Protein Intake at Twice the RDA in Older Men Increases Circulatory Concentrations of the Microbiome Metabolite Trimethylamine-N-Oxide (TMAO)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Procedures
2.4. Dietary Analysis
2.5. Sample Collection and Analysis
2.6. OGTT
2.7. Anthropometry
2.8. Calculations
2.9. Statistical Analysis
3. Results
3.1. Dietary Intake
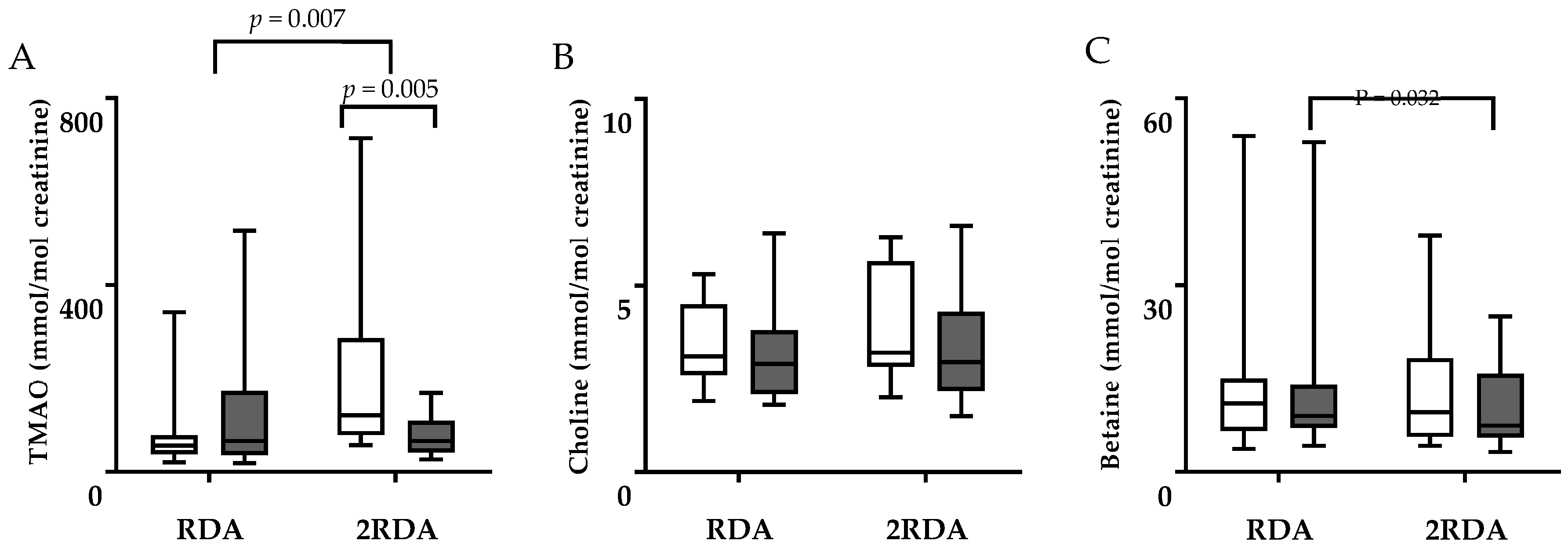
3.2. TMAO, Choline, Betaine, and Carnitine
3.3. Lipids and Lipoproteins
3.4. Metabolic and Inflammatory Biomarkers
3.5. Body Composition and Blood Pressure
3.6. Creatinine and Creatinine Clearance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- WHO. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- USDA. 2015–2020 Dietary Guidelines for Americans; USDA: Washington, DC, USA, 2015.
