Grandmother’s Diet Matters: Early Life Programming with Sucrose Influences Metabolic and Lipid Parameters in Second Generation of Rats
Abstract
1. Introduction
2. Materials and Methods
3. Results
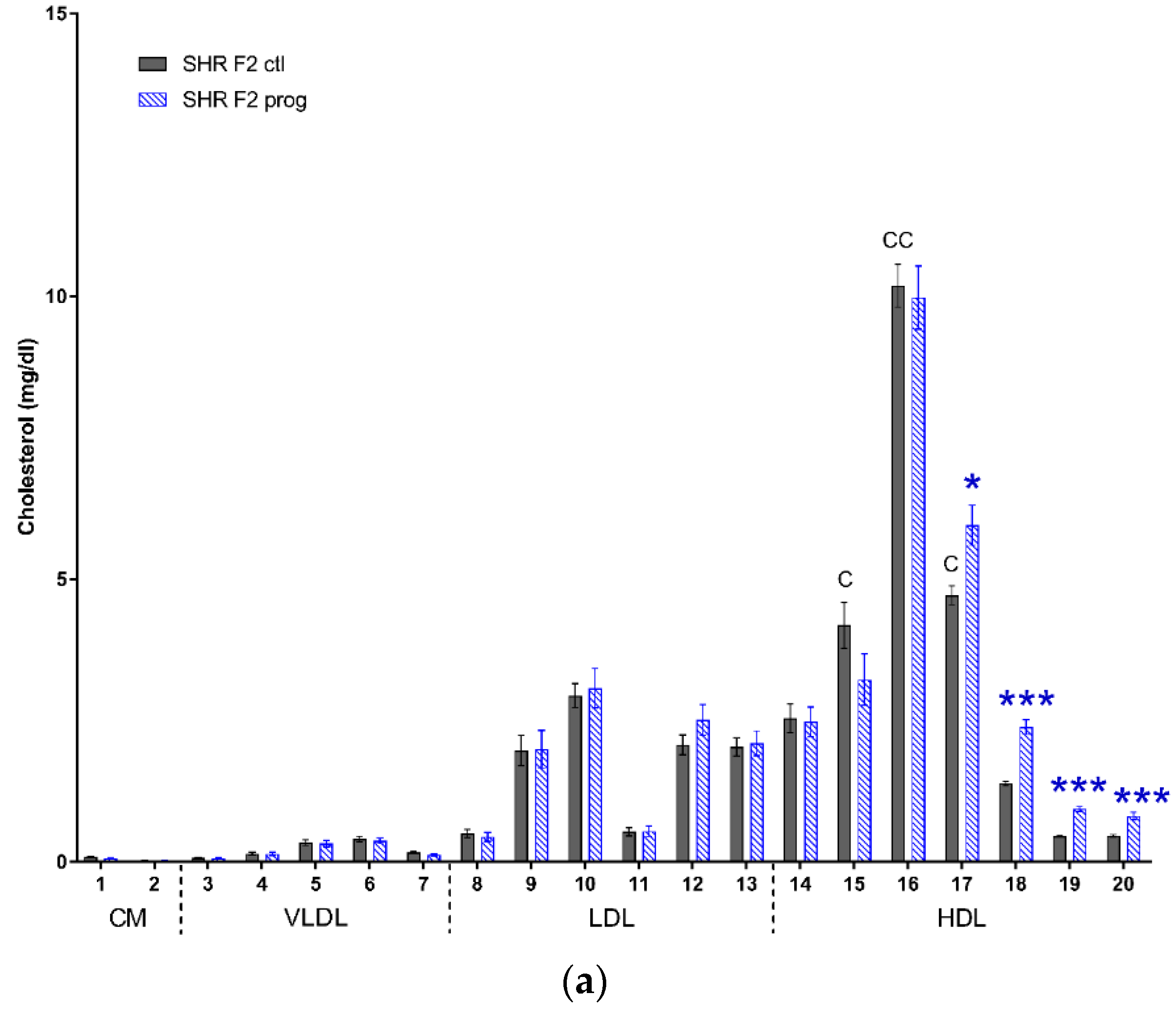
3.1. F1 Female Rats—Mothers
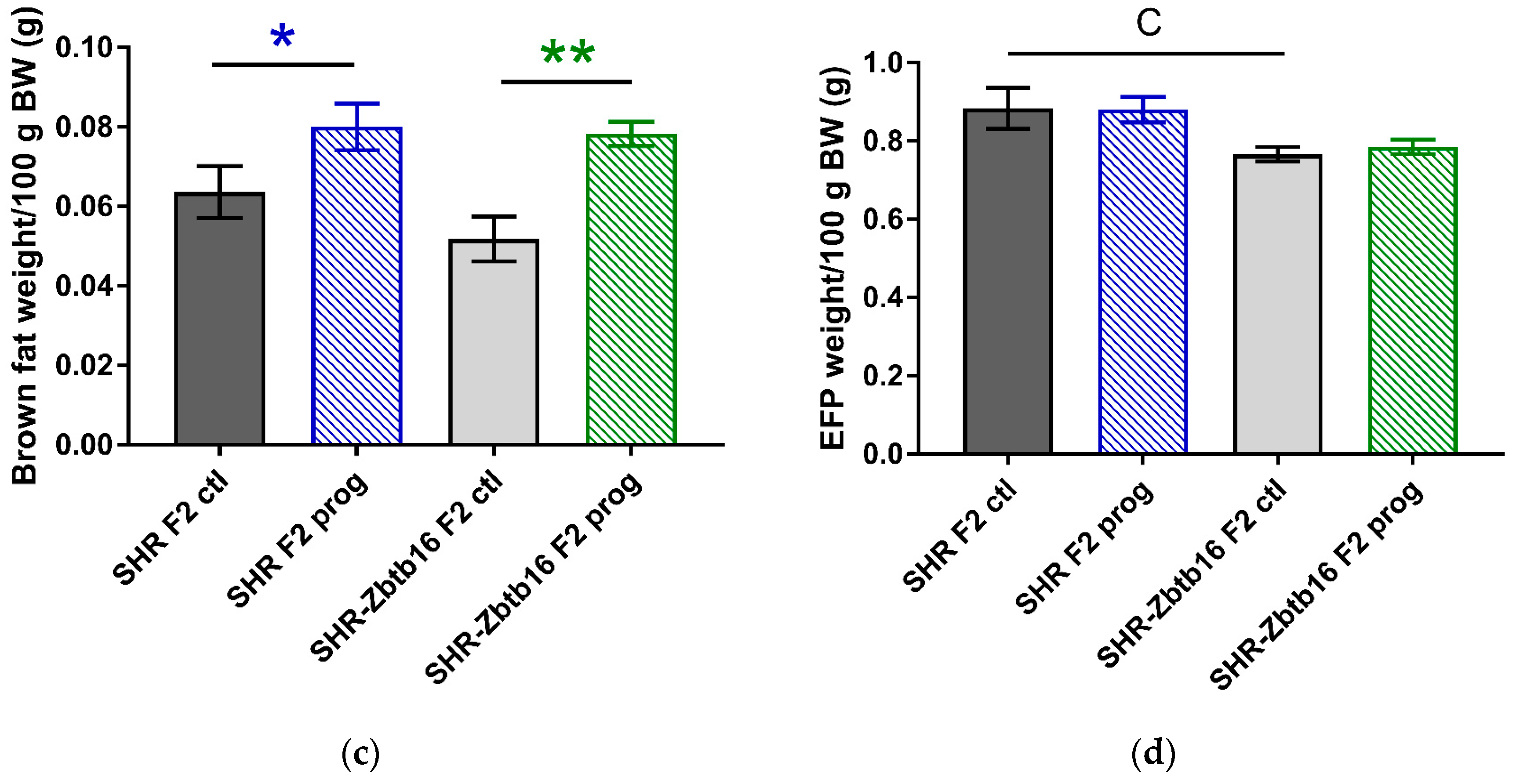
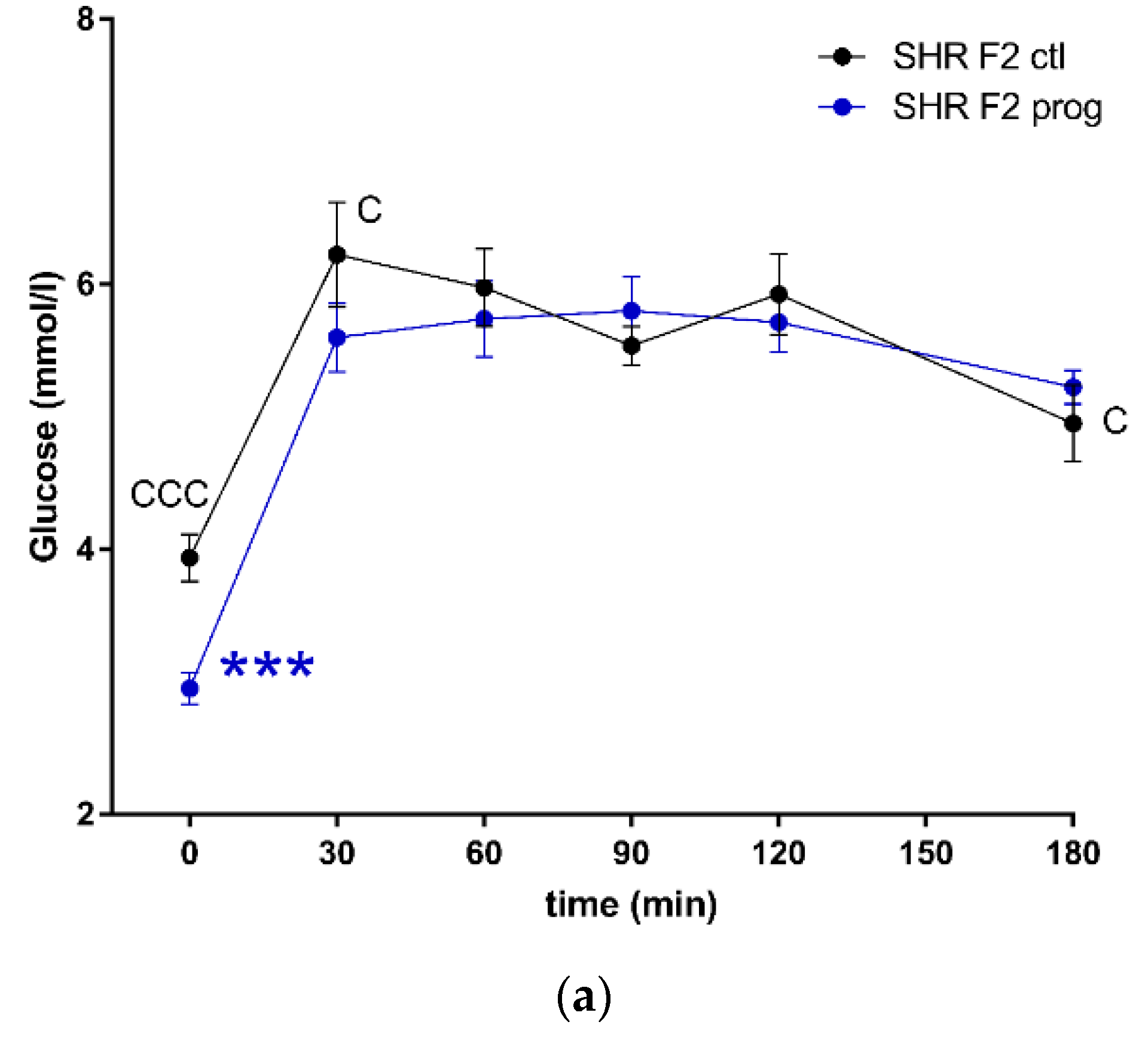
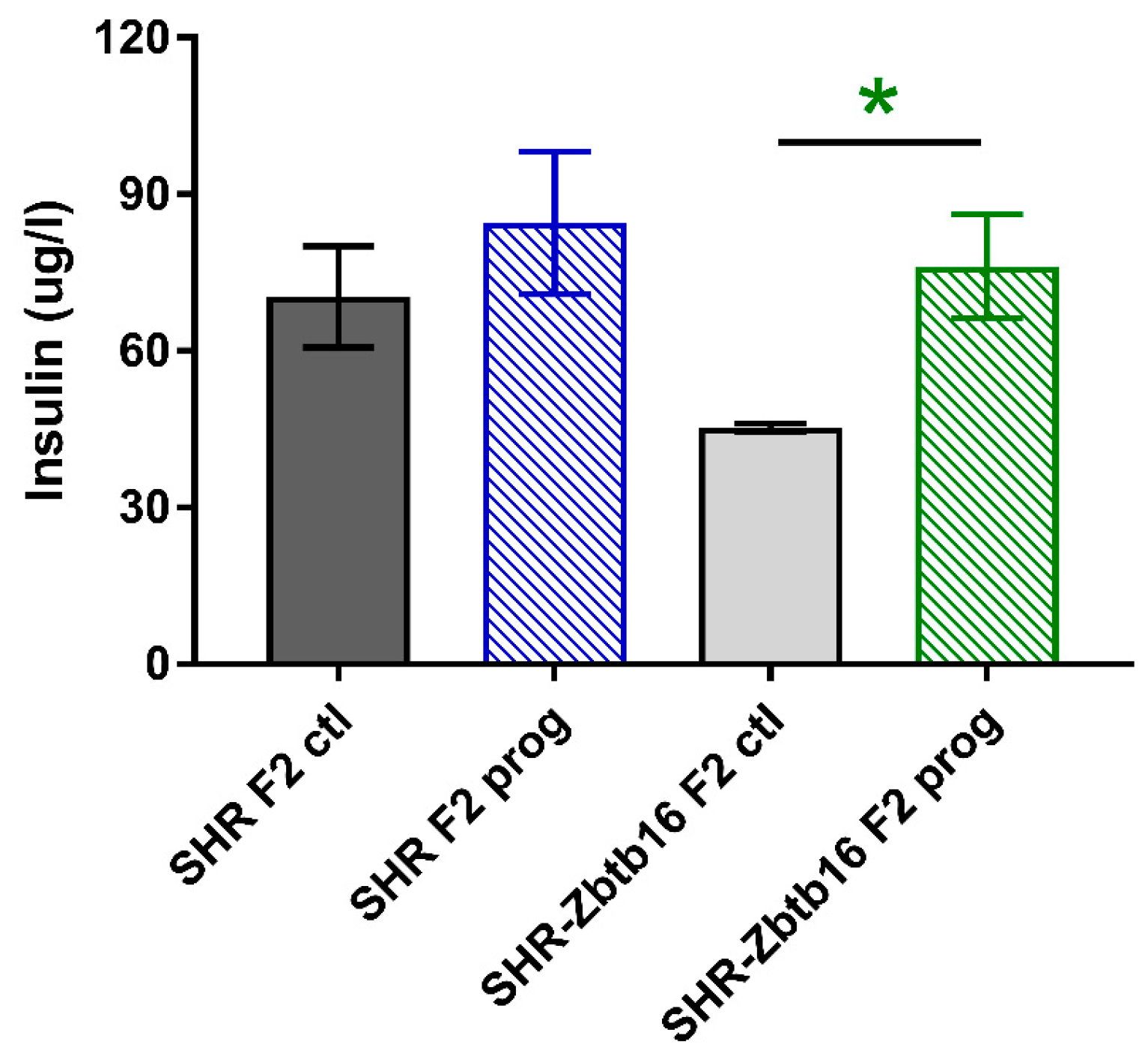
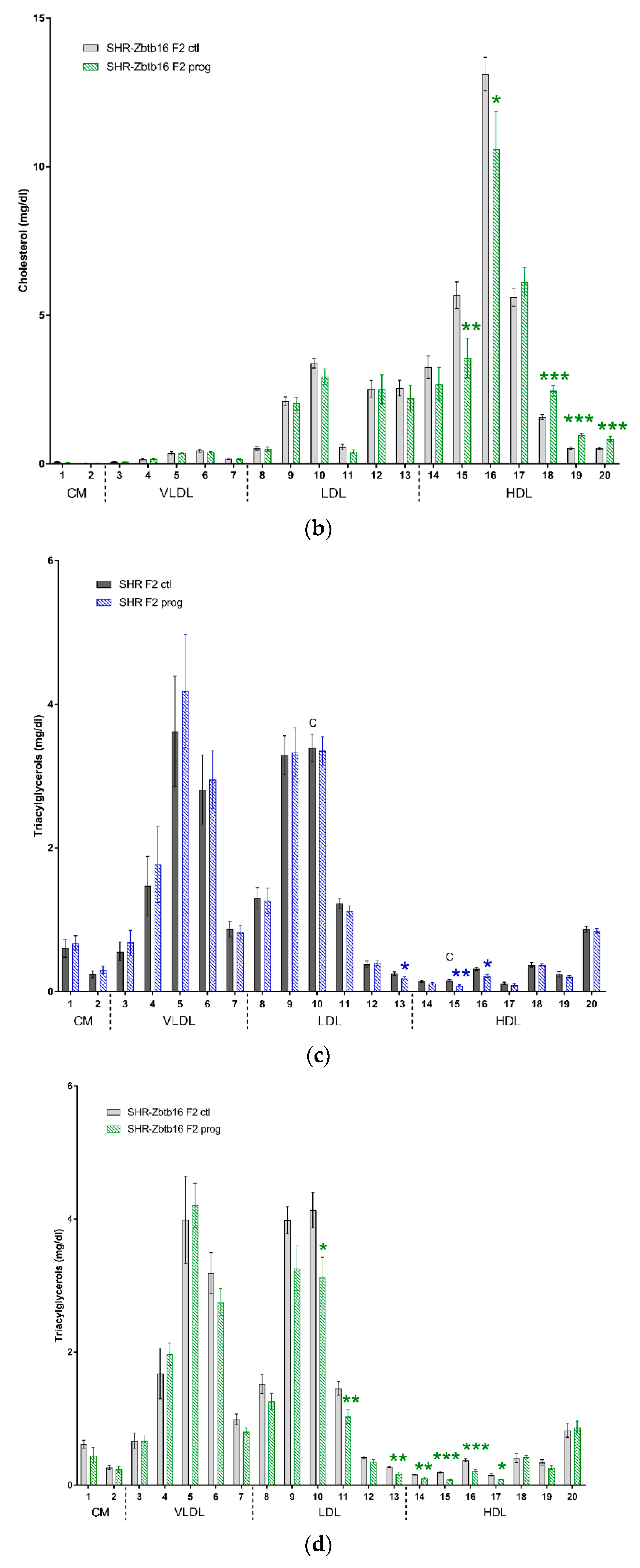
3.2. F2 Adult Male Offspring
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloomfield, F.H.; Harding, J.E. Experimental aspects of nutrition and fetal growth. Fetal Matern. Med. Rev. 1998, 10, 91–107. [Google Scholar] [CrossRef]
- Singal, R.; Ginder, G.D. DNA methylation. Blood 1999, 93, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Delage, B.; Dashwood, R.H. Dietary manipulation of histone structure and function. Annu. Rev. Nutr. 2008, 28, 347–366. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Skinner, M.K. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res. C Embryo Today 2011, 93, 51–55. [Google Scholar] [CrossRef]
- Skinner, M.K. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 2011, 6, 838–842. [Google Scholar]
