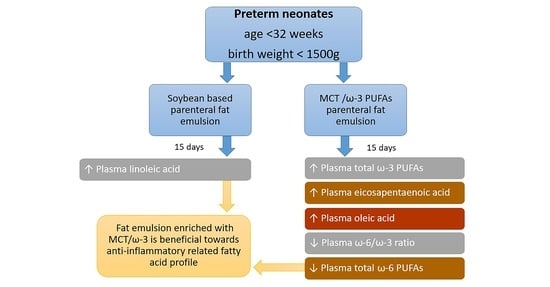
Administration of an Intravenous Fat Emulsion Enriched with Medium-Chain Triglyceride/ω-3 Fatty Acids is Beneficial Towards Anti-Inflammatory Related Fatty Acid Profile in Preterm Neonates: A Randomized, Double-Blind Clinical Trial
Abstract
1. Introduction
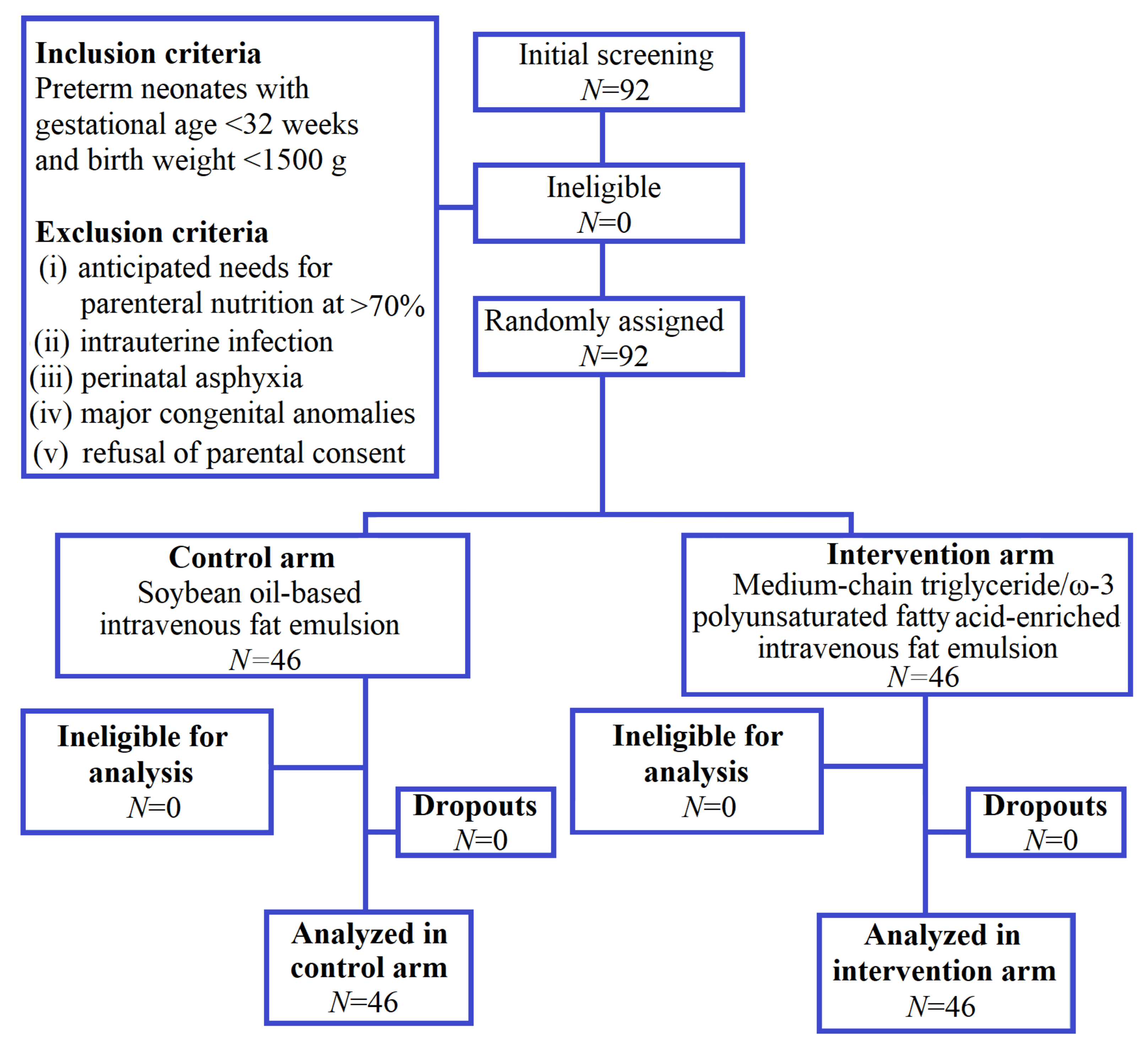
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Bioethics
2.4. Intervention Procedures
2.5. Clinical Data
2.6. Fatty Acid Assessment with Gas Chromatography
2.7. Serum Interleukin-6 Measurement
2.8. Randomization, Sequence Generation, and Implementation
2.9. Primary Outcomes and Sample Size
2.10. Statistical Analysis
3. Results
3.1. Dietary Intake
3.2. Plasma Fatty Acids
3.3. Polyunsaturated Fatty Acids
3.4. Monounsaturated Fatty Acids
3.5. Saturated Fatty Acids
3.6. Serum IL-6 Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, J.B.C.; Boskovic, D.; Angeles, D.M. The Energy Costs of Prematurity and the Neonatal Intensive Care Unit (NICU) Experience. Antioxidants 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Brown, L.D.; Georgieff, M.K. The Impact of Neonatal Illness on Nutritional Requirements: One Size Does Not Fit All. Curr. Pediatr. Rep. 2014, 2, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Das, A.; Wrage, L.A.; Poindexter, B.B.; Higgins, R.D.; Stoll, B.J.; Oh, W. Early Nutrition Mediates the Influence of Severity of Illness on Extremely Low Birth Weight Infants. Pediatr. Res. 2011, 69, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The Relationship of Poor Linear Growth Velocity with Neonatal Illness and Two-Year Neurodevelopment in Preterm Infants. Neonatology 2012, 102, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the Neonatal Intensive Care Unit Influences Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef]
- MacFie, J.; Smith, R.C.; Hill, G.L. Glucose or fat as a nonprotein energy source? A controlled clinical trial in gastroenterological patients requiring intravenous nutrition. Gastroenterology 1981, 80, 103–107. [Google Scholar] [CrossRef]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Sunehag, A.L. The Role of Parenteral Lipids in Supporting Gluconeogenesis in Very Premature Infants. Pediatr. Res. 2003, 54, 480–486. [Google Scholar] [CrossRef]