- Mitchell, C.J.; Milan, A.M.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; Sjödin, A.; Wagner, K.-H.; et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: A 10-wk randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.; Chapman, K.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary Protein Requirement of Men >65 Years Old Determined by the Indicator Amino Acid Oxidation Technique is Higher than the Current Estimated Average Requirement. J. Nutr. 2015, 146, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.J.; Hunt, W.C.; Romero, L.J.; Koehler, K.M.; Baumgartner, R.N.; Garry, P.J. Changes in nutritional status and patterns of morbidity among free-living elderly persons: A 10-year longitudinal study. Nutrition 1997, 13, 515–519. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Beasley, J.M.; Wertheim, B.C.; Lacroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Kritchevsky, S.; Shikany, J.M.; Eaton, C.; Chen, Z.; et al. Biomarker-Calibrated Protein Intake and Physical Function in the Women’s Health Initiative. J. Am. Geriatr. Soc. 2013, 61, 1863–1871. [Google Scholar] [CrossRef]
- Arango-Lopera, V.E.; Arroyo, P.; Gutiérrez-Robledo, L.M.; Perez-Zepeda, M.U.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary protein and risk of ischemic heart disease in women. Am. J. Clin. Nutr. 1999, 70, 221–227. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hajifaraji, M.; Bahadoran, Z.; Sarvghadi, F.; Azizi, F. Dietary protein intake is associated with favorable cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. Nutr. Res. 2012, 32, 169–176. [Google Scholar] [CrossRef]
- Anderson, J.W.; Fuller, J.; Patterson, K.; Blair, R.; Tabor, A. Soy compared to casein meal replacement shakes with energy-restricted diets for obese women: Randomized controlled trial. Metabolism 2007, 56, 280–288. [Google Scholar] [CrossRef]
- Clifton, P.; Bastiaans, K.; Keogh, J.; Clifton, P.; Keogh, J. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Noakes, M.; Keogh, J.B.; Foster, P.R.; Clifton, P.M. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am. J. Clin. Nutr. 2005, 81, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Boileau, R.A.; Erickson, D.J.; Painter, J.E.; Shiue, H.; Sather, C.; Christou, D.D. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J. Nutr. 2003, 133, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed Red and Processed Meats and Risk of Coronary Artery Disease and Type 2 Diabetes—An Updated Review of the Evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Hsieh, C.; Trichopoulos, D. Low-carbohydrate–high-protein diet and long-term survival in a general population cohort. Eur. J. Clin. Nutr. 2007, 61, 575. [Google Scholar] [CrossRef]
- Lagiou, P.; Sandin, S.; Lof, M.; Trichopoulos, D.; Adami, H.-O.; Weiderpass, E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: Prospective cohort study. BMJ 2012, 344, e4026. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, P.; Becker, W.; Warensjö, E.; Olsson, E.; Byberg, L.; Gustafsson, I.-B.; Karlström, B.; Cederholm, T. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: A cohort study in Sweden. Am. J. Clin. Nutr. 2010, 92, 967–974. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The women’s health study. Diabetes Care 2004, 27, 2108–2115. [Google Scholar] [CrossRef]
- Pounis, G.; Tyrovolas, S.; Antonopoulou, M.; Zeimbekis, A.; Anastasiou, F.; Bountztiouka, V.; Metallinos, G.; Gotsis, E.; Lioliou, E.; Polychronopoulos, E.; et al. Long-term animal-protein consumption is associated with an increased prevalence of diabetes among the elderly: The Mediterranean islands (MEDIS) study. Diabetes Metab. 2010, 36, 484–490. [Google Scholar] [CrossRef]
