4 | 8.0 ± 1.3 | 0.115 |
| Blood glucose in diabetic patients (mmol∙L−1) | 9.3 ± 1.1 | 9.1 ± 1.0 | 0.682 |
| Presence of mild hypoglycaemia (3–3.8 mmol∙L−1; cases) | 2 | 0 | 0.345 |
| Dose of insulin (mIU∙day−1) | 68 ± 57 | 43 ± 36 | 0.205 |
| Dose of insulin in diabetic patients (mIU∙day−1) | 82 ± 69 | 71 ± 43 | 0.418 |
| Administration of insulin (% of patients) | 68 | 52 | 0.314 |
| Serum insulin levels (μIU∙mL−1) | 40 ± 59 | 25 ± 44 | 0.215 |
| Serum insulin levels in diabetic patients (μIU∙mL−1) | 43 ± 71 | 36 ± 32 | 0.575 |
| Variable | Group | Day 0 | Day 1 | Day 3 | Day 6 | Day 9 | Day 14 | Day 28 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| C12:0 | GG | 0.22 ± 0.23 | 0.13 ± 0.10 | 0.15 ± 0.11 | 0.14 ± 0.08 | 0.14 ± 0.06 | 0.14 ± 0.10 | 0.10 ± 0.02 | n.s. |
| LL | 0.12 ± 0.05 | 0.11 ± 0.05 | 0.10 ± 0.04 | 0.11 ± 0.07 | 0.11 ± 0.10 | 0.11 ± 0.04 | 0.09 ± 0.03 | n.s. | |
| C14:0 | GG | 1.69 ± 0.78 | 1.38 ± 0.86 | 1.69 ± 0.71 | 1.61 ± 0.60 | 1.79 ± 0.81 | 2.06 ± 1.35 | 1.57 ± 0.62 | n.s. |
| LL | 1.21 ± 0.69 | 1.13 ± 0.56 | 1.16 ± 0.65 | 1.17 ± 0.71 | 1.05 ± 0.72 | 1.35 ± 0.93 | 0.97 ± 0.47 | n.s. | |
| C16:0 | GG | 32.82 ± 4.87 | 30.96 ± 3.78 | 32.07 ± 4.81 | 31.09 ± 3.85 | 31.59 ± 4.87 | 31.85 ± 5.74 | 30.28 ± 4.17 | n.s. |
| LL | 30.20 ± 4.62 | 29.39 ± 4.05 | 28.45 ± 4.55 | 27.78 ± 4.88 | 26.61 ± 3.86 | 27.08 ± 4.14 | 25.81 ± 2.20 | n.s. | |
| C16:1 | GG | 3.91 ± 2.14 | 4.16 ± 2.09 | 4.73 ± 2.14 | 5.69 ± 2.10 | 5.97 ± 2.04 | 7.61 ± 2.28 | 8.23 ± 3.21 | * |
| LL | 3.08 ± 1.53 | 3.05 ± 1.17 | 2.80 ± 1.00 | 2.43 ± 0.59 | 2.37 ± 0.69 | 2.36 ± 0.61 | 1.76 ± 0.57 | * | |
| C18:0 | GG | 7.25 ± 1.46 | 8.03 ± 1.40 | 7.42 ± 1.32 | 7.33 ± 1.30 | 7.37 ± 1.70 | 6.73 ± 2.07 | 7.67 ± 1.65 | n.s. |
| LL | 7.62 ± 1.65 | 7.94 ± 1.39 | 8.01 ± 1.37 | 7.65 ± 1.27 | 7.79 ± 0.93 | 8.22 ± 1.42 | 8.65 ± 1.24 | n.s. | |
| C18:1 | GG | 29.31 ± 5.76 | 32.39 ± 6.08 | 32.00 ± 5.70 | 34.22 ± 4.89 | 33.83 ± 5.79 | 34.29 ± 4.50 | 35.29 ± 1.89 | n.s. |
| LL | 29.96 ± 4.80 | 32.65 ± 4.86 | 31.12 ± 4.26 | 30.89 ± 3.34 | 30.53 ± 4.47 | 29.62 ± 2.80 | 29.38 ± 3.40 | n.s. | |
| C18:2 n6 | GG | 17.22 ± 5.64 | 14.28 ± 7.78 | 13.36 ± 6.62 | 10.96 ± 4.90 | 10.54 ± 5.59 | 9.18 ± 4.13 | 8.29 ± 3.35 | ** |
| LL | 18.71 ± 7.59 | 16.75 ± 9.89 | 19.12 ± 6.73 | 21.45 ± 1.95 | 21.77 ± 1.92 | 21.91 ± 1.63 | 22.99 ± 2.88 | ** | |
| C18:3 n6 | GG | 0.34 ± 0.21 | 0.41 ± 0.30 | 0.74 ± 0.21 | 0.43 ± 0.23 | 0.48 ± 0.33 | 0.48 ± 0.36 | 0.43 ± 0.27 | n.s. |
| LL | 0.30 ± 0.17 | 0.30 ± 0.20 | 0.34 ± 0.19 | 0.34 ± 0.20 | 0.33 ± 0.15 | 0.35 ± 0.18 | 0.31 ± 0.20 | n.s. | |
| C18:3 n3 | GG | 0.30 ± 0.24 | 0.49 ± 0.30 | 0.71 ± 0.40 | 0.86 ± 0.47 | 0.71 ± 0.36 | 0.72 ± 0.33 | 0.64 ± 0.26 | n.s. |
| LL | 0.42 ± 0.21 | 0.46 ± 0.23 | 0.46 ± 0.29 | 0.49 ± 0.33 | 0.45 ± 0.24 | 0.50 ± 0.18 | 0.59 ± 0.43 | n.s. | |
| C20:3 n6 | GG | 0.79 ± 0.28 | 1.06 ± 0.39 | 1.28 ± 0.53 | 1.48 ± 0.58 | 1.51 ± 0.64 | 1.32 ± 0.60 | 1.54 ± 0.65 | n.s. |