- Lapillonne, A.; Mis, N.F.; Goulet, O.; Akker, C.H.V.D.; Wu, J.; Koletzko, B.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Engler, M.M.; Engler, M.B. Omega-3 Fatty Acids: Role in Cardiovascular Health and Disease. J. Cardiovasc. Nurs. 2006, 21, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Périnat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M. Placental delivery of arachidonic and docosahexaenoic acids: Implications for the lipid nutrition of preterm infants. Am. J. Clin. Nutr. 2000, 71, 275S–284S. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Placental Regulation of Fatty Acid Delivery and its Effect on Fetal Growth—A Review. Placenta 2002, 23, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef]
- Lapillonne, A. Enteral and Parenteral Lipid Requirements of Preterm Infants. World Rev. Nutr. Diet 2014, 110, 82–98. [Google Scholar] [CrossRef]
- Crawford, M.A.; Costeloe, K.; Ghebremeskel, K.; Phylactos, A.; Skirvin, L.; Stacey, F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am. J. Clin. Nutr. 1997, 66, 1032S–1041S. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased Postnatal Docosahexaenoic and Arachidonic Acid Blood Levels in Premature Infants are Associated with Neonatal Morbidities. J. Pediatr. 2011, 159, 743–749. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef]
- Vlaardingerbroek, H.; Van Goudoever, J. Intravenous Lipids in Preterm Infants: Impact on Laboratory and Clinical Outcomes and Long-Term Consequences. World Rev. Nutr. Diet 2014, 112, 71–80. [Google Scholar] [CrossRef]
- Shoji, H.; Hisata, K.; Suzuki, M.; Yoshikawa, N.; Suganuma, H.; Ohkawa, N.; Shimizu, T. Effects of parenteral soybean oil lipid emulsion on the long-chain polyunsaturated fatty acid profile in very-low-birth-weight infants. Acta Paediatr. 2011, 100, 972–976. [Google Scholar] [CrossRef]
- Lyons, J.L.; Tovar-Y-Romo, L.B.; Thakur, K.T.; McArthur, J.C.; Haughey, N.J. Pathobiology of CNS human immunodeficiency virus infection. In Neurobiology of Brain Disorders, 1st ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 444–466. [Google Scholar]
- Malmsten, C.L. Prostaglandins, thromboxanes, and leukotrienes in inflammation. Am. J. Med. 1986, 80, 11–17. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Shapiro, H.; Theilla, M.; Anbar, R.; Singer, J.; Cohen, J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: Novel mechanisms and an integrative perspective. Intensiv. Care Med. 2008, 34, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- Rodri’guez-Palmero, M.; López-Sabater, M.; Castellote, A.; De La Torre-Boronat, M.; Rivero-Urgell, M. Comparison of two methods for the determination of fatty acid profiles in plasma and erythrocytes. J. Chromatogr. A 1997, 778, 435–439. [Google Scholar] [CrossRef]