- Friedman, A.N. High-protein diets: Potential effects on the kidney in renal health and disease. Am. J. Kidney Dis. 2004, 44, 950–962. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine N -Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Da Costa, K.-A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Demarquoy, J.; Georges, B.; Rigault, C.; Royer, M.-C.; Clairet, A.; Soty, M.; Lekounoungou, S.; Le Borgne, F. Radioisotopic determination of l-carnitine content in foods commonly eaten in Western countries. Food Chem. 2004, 86, 137–142. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2016, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.; Merz, B.; Rist, M.J.; Ferrario, P.G.; Bub, A.; Kulling, S.E.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61, 1700363. [Google Scholar] [CrossRef]
- Rohrmann, S.; Allenspach, M.; Linseisen, J.; Von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2015, 146, 283–289. [Google Scholar] [CrossRef]
- Miller, C.A.; Corbin, K.D.; Da Costa, K.-A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef]
- Morley, J.E.; Argilés, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional Recommendations for the Management of Sarcopenia. J. Am. Med Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gerrior, S.; Juan, W.; Peter, B. An Easy Approach to Calculating Estimated Energy Requirements. Prev. Chronic Dis. 2006, 3, 3. [Google Scholar]
- Zhao, X.; Zhang, S.; Zeisel, S.H.; Zeisel, S.H.; Zeisel, S. Rapid LC-MRM-MS assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamineN-oxide, and creatinine in human plasma and urine. Electrophoresis 2015, 36, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.; Clark, N.G.; Bistrian, B.R.; Flatt, J.P.; Hallowell, E.M.; Blackburn, G.L. A simple method for estimating nitrogen balance in hospitalized patients: A review and supporting data for a previously proposed technique. J. Am. Coll. Nutr. 1985, 4, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Matthews, D.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Luscombe-Marsh, N.D.; Noakes, M.; Wittert, G.A.; Keogh, J.B.; Foster, P.; Clifton, P.M. Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am. J. Clin. Nutr. 2005, 81, 762–772. [Google Scholar] [CrossRef]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Hear. J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Health, M.O. Food and Nutrition Guidelines for Healthy Older People: A background Paper; Ministry of Health: Wellington, New Zealand, 2013.
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; la Storia, A.; Laghi, L.; Serrazanetti, D.I.; di Cagno, R.; Ferrocino, I.; Lazzi, C. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Samulak, J.J.; Sawicka, A.K.; Hartmane, D.; Grinberga, S.; Pugovics, O.; Lysiak-Szydlowska, W.; Olek, R.A. l-Carnitine Supplementation Increases Trimethylamine-N-Oxide but not Markers of Atherosclerosis in Healthy Aged Women. Ann. Nutr. Metab. 2019, 74, 11–17. [Google Scholar] [CrossRef]
- Stanstrup, J.; Schou, S.S.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Whey Protein Delays Gastric Emptying and Suppresses Plasma Fatty Acids and Their Metabolites Compared to Casein, Gluten, and Fish Protein. J. Proteome Res. 2014, 13, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Levison, B.S.; Hazen, J.E.; Donahue, L.; Li, X.-M.; Hazen, S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014, 455, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Y.; Gua, C.; Li, X. Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats through Vascular Inflammation and Oxidative Stress. Front. Physiol. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. mBio 2015, 6, 02481. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.H.; Yeung, C.K.; Peter, R.M.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.M.; Rettie, A.E. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Wishnok, J.S.; Blusztajn, J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 1983, 225, 320–324. [Google Scholar] [PubMed]
- Missailidis, C.; Hallqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef]