- Skolnikova, E.; Sedova, L.; Krenova, D.; Kren, V.; Seda, O. Mutation in Zbtb16 gene plays a role in lipid profiles of pregnant rats and their offspring after high-Sucrose diet feeding. Atherosclerosis 2017, 263, E36–E37. [Google Scholar] [CrossRef]
- Warrington, N.M.; Beaumont, R.N.; Horikoshi, M.; Day, F.R.; Helgeland, Ø.; Laurin, C.; Bacelis, J.; Peng, S.; Hao, K.; Feenstra, B.; et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-Metabolic risk factors. Nat. Genet. 2019, 51, 804–814. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 2014 157, 95–109. [Google Scholar] [CrossRef]
- Skinner, M.K. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 2008, 25, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.Y.; Lönnerdal, B.; Philipps, A.F. Maternal zinc restriction affects postnatal growth and glucose homeostasis in rat offspring differently depending upon adequacy of their nutrient intake. Pediatr. Res. 2012, 71, 228e34. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Kurpad, A.V.; Duggan, C.P.; Bosch, R.J.; Dwarkanath, P.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Vaz, M.; Bhat, S.; et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur. J. Clin. Nutr. 2006, 60, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef]
- Ainge, H.; Thompson, C.; Ozanne, S.E.; Rooney, K.B. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int. J. Obes. 2011, 35, 325–335. [Google Scholar] [CrossRef]
- Bocarsly, M.E.; Barson, J.R.; Hauca, J.M.; Hoebel, B.G.; Leibowitz, S.F.; Avena, N.M. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol. Behav. 2012, 107, 568–575. [Google Scholar] [CrossRef]
- Ng, S.F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-Fat diet in fathers programs beta-Cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef]
- Šeda, O.; Šedová, L.; Včelák, J.; Vaňková, M.; Liška, F.; Bendlová, B. ZBTB16 and metabolic syndrome: A network perspective. Physiol. Res. 2017, 66, S357–S365. [Google Scholar] [CrossRef]
- Chen, S.; Qian, J.; Shi, X.; Gao, T.; Liang, T.; Liu, C. Control of hepatic gluconeogenesis by the promyelocytic leukemia zinc finger protein. Mol. Endocrinol. 2014, 28, 1987–1998. [Google Scholar] [CrossRef]
