The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. MTT Assay for Assessment of Cytotoxicity
2.4. Cell Proliferation Assay
2.5. Determination of Collagen Biosynthesis
2.6. Analysis of Activity of MMP-1 and MMP-2 by Zymography
2.7. Western Immunoblotting
2.8. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.9. Statistical Analysis
3. Results
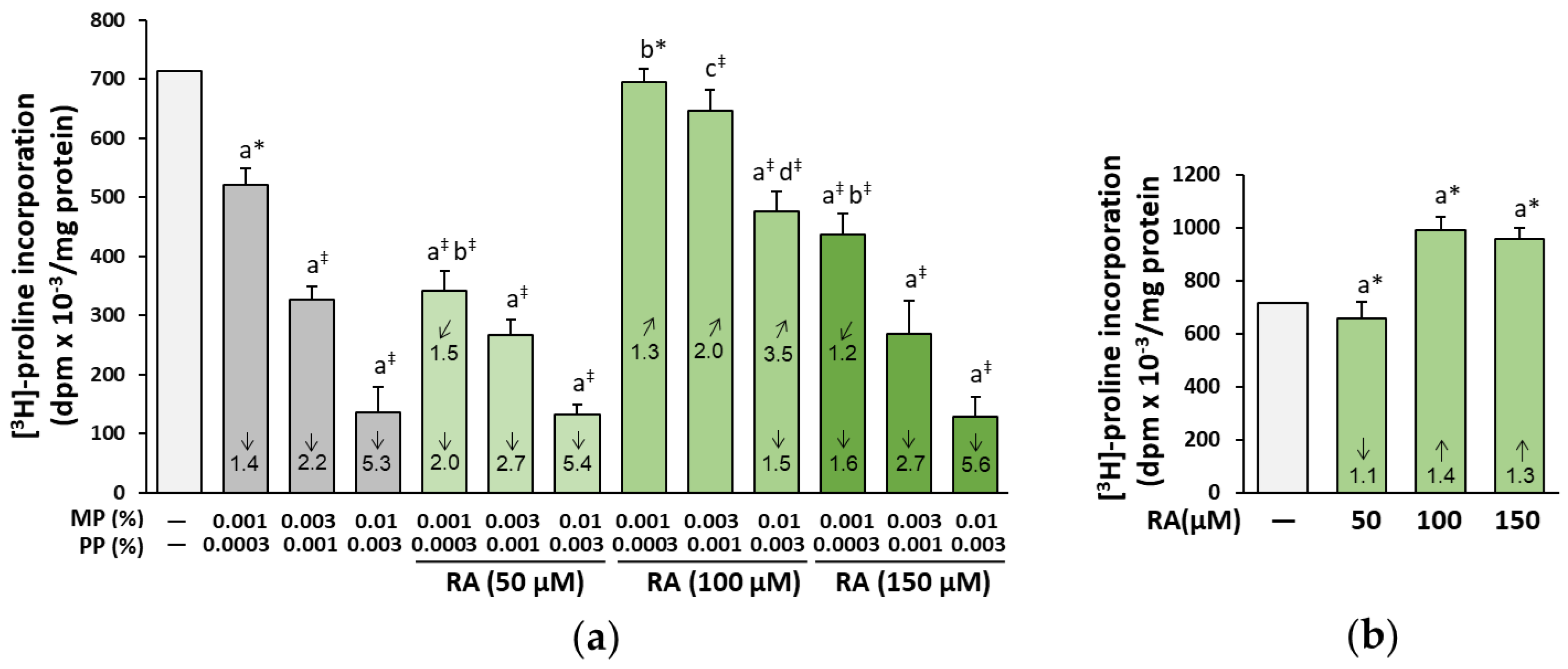
3.1. The Influence of RA on Biosynthesis of Collagen in the Fibroblasts Treated with Parabens
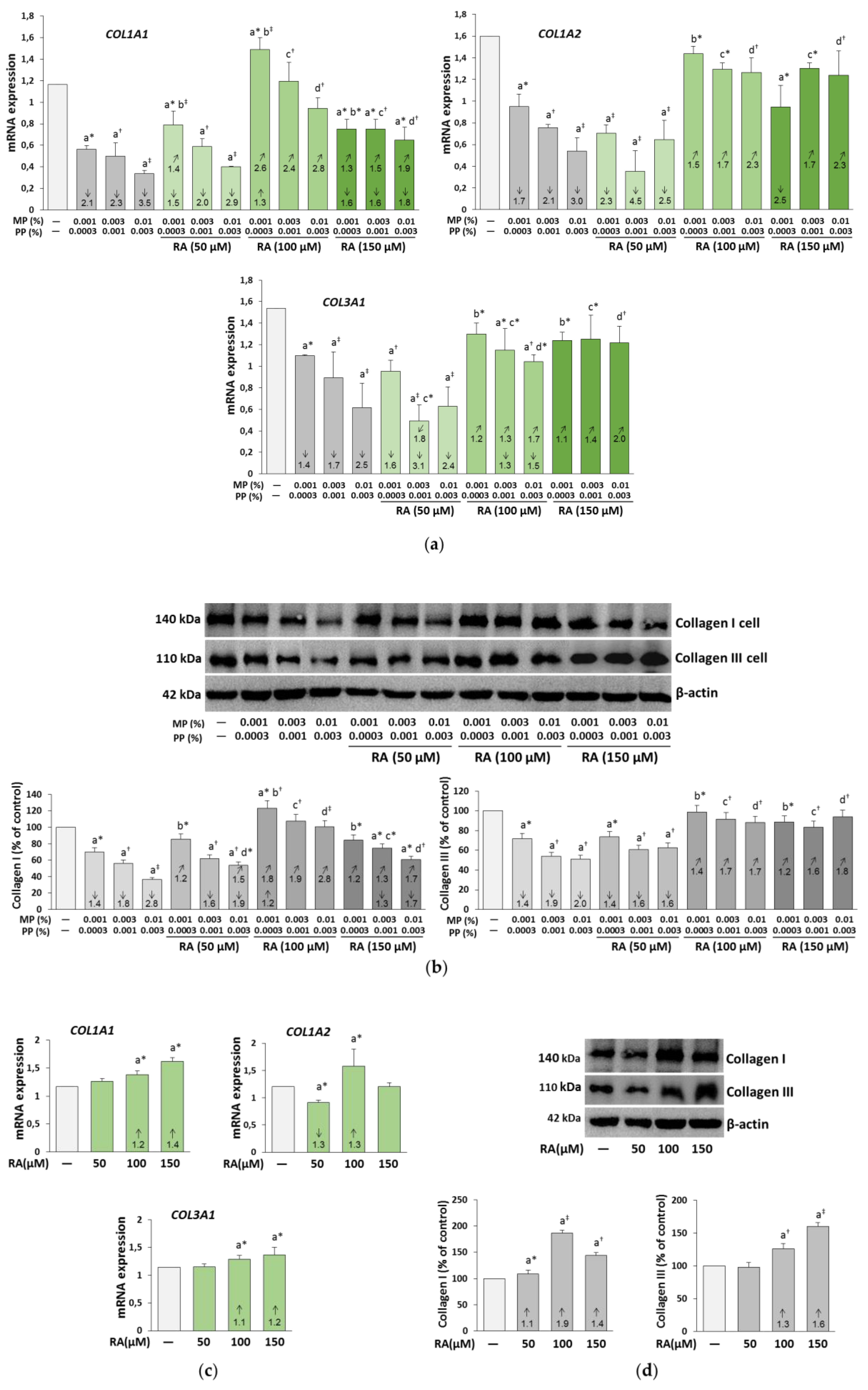
3.2. The Influence of RA on the Expression of Collagen I and III in the Fibroblasts Treated with Parabens
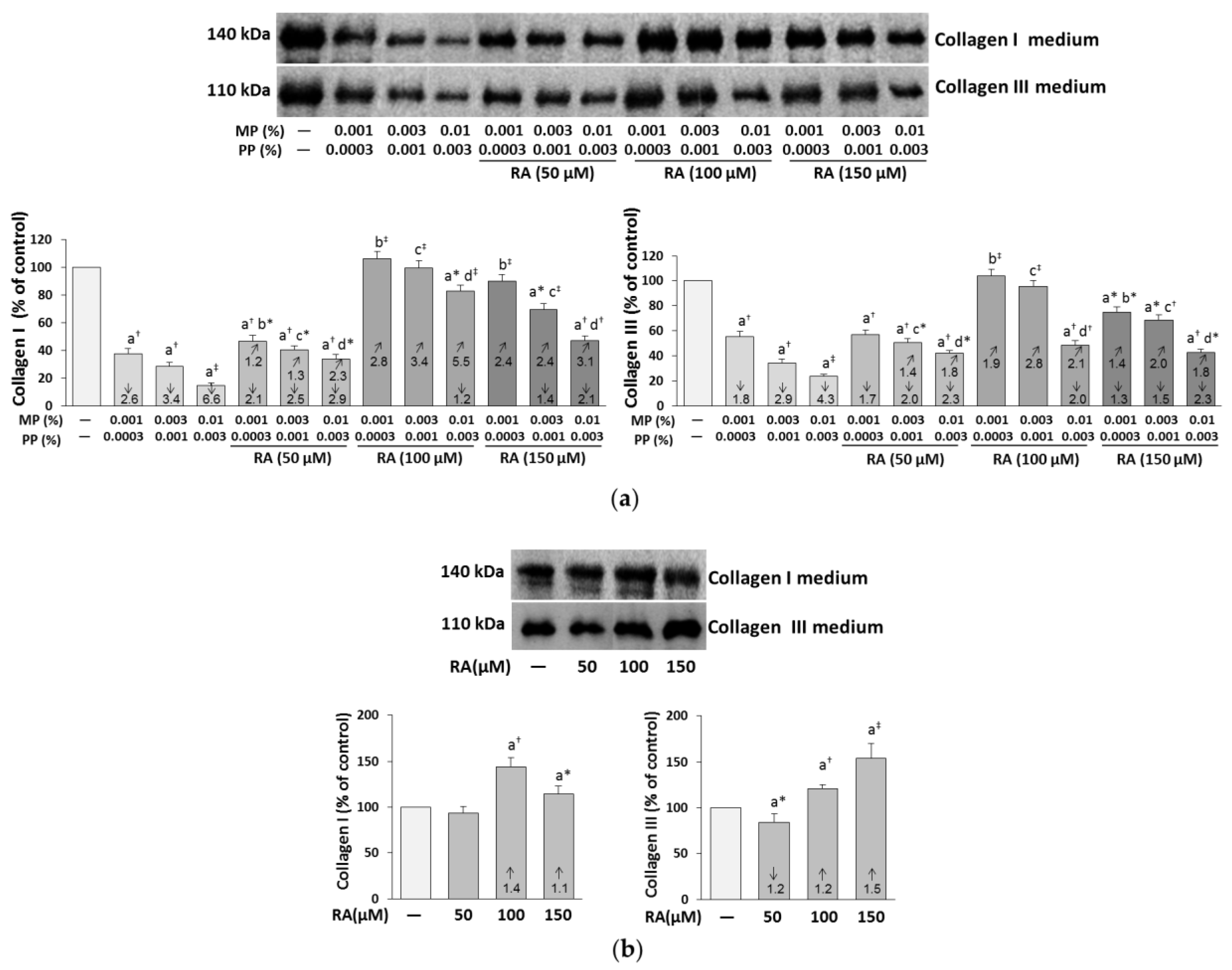
3.3. The Influence of RA on the Content of Collagen I and III in the Medium of the Fibroblasts Treated with Parabens
3.4. The Influence of RA on the Expression of ERK1/2 in the Fibroblasts Treated with Parabens
3.5. The Influence of RA on the Expression of CTHRC1 and HSP47 in the Fibroblasts Treated with Parabens
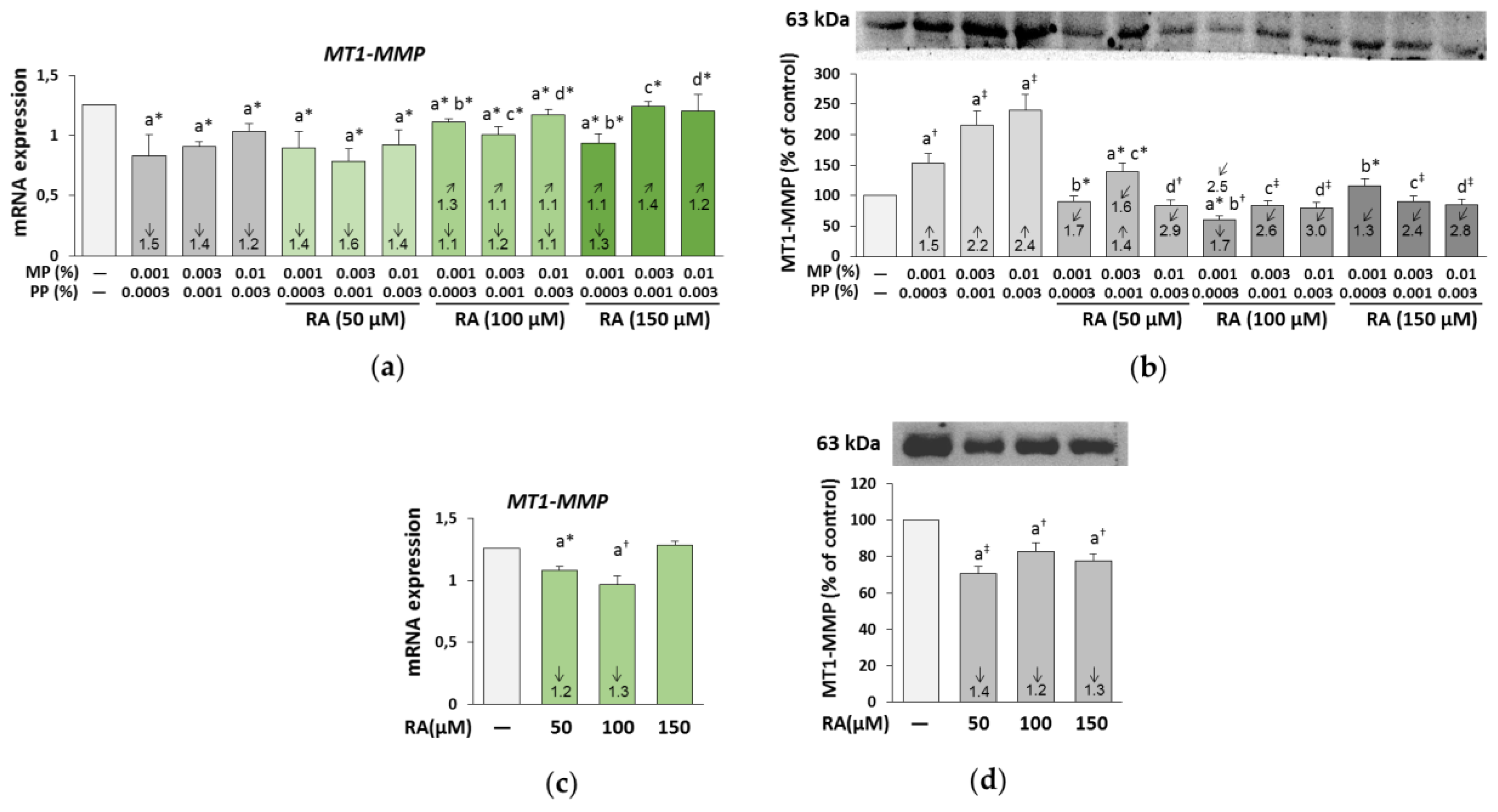
3.6. The Influence of RA on the Expression and Activity of MMP-1, MMP-2, and MT1-MMP in the Fibroblasts Treated with Parabens
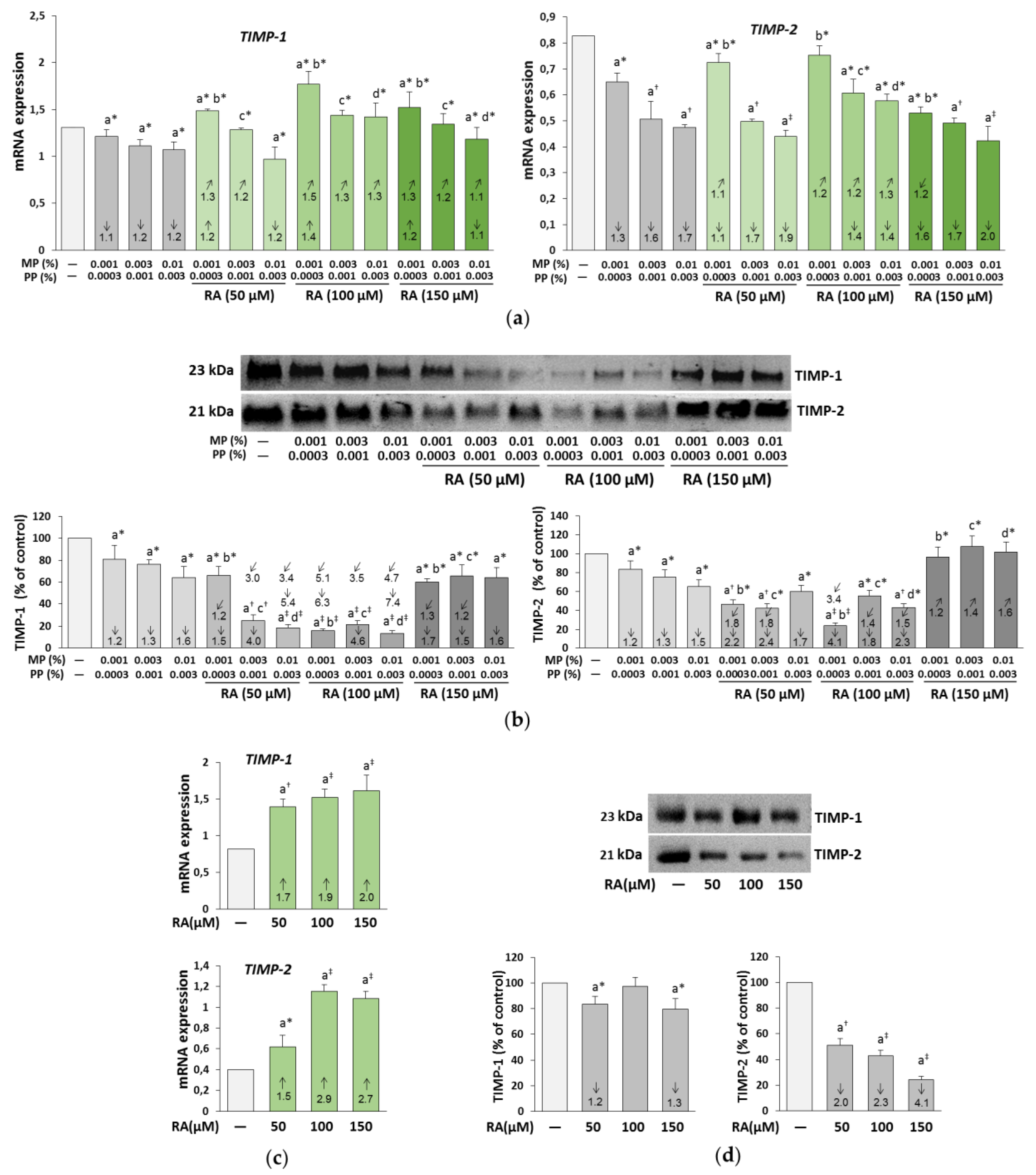
3.7. The Influence of RA on the Expression of TIMP-1 and TIMP-2 in the Fibroblasts Treated with Parabens
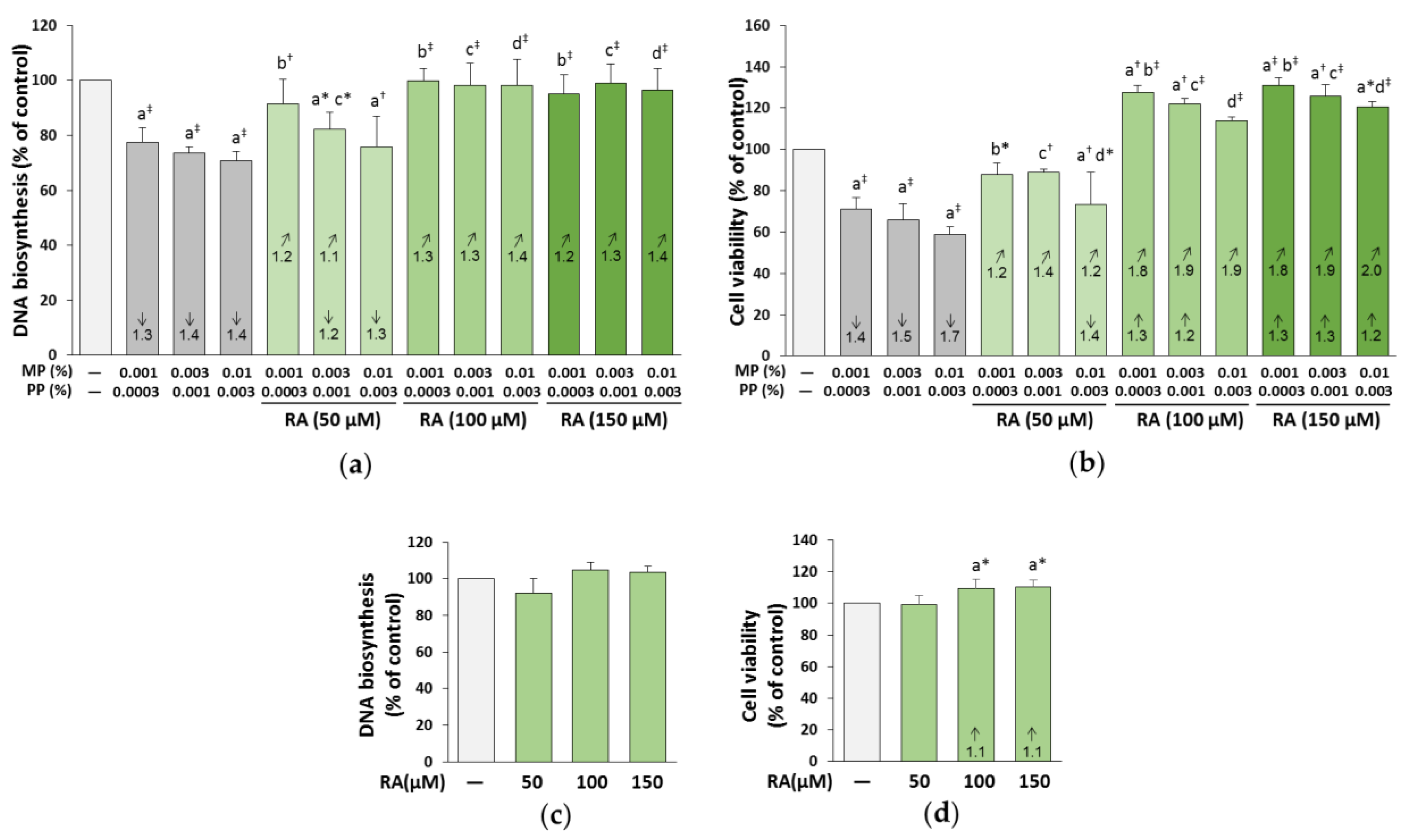
3.8. The Influence of RA on the Proliferation and Viability of the Fibroblasts Treated with Parabens
3.9. The Influence of RA on the Expression of Bcl-xL, Bax, and Caspase-3 in the Fibroblasts Treated with Parabens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoah, S.K.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic acid--pharmaceutical and clinical aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakabe, N.; Takano, H.; Sanbongi, C.; Yasuda, A.; Yanagisawa, R.; Inoue, K.; Yoshikawa, T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 2004, 21, 127–131. [Google Scholar] [CrossRef]
- Zou, Z.W.; Xu, L.N.; Tian, J.Y. Antithrombotic and antiplatelet effects of rosmarinic acid, a water-soluble component isolated from radix Salviae miltiorrhizae (danshen). Yao Xue Xue Bao 1993, 28, 241–245. [Google Scholar]