- Skouroliakou, M.; Konstantinou, D.; Agakidis, C.; Kaliora, A.; Kalogeropoulos, N.; Massara, P.; Antoniadi, M.; Panagiotakos, D.; Karagiozoglou-Lampoudi, T. Parenteral MCT/ω-3 Polyunsaturated Fatty Acid–Enriched Intravenous Fat Emulsion is Associated with Cytokine and Fatty Acid Profiles Consistent with Attenuated Inflammatory Response in Preterm Neonates. Nutr. Clin. Pr. 2015, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Jensen, G.L.; Koletzko, B.V.; Singer, P.; Wanten, G.J.A. Lipid emulsions in parenteral nutrition of intensive care patients: Current thinking and future directions. Intensiv. Care Med. 2010, 36, 735–749. [Google Scholar] [CrossRef]
- Krohn, K.; Koletzko, B. Parenteral lipid emulsions in paediatrics. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 319–323. [Google Scholar] [CrossRef]
- Goulet, O.J.; Cai, W.; Seo, J. Lipid Emulsion Use in Pediatric Patients Requiring Long-Term Parenteral Nutrition. J. Parenter. Enter. Nutr. 2020, 44, S55–S67. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Malviya, M.N.; Soll, R.F. Lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst. Rev. 2019, 6, CD013163. [Google Scholar] [CrossRef] [PubMed]
- Rostas, S.E.; McPherson, C. Intravenous Lipid Emulsions in Infants: Is Balanced Better? Neonatal Netw. 2019, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gura, K.M.; Duggan, C.P.; Collier, S.B.; Jennings, R.W.; Folkman, J.; Bistrian, B.R.; Puder, M. Reversal of Parenteral Nutrition-Associated Liver Disease in Two Infants with Short Bowel Syndrome Using Parenteral Fish Oil: Implications for Future Management. Pediatrics 2006, 118. [Google Scholar] [CrossRef] [PubMed]
- Gura, K.M.; Lee, S.; Valim, C.; Zhou, J.; Kim, S.; Modi, B.P.; Arsenault, D.A.; Strijbosch, R.A.M.; Lopes, S.; Duggan, C.; et al. Safety and Efficacy of a Fish-Oil-Based Fat Emulsion in the Treatment of Parenteral Nutrition-Associated Liver Disease. Pediatrics 2008, 121, e678–e686. [Google Scholar] [CrossRef]
- Cheung, H.M.; Lam, H.S.; Tam, Y.H.; Lee, K.H.; Ng, P.C. Rescue treatment of infants with intestinal failure and parenteral nutrition-associated cholestasis (PNAC) using a parenteral fish-oil-based lipid. Clin. Nutr. 2009, 28, 209–212. [Google Scholar] [CrossRef]