- Liška, F.; Landa, V.; Zídek, V.; Mlejnek, P.; Šilhavý, J.; Šimáková, M.; Strnad, H.; Trnovská, J.; Škop, V.; Kazdová, L. Downregulation of Plzf gene ameliorates metabolic and cardiac traits in the spontaneously hypertensive rat. Hypertension 2017, 69, 1084–1091. [Google Scholar] [CrossRef]
- Aitman, T.J.; Gotoda, T.; Evans, A.L.; Imrie, H.; Heath, K.E.; Trembling, P.M.; Truman, H.; Wallace, C.A.; Rahman, A.; Doré, C.; et al. Quantitative trait loci for cellular defects in glucose and fatty acid metabolism in hypertensive rats. Nat. Genet. 1997, 16, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Pravenec, M.; Křen, V.; Landa, V.; Mlejnek, P.; Musilová, A.; Šilhavý, J.; Šimáková, M.; Zídek, V. Recent progress in the genetics of spontaneously hypertensive rats. Physiol. Res. 2014, 63, S1–S8. [Google Scholar]
- Sedova, L.; Kazdova, L.; Seda, O.; Krenova, D.; Kren, V. Rat inbred PD/Cub strain as a model of dyslipidemia and insulin resistance. Folia Biol. (Praha) 2000, 46, 99–106. [Google Scholar] [PubMed]