- Radziejewska, I.; Supruniuk, K.; Nazaruk, J.; Karna, E.; Popławska, B.; Bielawska, A.; Galicka, A. Rosmarinic acid influences collagen, MMPs, TIMPs, glycosylation and MUC1 in CRL-1739 gastric cancer cell line. Biomed. Pharmacother. 2018, 107, 397–407. [Google Scholar] [CrossRef]
- Choi, S.H.; Jang, G.W.; Choi, S.I.; Jung, T.D.; Cho, B.Y.; Sim, W.S.; Han, X.; Lee, J.S.; Kim, D.Y.; Kim, D.B.; et al. Development and validation of an analytical method for carnosol, carnosic acid and rosmarinic acid in food matrices and evaluation of the antioxidant activity of rosemary extract as a food additive. Antioxidants 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective effect of a water-soluble extract of Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and reconstructed skin. Eur. J. Dermatol. 2008, 18, 128–135. [Google Scholar] [CrossRef]
- Fernando, P.M.D.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.K.M.; Chae, S.W.; Hyun, J.W. Rosmarinic acid attenuates cell damage against UVB radiation-induced oxidative stress via enhancing antioxidant effects in human HaCaT cells. Biomol. Ther. (Seoul) 2016, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M. Understanding the structure and function of the skin. Nurs. Times 2003, 99, 46–48. [Google Scholar] [PubMed]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W.A. Collagen supplement improves skin hydration, elasticity, roughness, and density: Results of a randomized, placebo-controlled, blind study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadoon, S.; Karim, S.; Asad, M.H.H.B.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxid. Med. Cell. Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S.; Benefits, M. Applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- Cowan-Ellsberry, C.E.; Robison, S.H. Refining aggregate exposure: Example using parabens. Regul. Toxicol. Pharmacol. 2009, 55, 321–329. [Google Scholar] [CrossRef] [PubMed]
- EC. Regulation No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; Official Journal of the European Union: Luxembourg, 2009. [Google Scholar]