- Puder, M.; Valim, C.; Meisel, J.A.; Le, H.D.; De Meijer, V.E.; Robinson, E.M.; Zhou, J.; Duggan, C.; Gura, K.M. Parenteral Fish Oil Improves Outcomes in Patients With Parenteral Nutrition-Associated Liver Injury. Trans. Meet. Am. Surg. Assoc. 2009, 127, 39–46. [Google Scholar] [CrossRef]
- Wanten, G.; Beunk, J.; Naber, A.; Swinkels, D. Tocopherol isoforms in parenteral lipid emulsions and neutrophil activation. Clin. Nutr. 2002, 21, 417–422. [Google Scholar] [CrossRef]
- Abdelkareem, M.; Wahba, Y.; Shouman, B.; Mesbah, A. Comparison of Soybean-based Oil and MCT-olive-fish-soy Oil Intravenous Lipid Emulsions on Soluble Adhesion Markers in Preterm Neonates with Sepsis: A Randomized Controlled Trial. Indian Pediatr. 2019, 56, 841–844. [Google Scholar] [CrossRef]
- Özkan, H.; Koksal, N.; Dorum, B.A.; Kocael, F.; Ozarda, Y.; Bozyigit, C.; Dogan, P.; Varal, I.G.; Bagci, O. New-generation fish oil and olive oil lipid for prevention of oxidative damage in preterm infants: Single center clinical trial at university hospital in Turkey. Pediatr. Int. 2019, 61, 388–392. [Google Scholar] [CrossRef]
- Vlaardingerbroek, H.; Vermeulen, M.J.; Carnielli, V.P.; Vaz, F.M.; Akker, C.H.P.V.D.; Van Goudoever, J.B. Growth and Fatty Acid Profiles of VLBW Infants Receiving a Multicomponent Lipid Emulsion From Birth. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Rayyan, M.; Devlieger, H.; Jochum, P.D.F.; Allegaert, K. Short-Term Use of Parenteral Nutrition with a Lipid Emulsion Containing a Mixture of Soybean Oil, Olive Oil, Medium-Chain Triglycerides, and Fish Oil. J. Parenter. Enter. Nutr. 2012, 36, 81S–94S. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, R.; Savini, S.; Biagetti, C.; Bellagamba, M.P.; Marchionni, P.; Pompilio, A.; Cogo, P.E.; Carnielli, V.P. Higher Docosahexaenoic acid, lower Arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: A randomized clinical trial. Clin. Nutr. 2014, 33, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
| Parameters | n | Control Group (SO–IVFE) | n | Intervention Group (MCT/ω-3 PUFA-IVFE) | p |
|---|---|---|---|---|---|
| N | 46 | - | 46 | - | - |
| Female | 19 | - | 19 | - | - |
| Male | 27 | - | 27 | - | - |
| Gestational age (weeks) | 46 | 29.76 ± 2.17 | 46 | 29.10 ± 1.83 | 0.126 |
| Birth weight (g) | 46 | 1223.04 ± 215.42 | 46 | 1222.74 ± 211.90 | 0.995 |
| Serum IL-6 (pg/mL) | 29 | 19.46 ± 12.24 | 30 | 17.98 ± 15.46 | 0.383 |