- Seda, O.; Liska, F.; Sedova, L.; Kazdova, L.; Krenova, D.; Kren, V. A 14-Gene region of rat chromosome 8 in SHR-Derived polydactylous congenic substrain affects muscle-Specific insulin resistance, dyslipidaemia and visceral adiposity. Folia Biol.-Prague 2005, 51, 53–61. [Google Scholar]
- Krupková, M.; Liška, F.; Kazdová, L.; Šedová, L.; Kábelová, A.; Křenová, D.; Křen, V.; Šeda, O. Single-Gene Congenic Strain Reveals the Effect of Zbtb16 on Dexamethasone-Induced Insulin Resistance. Front. Endocrinol. (Lausanne) 2018, 9, 185. [Google Scholar] [CrossRef]
- Usui, S.; Hara, Y.; Hosaki, S.; Okazaki, M. A new on-Line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 2002, 43, 805–814. [Google Scholar]
- Heikkinen, S.; Argmann, C.A.; Champy, M.F.; Auwerx, J. Evaluation of glucose homeostasis. Curr. Protoc. Mol. Biol. 2007. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr. Res. 2004, 56, 311–317. [Google Scholar] [CrossRef]
- Kisioglu, B.; Nergiz-Unal, R. Potential effect of maternal dietary sucrose or fructose syrup on CD36, leptin, and ghrelin-Mediated fetal programming of obesity. Nutr. Neurosci. 2018. [Google Scholar] [CrossRef]
- Lowette, K.; Roosen, L.; Tack, J.; Vanden Berghe, P. Effects of high-fructose diets on central appetite signaling and cognitive function. Front. Nutr. 2015, 2, 5. [Google Scholar] [CrossRef]