- EC. Regulation No 1004/2014 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and the Council on Cosmetic Products; Official Journal of the European Union: Luxembourg, 2014. [Google Scholar]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Cashman, A.L.; Warshaw, E.M. Parabens: A review of epidemiology, structure, alergenicity, and hormonal properties. Dermatitis 2005, 16, 57–66. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- Matwiejczuk, N.; Galicka, A.; Brzóska, M.M. Review of the safety of application of cosmetic products containing parabens. J. Appl. Toxicol. 2020, 40, 176–210. [Google Scholar] [CrossRef] [PubMed]
- Majewska, N.; Zaręba, I.; Surażyński, A.; Galicka, A. Methylparaben-induced decrease in collagen production and viability of cultured human dermal fibroblasts. J. Appl. Toxicol. 2017, 37, 1117–1124. [Google Scholar] [CrossRef]
- Rastogi, S.C.; Schouten, A.; de Kruijf, N.; Weijland, J.W. Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact Dermat. 1995, 32, 28–30. [Google Scholar] [CrossRef]
- Ishiwatari, S.; Suzuki, T.; Hitomi, T.; Yoshino, T.; Matsukuma, S.; Tsuji, T. Effects of methyl paraben on skin keratinocytes. J. Appl. Toxicol. 2007, 27, 1–9. [Google Scholar] [CrossRef]
- Handa, O.; Kokura, S.; Adachi, S.; Takagi, T.; Naito, Y.; Tanigawa, T.; Yoshida, N.; Yoshikawa, T. Methylparaben potentiates UV-induced damage of skin keratinocytes. Toxicology 2006, 227, 62–72. [Google Scholar] [CrossRef]
- Pedersen, S.; Marra, F.; Micoli, S.; Santi, P. In vitro skin permeation and retention of parabens from cosmetic formulations. Int. J. Cosmet. Sci. 2007, 29, 361–367. [Google Scholar] [CrossRef]
- Soni, M.G.; Taylor, S.L.; Greenberg, N.A.; Burdock, G.A. Evaluation of the health aspects of methyl paraben: A review of the published literature. Food Chem. Toxicol. 2002, 40, 1335–1373. [Google Scholar] [CrossRef]