| Fatty acids (% w/w) | |||||
| C14:0 | 45 | 0.65 ± 0.16 | 46 | 0.68 ± 0.11 | 0.350 |
| C15:0 | 40 | 0.26 ± 0.13 | 45 | 0.25 ± 0.17 | 0.142 |
| C16:0 | 46 | 26.52 ± 2.46 | 46 | 25.65 ± 2.08 | 0.070 |
| C16:1 ω-9 | 46 | 0.76 ± 0.20 | 46 | 0.79 ± 0.19 | 0.507 |
| C16:1 ω-7 | 46 | 3.38 ± 1.17 | 46 | 3.47 ± 1.14 | 0.888 |
| C17:0 | 46 | 0.25 ± 0.10 | 46 | 0.26 ± 0.11 | 0.592 |
| C17:1 | 41 | 0.21 ± 0.21 | 45 | 0.19 ± 0.24 | 0.343 |
| C18:0 | 46 | 9.93 ± 1.92 | 46 | 9.60 ± 1.90 | 0.323 |
| C18:1 ω-9 | 46 | 19.75 ± 3.61 | 46 | 20.36 ± 3.03 | 0.385 |
| C18:1 ω-7 | 46 | 2.75 ± 0.60 | 46 | 2.82 ± 0.58 | 0.579 |
| C18:2 ω-6 | 46 | 8.73 ± 4.90 | 46 | 8.51 ± 2.96 | 0.717 |
| C18:3 ω-6 | 45 | 0.43 ± 0.22 | 46 | 0.42 ± 0.23 | 0.533 |
| C18:3 ω-3 | 40 | 0.33 ± 0.55 | 46 | 0.33 ± 0.26 | 0.134 |
| C20:0 | 46 | 0.45 ± 0.15 | 46 | 0.43 ± 0.17 | 0.520 |
| C20:1 ω-9 | 40 | 0.16 ± 0.15 | 45 | 0.19 ± 0.18 | 0.251 |
| C20:2 ω-6 | 46 | 0.49 ± 0.34 | 46 | 0.46 ± 0.33 | 0.614 |
| C20:4 ω-6 | 46 | 10.36 ± 2.73 | 46 | 9.79 ± 2.81 | 0.329 |
| C20:5 ω-3 | 46 | 0.24 ± 0.25 | 46 | 0.30 ± 0.50 | 0.529 |
| C23:0 | 40 | 0.30 ± 0.30 | 44 | 0.48 ± 0.50 | 0.144 |
| C24:0 | 46 | 0.62 ± 0.12 | 46 | 0.57 ± 0.14 | 0.090 |
| C22:4 ω-6 | 45 | 0.35 ± 0.11 | 46 | 0.32 ± 0.09 | 0.130 |
| C22:5 ω-6 | 45 | 0.29 ± 0.23 | 46 | 0.32 ± 0.34 | 0.640 |
| C24:1 ω-9 | 46 | 1.41 ± 0.39 | 46 | 1.49 ± 0.35 | 0.303 |
| C22:6 ω-3 | 46 | 3.24 ± 3.67 | 46 | 3.22 ± 3.28 | 0.970 |
| SFA | 46 | 39.42 ± 4.60 | 46 | 38.70 ± 3.94 | 0.420 |
| MUFA | 46 | 28.81 ± 4.22 | 46 | 30.00 ± 4.20 | 0.179 |
| PUFA | 46 | 25.97 ± 4.64 | 46 | 25.45 ± 3.16 | 0.526 |
| ω-6 | 46 | 23.04 ± 4.57 | 46 | 21.78 ± 3.08 | 0.124 |
| ω-3 | 46 | 2.94 ± 0.78 | 46 | 3.22 ± 0.80 | 0.120 |
| ω-6/ω-3 ratio | 46 | 7.72 ± 2.31 | 46 | 7.28 ± 2.47 | 0.226 |
| Dietary Intake | Group | Day 0 | Day 15 | p | * p |
|---|---|---|---|---|---|
| PN energy, kcal/kg/day | Intervention (n=46) | 51.69 ± 17.03 | 54.59 ± 28.43 | 0.591 | 0.551 |
| Control (n = 46) | 51.47 ± 16.26 | 58.22 ± 23.11 | 0.086 | ||
| Milk energy, kcal/kg/day | Intervention (n = 46) | 0.00 ± 0.00 | 48.09 ± 34.32 | 0.000 | 0.693 |
| Control (n = 46) | 0.00 ± 0.00 | 43.85 ± 34.16 | 0.000 | ||
| Total energy, kcal/kg/day | Intervention (n = 46) | 51.69 ± 17.03 | 102.69 ± 9.46 | 0.000 | 0.944 |
| Control (n = 46) | 51.47 ± 16.26 | 101.59 ± 18.30 | 0.000 |