- Tschritter, O.; Fritsche, A.; Shirkavand, F.; Machicao, F.; Häring, H.; Stumvoll, M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. The developmental origins of the metabolic syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Spencer, H.G. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 2005, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.J.; Barter, P.J. Role of triglyceride-Rich lipoproteins and hepatic lipase in determining the particle size and composition of high density lipoproteins. J. Lipid Res. 1986, 27, 1265–1277. [Google Scholar]
- Dumortier, O.; Roger, E.; Pisani, D.F.; Casamento, V.; Gautier, N.; Lebrun, P.; Johnston, H.; Lopez, P.; Amri, E.Z.; Jousse, C. Age-dependent control of energy homeostasis by brown adipose tissue in progeny subjected to maternal diet-Induced fetal programming. Diabetes 2017, 66, 627–639. [Google Scholar] [CrossRef]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr. 2016, 3, 10. [Google Scholar] [CrossRef]
- Liska, F.; Snajdr, P.; Sedova, L.; Seda, O.; Chylikova, B.; Slamova, P.; Krejci, E.; Sedmera, D.; Grim, M.; Krenova, D.; et al. Deletion of a conserved noncoding sequence in Plzf intron leads to Plzf down-Regulation in limb bud and polydactyly in the rat. Dev. Dyn. 2009, 238, 673–684. [Google Scholar] [CrossRef]
- Sedova, L.; Seda, O.; Kazdová, L.; Chylíková, B.; Hamet, P.; Tremblay, J.; Kren, V.; Krenová, D. Sucrose feeding during pregnancy and lactation elicits distinct metabolic response in offspring of an inbred genetic model of metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2017, 292, E1318–E1324. [Google Scholar] [CrossRef]

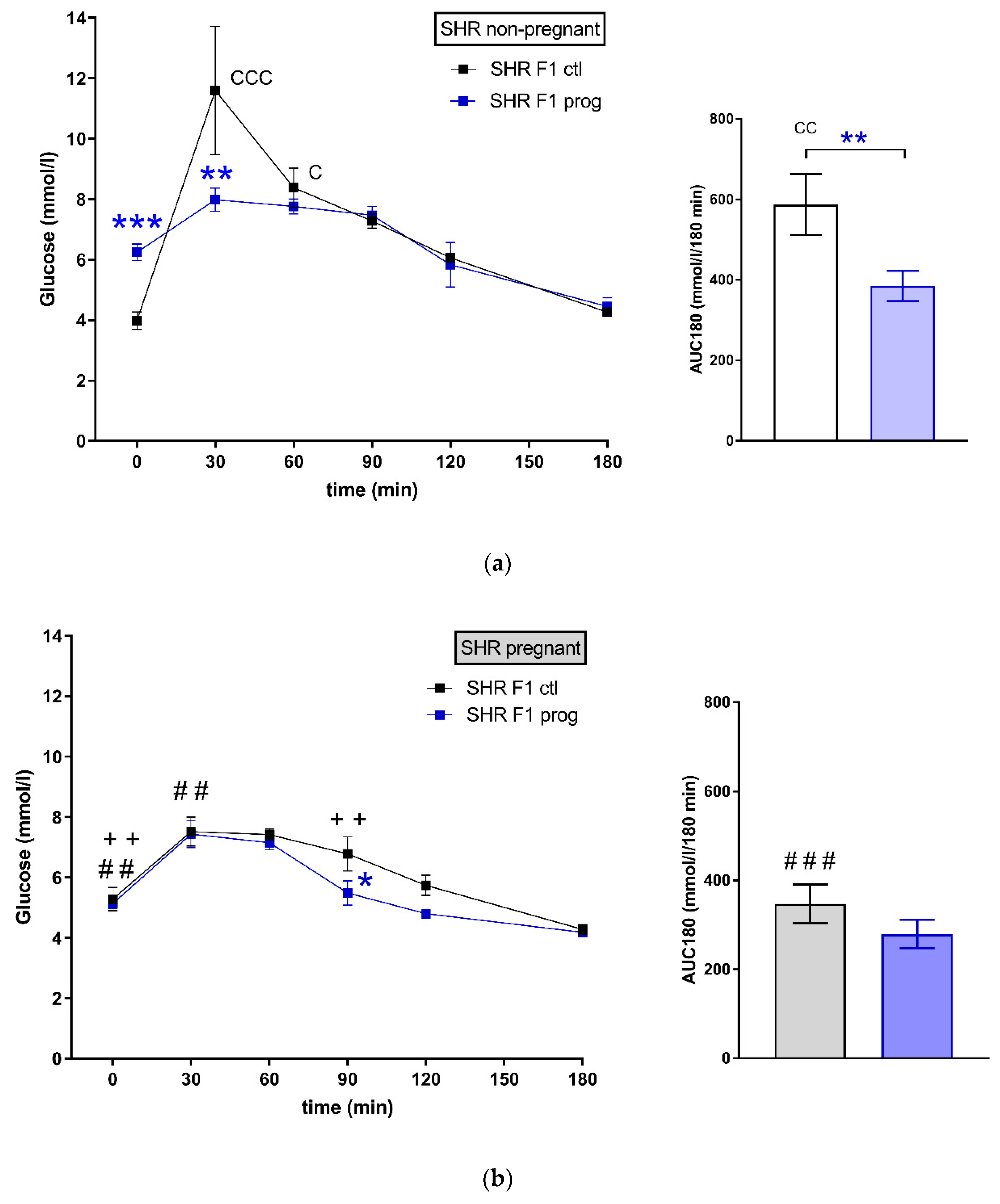

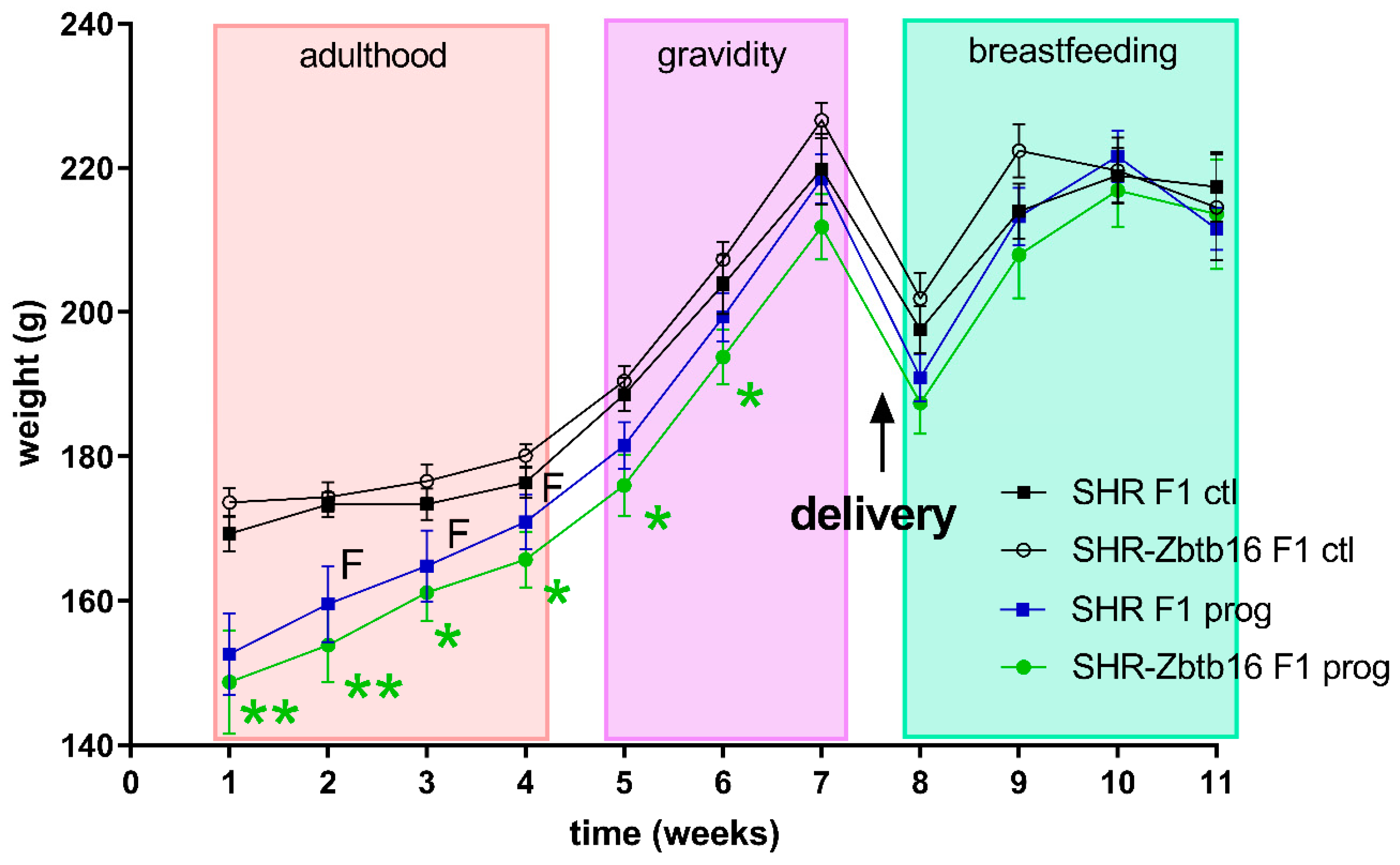
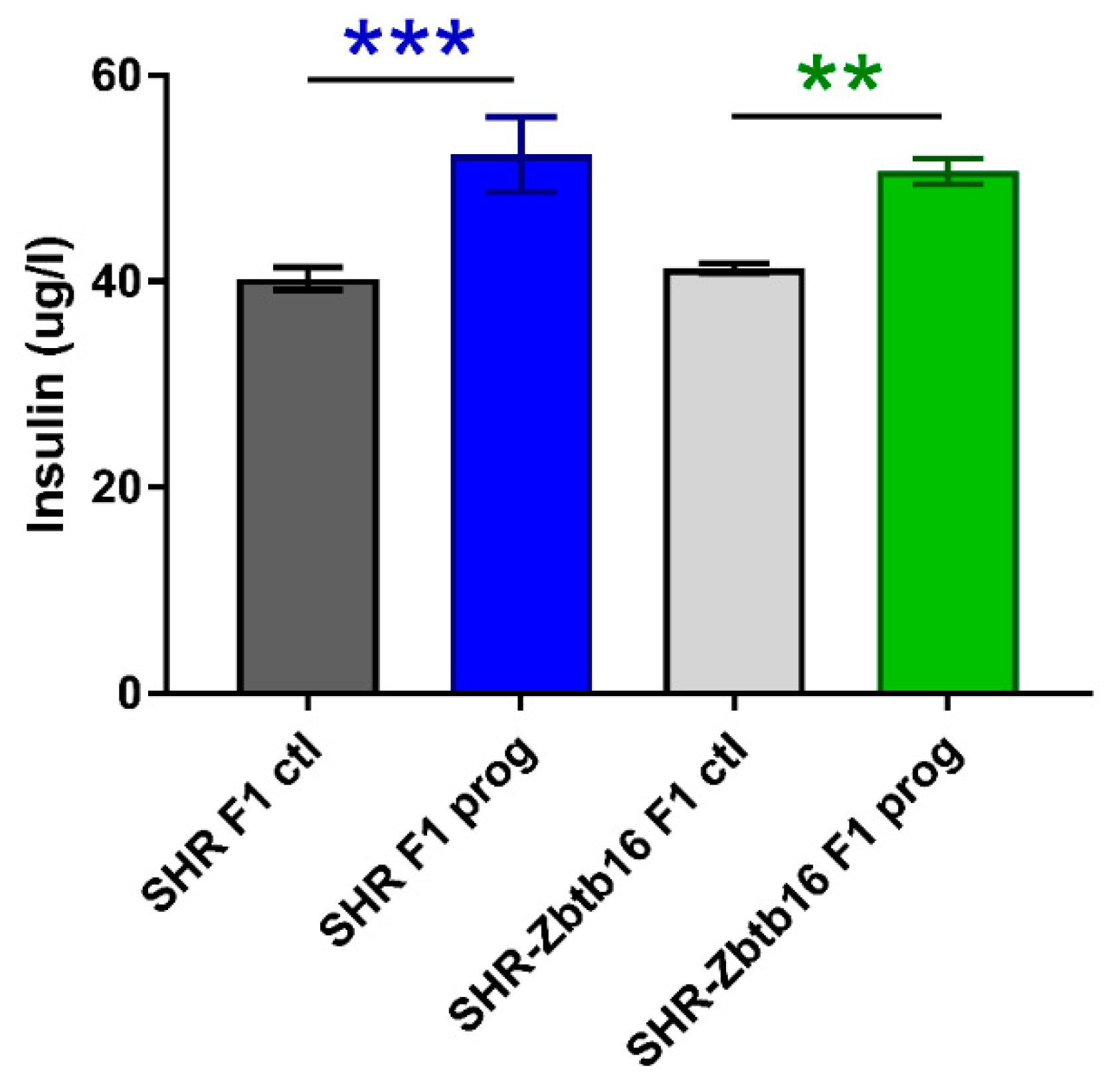
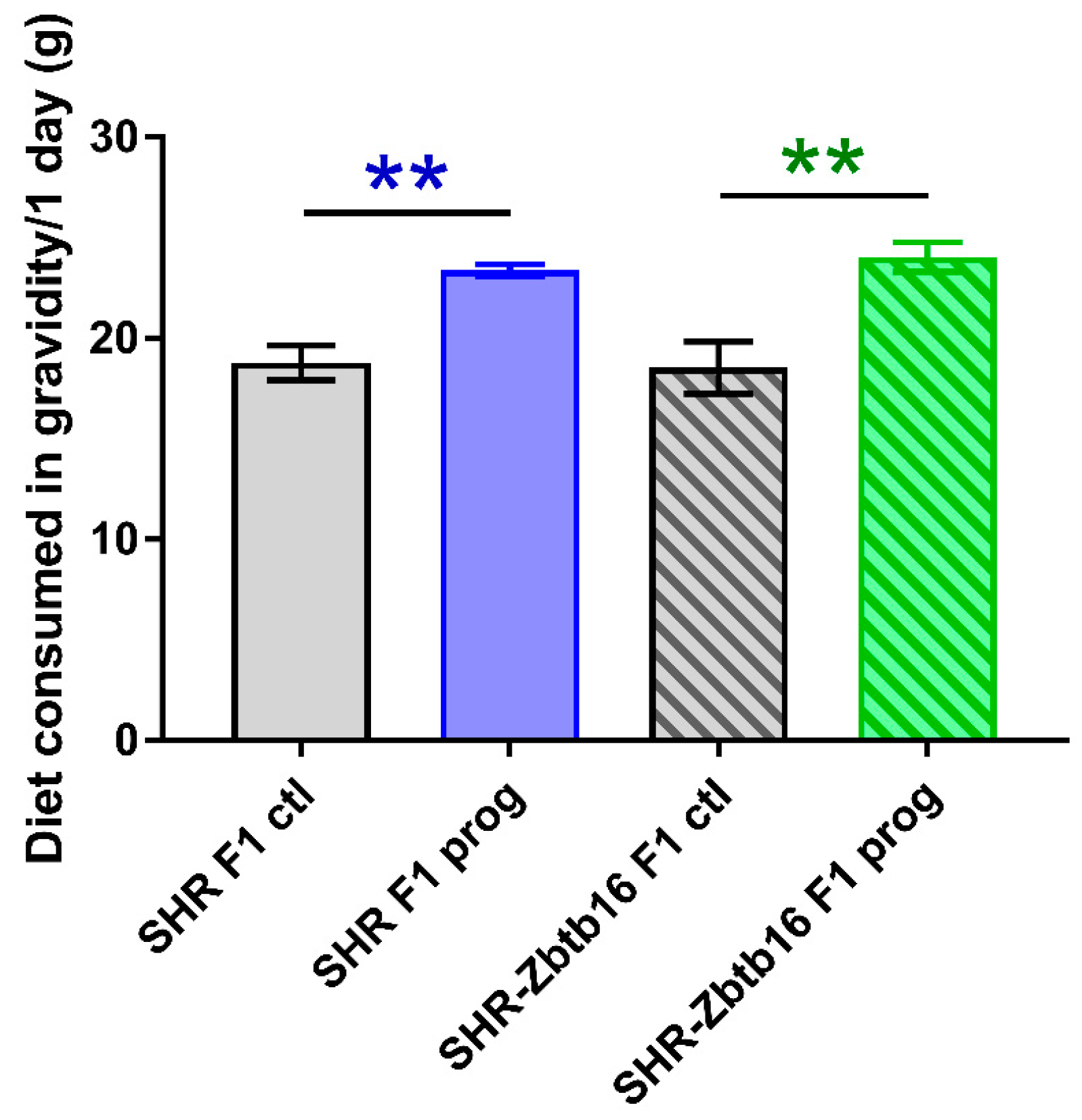


| AUC (mmol/L/180 min), mean ± SEM | ||
|---|---|---|
| Adulthood | Pregnancy | |
| F1 mothers | ||
| SHR F1 control | 587 ± 76 | 347 ± 44 |
| SHR-Zbtb16 F1 control | 383 ± 42 | 211 ± 43 |
| SHR F1 programmed | 385 ± 38 | 280 ± 32 |
| SHR-Zbtb16 F1 programmed | 345 ± 42 | 244 ± 36 |
| F2 male offspring | ||
| SHR F2 control | 372 ± 32 | |
| SHR-Zbtb16 F2 control | 458 ± 19 | |