- Peterkofsky, B.; Chojkier, M.; Bateman, J. Determination of collagen synthesis in tissue and cell culture system. In Immuno-Chemistry of the Extracellular Matrix, 2nd ed.; Furthmayr, H., Ed.; CRC Press: Boca Raton, FL, USA, 1982; pp. 19–47. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. Extract from Aronia melanocarpa L. berries protects against cadmium-induced lipid peroxidation and oxidative damage to proteins and DNA in the liver: A study in a rat model of environmental human exposure to this xenobiotic. Nutrients 2019, 11, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.J.; Chen, S.Y.; Qu, X.Y.; Abdul-Fattah, B.; Lai, T.; Xie, M.; Wu, S.D.; Zhou, Y.W.; Huang, C.Z. Increased Cthrc1 activates normal fibroblasts and suppresses keloid fibroblasts by inhibiting TGF-β/Smad signal pathway and modulating YAP subcellular location. Curr. Med. Sci. 2018, 38, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Hosokawa, M.; Itohara, S.; Adachi, E.; Matsushita, T.; Hosokawa, N.; Nagata, K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell. Biol. 2000, 150, 1499–1506. [Google Scholar] [CrossRef] [Green Version]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [Green Version]
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Ock, S.M.; Kim, H.J.; Park, H.J.; Lee, Y.B.; Choi, J.M.; Cho, C.S.; Lee, J.Y.; Cho, B.K.; Cho, D.H.; et al. Vitamin C attenuates ERK signalling to inhibit the regulation of collagen production by LL-37 in human dermal fibroblasts. Exp. Dermatol. 2010, 19, e258–e264. [Google Scholar] [CrossRef]
- Reunanen, N.; Foschi, M.; Han, J.; Kahari, V.M. Activation of extracellular signal-regulated kinase 1/2 inhibits type I collagen expression by human skin fibroblasts. J. Biol. Chem. 2000, 275, 34634–34639. [Google Scholar] [CrossRef] [Green Version]
- Dubey, D.; Chopra, D.; Singh, J.; Srivastav, A.K.; Kumari, S.; Verma, A.; Ray, R.S. Photosensitized methyl paraben induces apoptosis via caspase dependent pathway under ambient UVB exposure in human skin cells. Food Chem. Toxicol. 2017, 108, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Menezes, P.F.; Letenski, G.C.; Praes, C.E.; Feferman, I.H.; Lorencini, M. In vitro induction of apoptosis, necrosis and genotoxicity by cosmetic preservatives: Application of flow cytometry as a complementary analysis by NRU. Int. J. Cosmet. Sci. 2012, 34, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Bae, S.; Kim, K.; Kwon, S.B.; An, I.S.; Ahn, K.J.; Ryu, J.; Kim, H.; Ye, S.; Kim, B.; et al. Overdosage of methylparaben induces cellular senescence in vitro and in vivo. J. Investig. Dermatol. 