| Fatty Acids (% w/w) | n | Control Group (SO-IVFE) | n | Intervention Group (MCT/ω-3 PUFAs-IVFE) | * p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 15 | p | Day 0 | Day 15 | p | ||||
| C14:0 | 45 | 0.65 ± 0.16 | 0.97 ± 0.45 | 0.000 | 46 | 0.68 ± 0.11 | 0.86 ± 0.28 | 0.001 | 0.128 |
| C15:0 | 40 | 0.27 ± 0.13 | 0.17 ± 0.10 | 0.000 | 45 | 0.25 ± 0.17 | 0.17 ± 0.10 | 0.000 | 0.641 |
| C16:0 | 46 | 26.52 ± 2.46 | 23.87 ± 2.31 | 0.000 | 46 | 25.65 ± 2.08 | 23.60 ± 2.30 | 0.000 | 0.265 |
| C16:1 ω-9 | 46 | 0.76 ± 0.21 | 0.50 ± 0.14 | 0.000 | 46 | 0.79 ± 0.19 | 0.52 ± 0.11 | 0.000 | 0.944 |
| C16:1 ω-7 | 46 | 3.38 ± 1.17 | 3.22 ± 1.61 | 0.175 | 46 | 3.47 ± 1.14 | 3.08 ± 1.12 | 0.052 | 0.901 |
| C17:0 | 46 | 0.25 ± 0.10 | 0.20 ± 0.08 | 0.000 | 46 | 0.26 ± 0.11 | 0.19 ± 0.05 | 0.000 | 0.650 |
| C17:1 | 41 | 0.21 ± 0.21 | 0.17 ± 0.14 | 0.083 | 44 | 0.19 ± 0.24 | 0.11 ± 0.11 | 0.013 | 0.703 |
| C18:0 | 46 | 9.93 ± 1.92 | 9.32 ± 1.79 | 0.014 | 46 | 9.60 ± 1.90 | 9.29 ± 1.88 | 0.289 | 0.337 |
| C18:1 ω-9 | 46 | 19.75 ± 3.61 | 19.84 ± 2.52 | 0.933 | 46 | 20.36 ± 3.03 | 23.02 ± 2.86 | 0.000 | 0.003 |
| C18:1 ω-7 | 46 | 2.75 ± 0.60 | 2.29 ± 0.47 | 0.000 | 46 | 2.82 ± 0.58 | 2.21 ± 0.30 | 0.000 | 0.266 |
| C18:2 ω-6 | 46 | 8.73 ± 4.90 | 17.98 ± 4.35 | 0.000 | 46 | 8.51 ± 2.96 | 16.07 ± 2.70 | 0.000 | 0.006 |
| C18:3 ω-6 | 45 | 0.43 ± 0.22 | 0.57 ± 0.32 | 0.040 | 46 | 0.42 ± 0.23 | 0.44 ± 0.17 | 0.375 | 0.231 |
| C18:3 ω-3 | 40 | 0.33 ± 0.55 | 0.56 ± 0.39 | 0.000 | 46 | 0.33 ± 0.26 | 0.38 ± 0.19 | 0.030 | 0.006 |
| C20:0 | 46 | 0.45 ± 0.15 | 0.36 ± 0.17 | 0.001 | 46 | 0.43 ± 0.17 | 0.43 ± 0.26 | 0.850 | 0.059 |
| C20:1 ω-9 | 40 | 0.15 ± 0.14 | 0.16 ± 0.10 | 0.512 | 45 | 0.19 ± 0.18 | 0.18 ± 0.14 | 0.350 | 0.406 |
| C20:2 ω-6 | 46 | 0.49 ± 0.34 | 0.43 ± 0.23 | 0.288 | 46 | 0.46 ± 0.33 | 0.36 ± 0.20 | 0.029 | 0.417 |
| C20:4 ω-6 | 46 | 10.36 ± 2.73 | 6.46 ± 1.91 | 0.000 | 46 | 9.79 ± 2.81 | 5.16 ± 1.45 | 0.000 | 0.204 |
| C20:5 ω-3 | 46 | 0.24 ± 0.25 | 0.39 ± 0.26 | 0.001 | 46 | 0.30 ± 0.50 | 0.91 ± 0.58 | 0.000 | 0.000 |
| C23:0 | 40 | 0.28 ± 0.29 | 0.29 ± 0.33 | 0.421 | 44 | 0.48 ± 0.50 | 0.33 ± 0.35 | 0.319 | 0.053 |
| C24:0 | 46 | 0.62 ± 0.12 | 0.50 ± 0.19 | 0.001 | 46 | 0.57 ± 0.14 | 0.44 ± 0.11 | 0.000 | 0.585 |
| C22:4 ω-6 | 45 | 0.32 ± 0.10 | 0.24 ± 0.11 | 0.000 | 46 | 0.35 ± 0.11 | 0.23 ± 0.15 | 0.000 | 0.004 |
| C22:5 ω-6 | 45 | 0.30 ± 0.24 | 0.18 ± 0.23 | 0.000 | 46 | 0.32 ± 0.34 | 0.18 ± 0.29 | 0.000 | 0.927 |
| C24:1 ω-9 | 46 | 1.41 ± 0.39 | 1.13 ± 0.31 | 0.000 | 46 | 1.49 ± 0.35 | 1.34 ± 0.28 | 0.001 | 0.088 |