| LL | 1.10 ± 0.64 | 1.04 ± 0.46 | 1.01 ± 0.33 | 0.81 ± 0.28 | 0.86 ± 0.18 | 0.86 ± 0.35 | 0.82 ± 0.24 | n.s. | |
| C20:4 n6 | GG | 4.59 ± 1.87 | 4.90 ± 1.97 | 4.62 ± 1.88 | 4.50 ± 1.81 | 4.45 ± 1.94 | 4.02 ± 2.28 | 4.33 ± 2.04 | n.s. |
| LL | 5.22 ± 1.95 | 4.94 ± 1.51 | 4.82 ± 1.72 | 4.19 ± 1.50 | 4.74 ± 1.42 | 4.29 ± 1.47 | 4.31 ± 1.35 | n.s. | |
| C20:5 n3 | GG | 0.69 ± 0.43 | 0.79 ± 0.48 | 0.73 ± 0.47 | 0.89 ± 0.46 | 0.81 ± 0.38 | 0.90 ± 0.29 | 0.89 ± 0.43 | n.s. |
| LL | 0.88 ± 0.46 | 0.73 ± 0.32 | 0.89 ± 0.37 | 0.98 ± 0.40 | 0.99 ± 0.34 | 1.05 ± 0.52 | 1.02 ± 0.65 | n.s. | |
| C22:6 n3 | GG | 0.89 ± 0.48 | 1.00 ± 0.52 | 0.79 ± 0.46 | 0.79 ± 0.47 | 0.80 ± 0.48 | 0.69 ± 0.42 | 0.74 ± 0.53 | *** |
| LL | 1.19 ± 0.62 | 1.50 ± 0.87 | 1.73 ± 0.88 | 1.73 ± 1.00 | 2.40 ± 1.12 | 2.31 ± 1.23 | 3.29 ± 1.52 | *** | |
| AA to EPA | GG | 8.88 ± 5.61 | 8.29 ± 5.59 | 8.41 ± 5.02 | 6.17 ± 3.78 | 6.75 ± 4.84 | 4.71 ± 2.59 | 5.68 ± 2.78 | n.s. |
| LL | 7.64 ± 4.41 | 11.2 ± 17.47 | 6.37 ± 4.38 | 4.93 ± 3.03 | 5.67 ± 3.54 | 5.26 ± 3.64 | 6.44 ± 5.29 | n.s. | |
| n3 + n6 | GG | 24.52 ± 6.99 | 22.63 ± 7.31 | 21.55 ± 6.53 | 19.59 ± 5.03 | 18.94 ± 6.17 | 16.96 ± 5.62 | 16.64 ± 4.54 | **** |
| LL | 27.65 ± 8.06 | 25.57 ± 8.71 | 28.17 ± 6.67 | 29.79 ± 4.16 | 31.38 ± 3.73 | 31.06 ± 3.96 | 33.17 ± 5.04 | **** |
| Variable | Group | Day 0 | Day 1 | Day 3 | Day 6 | Day 9 | Day 14 | Day 28 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Resistin (ng·mL−1) | GG | 27.76 ± 6.86 | 26.28 ± 8.51 | 27.34 ± 7.64 | 24.36 ± 9.35 | 25.79 ± 7.11 | 27.97 ± 4.69 | 25.3 ± 9.21 | n.s. |
| LG | 25.83 ± 9.97 | 25.29 ± 9.5 | 23.88 ± 9.57 | 27.45 ± 6.46 | 24.46 ± 9.69 | 26.94 ± 9.99 | 24.55 ± 6.22 | n.s. | |
| Leptin (pg·mL−1) | GG | 4.86 ± 3.16 | 24.34 ± 11.9 | 20.49 ± 13.9 | 17.17 ± 11.47 | 21.48 ± 12.72 | 21.99 ± 9.73 | 16.69 ± 7.12 | n.s. |
| LG | 4.34 ± 4.11 | 4.45 ± 5.05 | 4.37 ± 3.52 | 2.98 ± 2.52 | 5.77 ± 6.69 | 4.19 ± 5.08 | 5.17 ± 6.20 | n.s. | |
| PAI-1 (ng·mL−1) | GG | 73.77 ± 38.59 | 90.66 ± 61.61 | 72.25 ± 40.21 | 66.33 ± 26.25 | 104.65 ± 82.71 | 85.25 ± 79.10 | 78.11 ± 57.23 | n.s. |
| LG | 50.39 ± 28.49 | 41.92 ± 25.34 | 46.21 ± 30.11 | 51.36 ± 46.31 | 68.65 ± 54.59 | 55.46 ± 33.67 | 51.18 ± 29.40 | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorepa, P.; Sobotka, O.; Vanek, J.; Ticha, A.; Fortunato, J.; Manak, J.; Blaha, V.; Horacek, J.M.; Sobotka, L. The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients. Nutrients 2020, 12, 1373. https://doi.org/10.3390/nu12051373
Skorepa P, Sobotka O, Vanek J, Ticha A, Fortunato J, Manak J, Blaha V, Horacek JM, Sobotka L. The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients. Nutrients. 2020; 12(5):1373. https://doi.org/10.3390/nu12051373
Chicago/Turabian StyleSkorepa, Pavel, Ondrej Sobotka, Jan Vanek, Alena Ticha, Joao Fortunato, Jan Manak, Vladimir Blaha, Jan M. Horacek, and Lubos Sobotka. 2020. "The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients" Nutrients 12, no. 5: 1373. https://doi.org/10.3390/nu12051373