2015, 135, 609–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cizauskaite, U.; Bernatoniene, J. Innovative natural ingredients–based multiple emulsions: The effect on human skin moisture, sebum content, pore size and pigmentation. Molecules 2018, 23, 1428. [Google Scholar] [CrossRef] [Green Version]
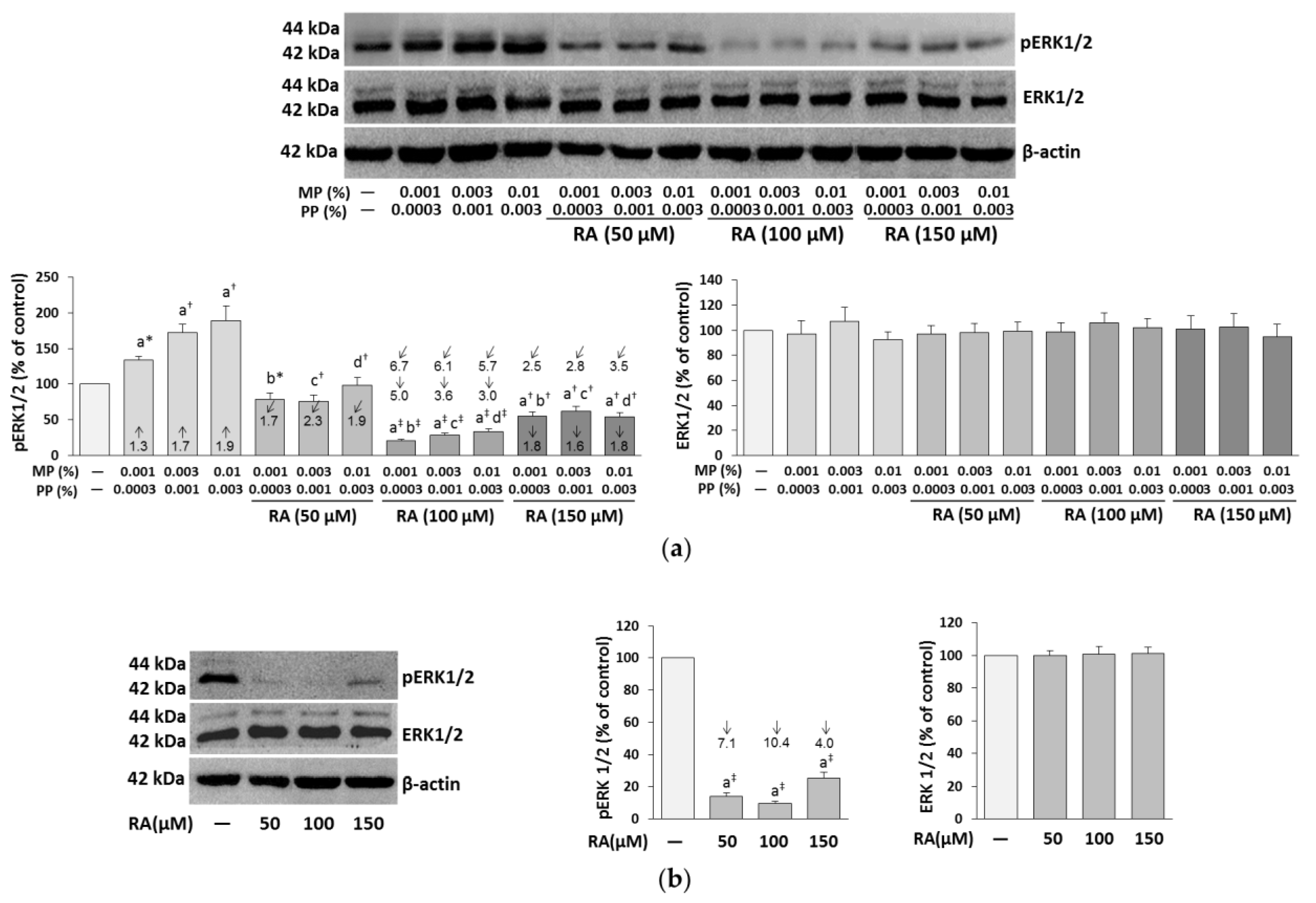
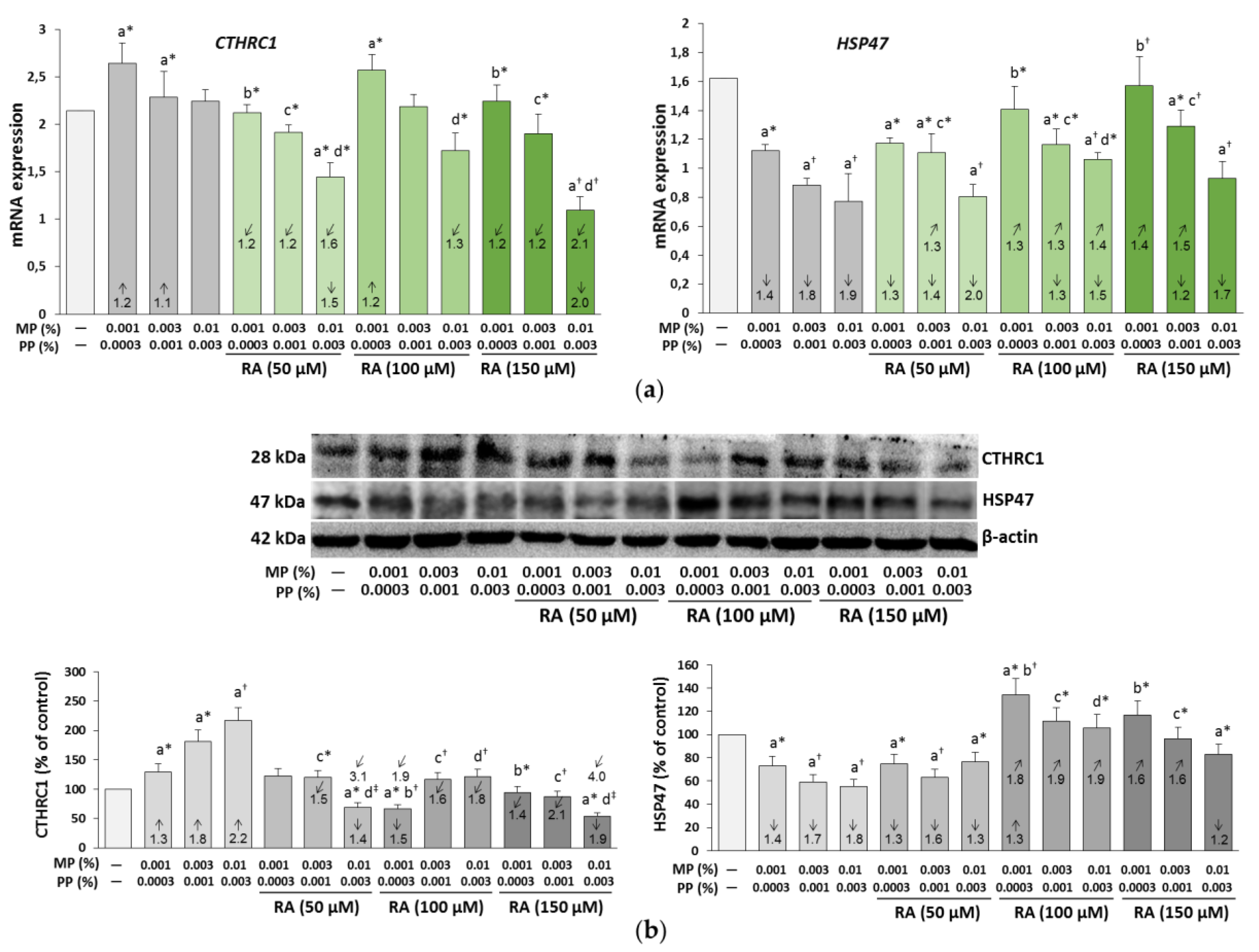

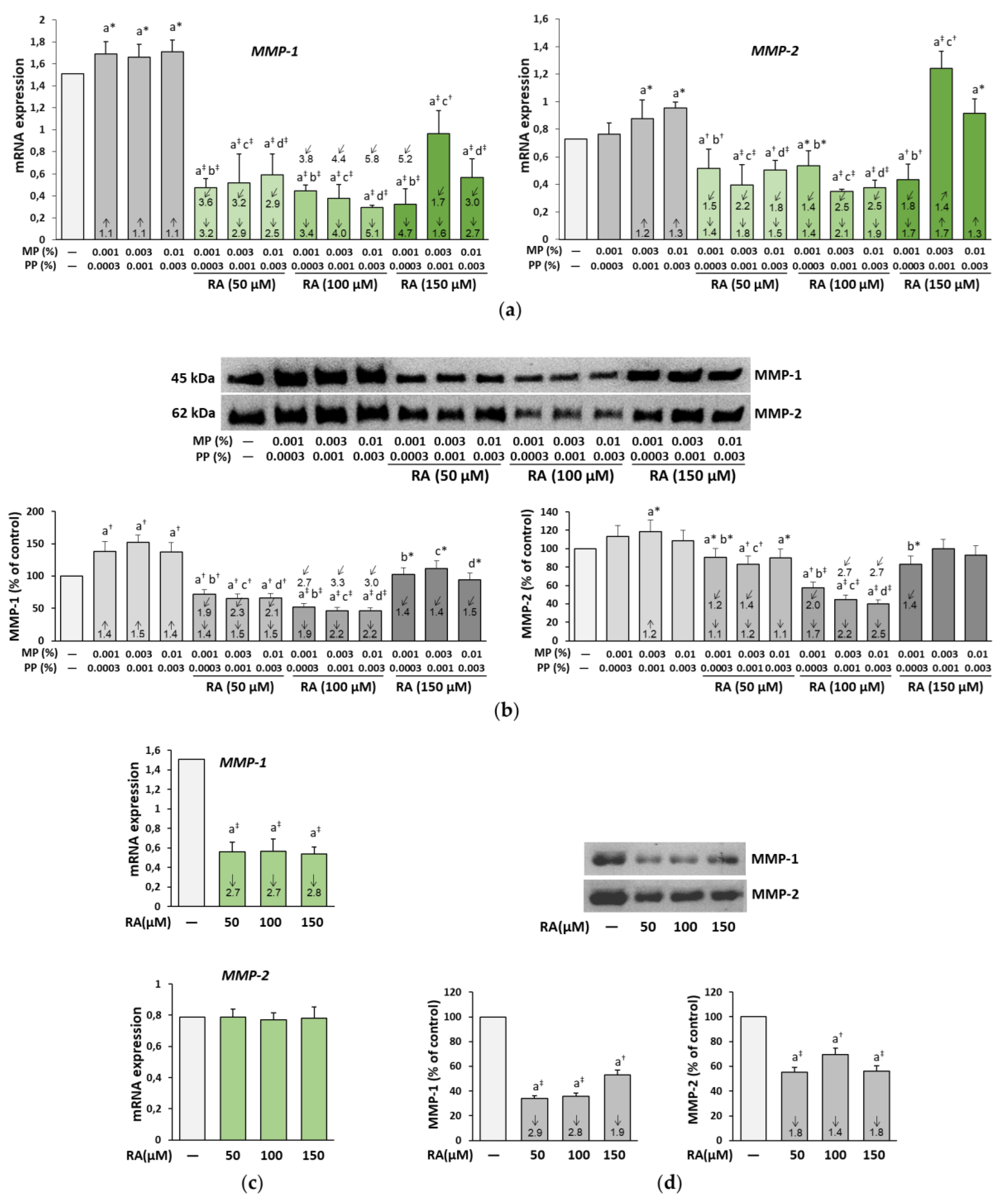
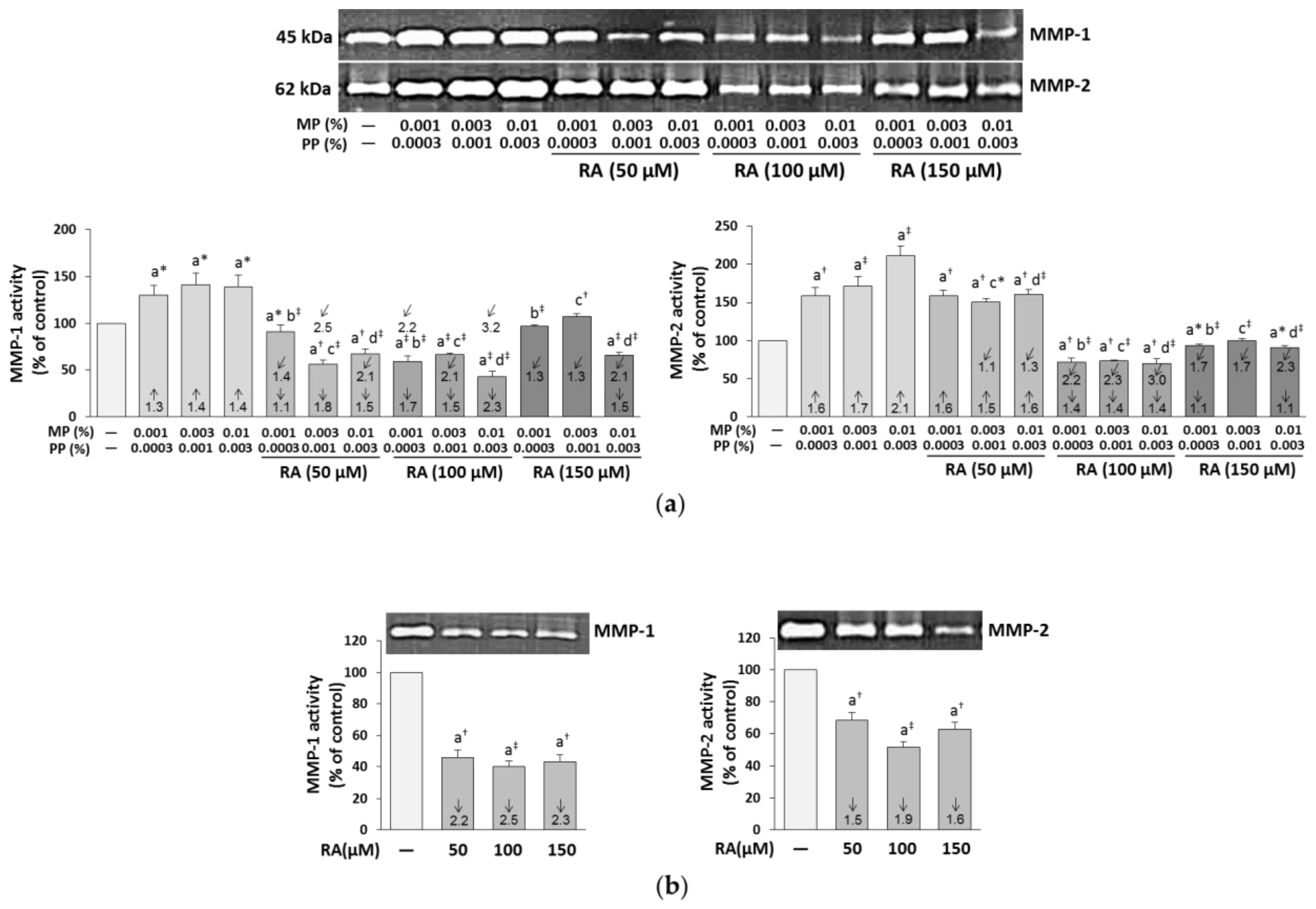


| Concentration of RA (μM) | Concentrations of Parabens (%) MP + PP | Parabens (MP + PP) + RA | |||
|---|---|---|---|---|---|
| Main Effect of Parabens | Main Effect of RA | Interactive Effect of Parabens—RA | Parabens + RA Effect vs. Parabens Effect + RA Effect Possible Character of Interaction | ||
| Biosynthesis of collagen | |||||
| 50 | 0.001 + 0.0003 | 32.02‡ | 23.85† | 10.45* | −2 3 vs. −1.4 + (−1.1); −2 vs. −2.5; Antagonistic action |
| 0.003 + 0.001 | 313.2‡ | 7.276† | NS | No interaction | |
| 0.01 + 0.003 | 576.6‡ | NS | NS | No interaction | |
| 100 | 0.001 + 0.0003 | 33.69‡ | 27.47‡ | 10.29* | 0 vs. −1.4 + (+1.4); 0 vs. 0; Additive action |
| 0.003 + 0.001 | 321.2‡ | 240.1‡ | NS | No interaction | |
| 0.01 + 0.003 | 706.8‡ | 226.5‡ | NS | No interaction | |
| 150 | 0.001 + 0.0003 | 84.89‡ | NS | 43.67‡ | −1.6 vs. −1.4 + (+1.3); −1.6 vs. −0.1 4 |
| 0.003 + 0.001 | 699.4‡ | 20.49† | 54.81‡ | −2.7 vs. −2.2 + (+1.3); −2.7 vs. −0.9 4 | |
| 0.01 + 0.003 | 1278‡ | 35.50‡ | 40.35‡ | −5.6 vs. −5.3 + (+1.3); −5.6 vs. −4 4 | |
| pERK1/2 expression | |||||
| 50 | 0.001 + 0.0003 | 192.6‡ | 391.3‡ | 19.21† | 0 vs. +1.3 + (−7.1); 0 vs. −5.8; Antagonistic action |
| 0.003 + 0.001 | 168.5‡ | 311.1‡ | NS | No interaction | |
| 0.01 + 0.003 | 189.2‡ | 195.4‡ | NS | No interaction | |
| 100 | 0.001 + 0.0003 | 58.05‡ | 1247‡ | 16.43† | −5 vs. +1.3 + (−10.4); −5 vs. −9.1; Antagonistic action |
| 0.003 + 0.001 | 4.208# | 147.5‡ | 264.2‡ | −3.6 vs. +1.7 + (−10.4); −3.6 vs. −8.7; Antagonistic action | |