| C22:6 ω-3 | 46 | 3.24 ± 3.67 | 2.28 ± 2.35 | 0.000 | 46 | 3.22 ± 3.28 | 2.45 ± 2.05 | 0.001 | 0.204 |
| SFA | 46 | 39.42 ± 4.60 | 36.28 ± 4.60 | 0.000 | 46 | 38.70 ± 3.94 | 36.29 ± 4.76 | 0.000 | 0.349 |
| MUFA | 46 | 28.81 ± 4.22 | 28.12 ± 4.14 | 0.390 | 46 | 30.00 ± 4.20 | 30.39 ± 3.29 | 0.575 | 0.308 |
| PUFA | 46 | 25.97 ± 4.64 | 30.70 ± 3.64 | 0.000 | 46 | 25.45 ± 3.16 | 28.45 ± 3.11 | 0.000 | 0.099 |
| ω-6 | 46 | 23.04 ± 4.57 | 28.03 ± 3.55 | 0.000 | 46 | 21.78 ± 3.08 | 24.51 ± 3.03 | 0.000 | 0.023 |
| ω-3 | 46 | 2.94 ± 0.78 | 2.70 ± 0.98 | 0.131 | 46 | 3.22 ± 0.80 | 3.61 ± 1.51 | 0.070 | 0.031 |
| ω-6/ω-3 ratio | 46 | 7.72 ± 2.31 | 12.66 ± 5.20 | 0.000 | 46 | 7.28 ± 2.47 | 8.07 ± 4.58 | 0.202 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, P.; Gioxari, A.; Ntountaniotis, D.; Korda, O.-N.; Skouroliakou, M.; Siahanidou, T. Administration of an Intravenous Fat Emulsion Enriched with Medium-Chain Triglyceride/ω-3 Fatty Acids is Beneficial Towards Anti-Inflammatory Related Fatty Acid Profile in Preterm Neonates: A Randomized, Double-Blind Clinical Trial. Nutrients 2020, 12, 3526. https://doi.org/10.3390/nu12113526
Papandreou P, Gioxari A, Ntountaniotis D, Korda O-N, Skouroliakou M, Siahanidou T. Administration of an Intravenous Fat Emulsion Enriched with Medium-Chain Triglyceride/ω-3 Fatty Acids is Beneficial Towards Anti-Inflammatory Related Fatty Acid Profile in Preterm Neonates: A Randomized, Double-Blind Clinical Trial. Nutrients. 2020; 12(11):3526. https://doi.org/10.3390/nu12113526
Chicago/Turabian StylePapandreou, Panos, Aristea Gioxari, Dimitrios Ntountaniotis, Olga-Natalia Korda, Maria Skouroliakou, and Tania Siahanidou. 2020. "Administration of an Intravenous Fat Emulsion Enriched with Medium-Chain Triglyceride/ω-3 Fatty Acids is Beneficial Towards Anti-Inflammatory Related Fatty Acid Profile in Preterm Neonates: A Randomized, Double-Blind Clinical Trial" Nutrients 12, no. 11: 3526. https://doi.org/10.3390/nu12113526
APA StylePapandreou, P., Gioxari, A., Ntountaniotis, D., Korda, O.-N., Skouroliakou, M., & Siahanidou, T. (2020). Administration of an Intravenous Fat Emulsion Enriched with Medium-Chain Triglyceride/ω-3 Fatty Acids is Beneficial Towards Anti-Inflammatory Related Fatty Acid Profile in Preterm Neonates: A Randomized, Double-Blind Clinical Trial. Nutrients, 12(11), 3526. https://doi.org/10.3390/nu12113526