| SHR F2 programmed | 379 ± 16 | |
| SHR-Zbtb16 F2 programmed | 356 ± 21 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Školníková, E.; Šedová, L.; Šeda, O. Grandmother’s Diet Matters: Early Life Programming with Sucrose Influences Metabolic and Lipid Parameters in Second Generation of Rats. Nutrients 2020, 12, 846. https://doi.org/10.3390/nu12030846
Školníková E, Šedová L, Šeda O. Grandmother’s Diet Matters: Early Life Programming with Sucrose Influences Metabolic and Lipid Parameters in Second Generation of Rats. Nutrients. 2020; 12(3):846. https://doi.org/10.3390/nu12030846
Chicago/Turabian StyleŠkolníková, Elena, Lucie Šedová, and Ondřej Šeda. 2020. "Grandmother’s Diet Matters: Early Life Programming with Sucrose Influences Metabolic and Lipid Parameters in Second Generation of Rats" Nutrients 12, no. 3: 846. https://doi.org/10.3390/nu12030846
APA StyleŠkolníková, E., Šedová, L., & Šeda, O. (2020). Grandmother’s Diet Matters: Early Life Programming with Sucrose Influences Metabolic and Lipid Parameters in Second Generation of Rats. Nutrients, 12(3), 846. https://doi.org/10.3390/nu12030846