| 0.01 + 0.003 | NS | 128.6‡ | 220.2‡ | −3 vs. +1.9 + (−10.4); −3 vs. −8.5; Antagonistic action | |
| 150 | 0.001 + 0.0003 | 59.37‡ | 345.0‡ | NS | No interaction |
| 0.003 + 0.001 | 91.66‡ | 263.3‡ | 9.480* | −1.6 vs. +1.7 + (−4); −1.6 vs. −2.3; Antagonistic action | |
| 0.01 + 0.003 | 91.91‡ | 288.3‡ | 23.84† | −1.8 vs. +1.9 + (−4); −1.8 vs. −2.1; Antagonistic action | |
| Concentrationof RA (μM) | Concentrations of Parabens (%) MP + PP | Parabens (MP + PP) + RA | |||
|---|---|---|---|---|---|
| Main Effect of Parabens | Main Effect ofRA | Interactive Effect of Parabens—RA | Parabens + RA Effect vs. Parabens Effect + RA Effect Possible Character of Interaction | ||
| Collagen I cell expression | |||||
| 50 | 0.001 + 0.0003 | 59.65‡ | 12.47† | NS | No interaction |
| 0.003 + 0.001 | 207.9‡ | 5.415* | NS | No interaction | |
| 0.01 + 0.003 | 393.9‡ | 19.31† | NS | No interaction | |
| 100 | 0.001 + 0.0003 | 188.7‡ | 424.8‡ | 24.20† | +1.2 3 vs. −1.4 + (+1.9); +1.2 vs. +0.5 4 |
| 0.003 + 0.001 | 159.3‡ | 90.38‡ | 915.4‡ | 0 vs. −1.8 + (+1.9); 0 vs. +0.1; Antagonistic action | |
| 0.01 + 0.003 | 75.81‡ | 69.71‡ | 1583‡ | 0 vs. −2.8 + (+1.9); 0 vs. −0.9; Antagonistic action | |
| 150 | 0.001 + 0.0003 | 243.4‡ | 99.59‡ | 25.42‡ | 0 vs. −1.4 + (+1.4); 0 vs. 0; Additive action |
| 0.003 + 0.001 | 560.5‡ | 170.9‡ | 28.82‡ | −1.3 vs. −1.8 + (+1.4); −1.3 vs. −0.4 4 | |
| 0.01 + 0.003 | 1172‡ | 252.5‡ | 21.27† | −1.7 vs. −2.8 + (+1.4); −1.7 vs. −1.4 4 | |
| Collagen I medium expression | |||||
| 50 | 0.001 + 0.0003 | 102.6‡ | NS | 4.000#5 | −2.1 vs. −2.6 + 0; −2.1 vs. −2.6; Antagonistic action |
| 0.003 + 0.001 | 48.80‡ | NS | NS | No interaction | |
| 0.01 + 0.003 | 303.7‡ | 6.228* | 21.01† | −2.9 vs. −6.6 + 0; −2.9 vs. −6.6; Antagonistic action | |
| 100 | 0.001 + 0.0003 | 144.8‡ | 89.11‡ | 8.216* | 0 vs. −2.6 + (+1.4); 0 vs. −1.2; Antagonistic action |
| 0.003 + 0.001 | 169.2‡ | 66.34‡ | NS | No interaction | |
| 0.01 + 0.003 | 274.3‡ | 94.72‡ | 5.207* | −1.2 vs. −6.6 + (+1.4); −1.2 vs. −5.2; Antagonistic action | |
| 150 | 0.001 + 0.0003 | 97.86‡ | 25.87‡ | NS | No interaction |
| 0.003 + 0.001 | 200.9‡ | 42.76‡ | NS | No interaction | |
| 0.01 + 0.003 | 361.1‡ | 52.90‡ | 5.986* | −2.1 vs. −6.6 + (+1.1); −2.1 vs. −5.5; Antagonistic action | |
| Collagen III cell expression | |||||
| 100 | 0.001 + 0.0003 | 88.74‡ | 80.66‡ | NS | No interaction |
| 0.003 + 0.001 | 194.8‡ | 148.6‡ | 35.04‡ | 0 vs. −1.9 + (+1.3); 0 vs. −0.6; Antagonistic action | |
| 0.01 + 0.003 | 646.4‡ | 455.0‡ | 73.99‡ | 0 vs. −2 + (+1.3); 0 vs. −0.7; Antagonistic action | |
| 150 | 0.001 + 0.0003 | 465.6‡ | 281.2‡ | 69.95‡ | 0 vs. −1.4 + (+1.6); 0 vs. 0.2; Antagonistic action |
| 0.003 + 0.001 | 153.8‡ | 77.97‡ | 16.78† | 0 vs. −1.9 + (+1.6); 0 vs. −0.3; Antagonistic action | |
| 0.01 + 0.003 | 419.2‡ | 335.3‡ | 9.450* | 0 vs. −2 + (+1.6); 0 vs. −0.4; Antagonistic action | |
| Collagen III medium expression | |||||
| 50 | 0.001 + 0.0003 | 34.77‡ | 10.08* | NS | No interaction |
| 0.003 + 0.001 | 132.0‡ | NS | 11.33† | −2 vs. -2.9 + (−1.2); −2 vs. −4.1; Antagonistic action | |
| 0.01 + 0.003 | 239.5‡ | NS | 10.42* | −2.3 vs. -4.3 + (−1.2); -2.3 vs. −5.5; Antagonistic action | |
| 100 | 0.001 + 0.0003 | 78.34‡ | 28.81* | NS | No interaction |
| 0.003 + 0.001 | 252.8‡ | 160.0‡ | NS | No interaction | |
| 0.01 + 0.003 | 415.5‡ | 67.61‡ | 12.57† | −2 vs. −4.3 + (+1.2); −2 vs. −3.1; Antagonistic action | |
| 150 | 0.001 + 0.0003 | 100.4‡ | 23.97† | 27.17‡ | −1.3 vs. −1.8 + (+1.5); −1.3 vs. −0.3 4 |
| 0.003 + 0.001 | 180.6‡ | 46.06‡ | 13.22† | −1.5 vs. −2.9 + (+1.5); −1.5 vs. −1.4 4 | |
| 0.01 + 0.003 | 289.5‡ | 37.71‡ | 18.37† | −2.3 vs. −4.3 + (+1.5); −2.3 vs. −2.8; Antagonistic action | |
| Concentrationof RA (μM) | Concentrations of Parabens (%) MP + PP | Parabens (MP + PP) + RA | |||
|---|---|---|---|---|---|
| Main Effect of Parabens | Main Effect ofRA | Interactive Effect of Parabens—RA | Parabens + RA Effect vs. Parabens Effect + RA Effect Possible Character of Interaction | ||
| [3H]thymidine incorporation | |||||
| 50 | 0.001 + 0.0003 | 224.1‡ | 369.8‡ | 687.8‡ | 0 vs. −1.3 3 + 0; 0 vs. −1.3; Antagonistic action |
| 0.003 + 0.001 | 77.71‡ | 244.3‡ | 356.0‡ | −1.2 vs. −1.4 + 0; −1.2 vs. −1.4; Antagonistic action | |
| 0.01 + 0.003 | 18.44† | 96.58‡ | 120.5‡ | −1.3 vs. −1.4 + 0; −1.3 vs. −1.4; Antagonistic action | |
| 100 | 0.001 + 0.0003 | 230.4‡ | 231.2‡ | 643.7‡ | 0 vs. −1.3 + 0; 0 vs. −1.3; Antagonistic action |
| 0.003 + 0.001 | 229.7‡ | 255.8‡ | 730.6‡ | 0 vs. −1.4 + 0; 0 vs. −1.4; Antagonistic action | |
| 0.01 + 0.003 | 93.16‡ | 104.2‡ | 336.2‡ | 0 vs. −1.4 + 0; 0 vs. −1.4; Antagonistic action | |
| 150 | 0.001 + 0.0003 | 238.3‡ | 322.4‡ | 708.3‡ | 0 vs. −1.3 + 0; 0 vs. −1.3; Antagonistic action |
| 0.003 + 0.001 | 245.8‡ | 260.7‡ | 841.1‡ | 0 vs. −1.4 + 0; 0 vs. −1.4; Antagonistic action | |
| 0.01 + 0.003 | 78.31‡ | 98.63‡ | 320.6‡ | 0 vs. −1.4 + 0; 0 vs. −1.4; Antagonistic action | |
| Viability of cells | |||||
| 50 | 0.001 + 0.0003 | 61.54‡ | 13.68† | 15.83† | 0 vs. −1.4 + 0; 0 vs. −1.4; Antagonistic action |
| 0.003 + 0.001 | 95.04‡ | 23.17† | 26.40‡ | 0 vs. −1.5 + 0; 0 vs. −1.5; Antagonistic action | |
| 0.01 + 0.003 | 93.02‡ | 8.118* | 9.413* | −1.4 vs. −1.7 + 0; −1.4 vs. −1.7; Antagonistic action | |
| 100 | 0.001 + 0.0003 | 4.286# | 207.7‡ | 106.5‡ | +1.3 vs. −1.4 + (+1.1); +1.3 vs. −0.3 4 |
| 0.003 + 0.001 | 12.29† | 241.0‡ | 169.6‡ | +1.2 vs. −1.5 + (+1.1); +1.2 vs. −0.4 4 | |
| 0.01 + 0.003 | 108.7‡ | 410.6‡ | 288.6‡ | 0 vs. −1.7 + (+1.1); 0 vs. −0.6; Antagonistic action | |
| 150 | 0.001 + 0.0003 | NS | 304.3‡ | 149.6‡ | +1.3 vs. −1.4 + (+1.1); +1.3 vs. −0.3 4 |
| 0.003 + 0.001 | 1312‡ | 306.1‡ | 147.3‡ | +1.3 vs. −1.5 + (+1.1); +1.3 vs. −0.4 4 | |
| 0.01 + 0.003 | 108.7‡ | 658.7‡ | 324.8‡ | +1.2 vs. −1.7 + (+1.1); +1.2 vs. −0.6 4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matwiejczuk, N.; Galicka, A.; Zaręba, I.; Brzóska, M.M. The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients 2020, 12, 1282. https://doi.org/10.3390/nu12051282
Matwiejczuk N, Galicka A, Zaręba I, Brzóska MM. The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients. 2020; 12(5):1282. https://doi.org/10.3390/nu12051282
Chicago/Turabian StyleMatwiejczuk, Natalia, Anna Galicka, Ilona Zaręba, and Małgorzata M. Brzóska. 2020. "The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts" Nutrients 12, no. 5: 1282. https://doi.org/10.3390/nu12051282