- James, G.D.; Sealey, J.E.; Alderman, M.; Ljungman, S.; Mueller, F.B.; Pecker, M.S.; Laragh, J.H. A Longitudinal Study of Urinary Creatinine and Creatinine Clearance in Normal Subjects: Race, Sex, and Age Differences. Am. J. Hypertens. 1988, 1, 124–131. [Google Scholar] [CrossRef]
- Møller, G.; Andersen, J.R.; Ritz, C.; Silvestre, M.P.; Navas-Carretero, S.; Jalo, E.; Christensen, P.; Simpson, E.; Taylor, M.; Martinez, J.A.; et al. Higher Protein Intake Is Not Associated with Decreased Kidney Function in Pre-Diabetic Older Adults Following a One-Year Intervention—A Preview Sub-Study. Nutrients 2018, 10, 54. [Google Scholar] [CrossRef]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary protein intake and renal function. Nutr. Metab. 2005, 2, 25. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.B.; Morse, B.L.; Djurdjev, O.; Tang, M.; Muirhead, N.; Barrett, B.; Holmes, D.T.; Madore, F.; Clase, C.M.; Rigatto, C. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016, 89, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Zhou, J.; Sayer, R.D.; Kim, J.E.; Campbell, W.W. Effects of a High-Protein Diet Including Whole Eggs on Muscle Composition and Indices of Cardiometabolic Health and Systemic Inflammation in Older Adults with Overweight or Obesity: A Randomized Controlled Trial. Nutrients 2018, 10, 946. [Google Scholar] [CrossRef]
- Pearce, K.L.; Clifton, P.M.; Noakes, M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br. J. Nutr. 2011, 105, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Akl, E.A.; Bianchi, M.; Mente, A.; Mustafa, R.; Heels-Ansdell, D.; Schünemann, H.J. Effects of higher- versus lower-protein diets on health outcomes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 780–788. [Google Scholar] [CrossRef]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar] [CrossRef]
- Beavers, K.M.; Nesbit, B.A.; Kiel, J.R.; Sheedy, J.L.; Arterburn, L.M.; Collins, A.E.; Ford, S.A.; Henderson, R.M.; Coleman, C.D.; Beavers, D.P. Effect of an Energy-Restricted, Nutritionally Complete, Higher Protein Meal Plan on Body Composition and Mobility in Older Adults with Obesity: A Randomized Controlled Trial. J. Gerontol. Ser. 2019, 74, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Mytton, O.T.; Nnoaham, K.; Eyles, H.; Scarborough, P.; Ni Mhurchu, C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Heal. 2014, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Arnett, D.K.; Coon, H.; Province, M.A.; Moore, L.L.; Ellison, R.C. Fruit and vegetable consumption and LDL cholesterol: The National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Clin. Nutr. 2004, 79, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.; Mosca, L. Epidemiology of coronary heart disease in women. Prog. Cardiovasc. Dis. 2004, 46, 287–295. [Google Scholar] [CrossRef] [PubMed]


| Parameter | RDA Group | 2RDA Group |
|---|---|---|
| Mean ± SD or Number | Mean ± SD or Number | |
| Count 1 | 15 | 14 |
| Age (years) | 74.7 ± 3.9 | 73.7 ± 3.3 |
| Height (cm) | 172.8 ± 8.2 | 171.7 ± 5.5 |
| Weight (kg) | 85.7 ± 20.5 | 83.0 ± 8.3 |
| BMI (kg/m2) | 28.4 ± 5.1 | 28.2 ± 3.3 |
| Medication usage (n) | ||
| Statin | 4 | 6 |
| ACE inhibitor | 4 | 3 |
| Aspirin | 4 | 2 |
| Calcium channel blocker | 1 | 2 |
| Proton pump inhibitors | 0 | 2 |
| Alpha blocker | 1 | 2 |
| Beta blocker | 1 | 2 |
| Xanthine oxidase inhibitor | 1 | 1 |
| Food Group | RDA (Mean ± SD) | 2RDA (Mean ± SD) | Effect (p) a | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Diet | Time × Diet | |
| Egg 1 | 0.7 ± 0.6 | 0.6 ± 0.4 | 0.7 ± 0.5 | 1. ± 0.4 † # | 0.047 * | 0.054 | 0.021 * |
| Fish 2 | 0.4 ± 0.4 | 0.1 ± 0.1 # | 0.4 ± 0.5 | 0.6 ± 0.3 † | 0.489 | 0.013 * | 0.002 * |
| Red meat 2 | 1.2 ± 0.9 | 0.2 ± 0.1 # | 1.3 ± 0.7 | 1.1 ± 0.3 † | <0.001 * | 0.003 * | 0.011 * |
| White meat 2 | 0.4 ± 0.5 | 0.2 ± 0.1 | 0.3 ± 0.4 | 0.9 ± 0.1 † # | 0.024 * | 0.001 * | <0.001 * |
| Dairy 3 | 1.1 ± 0.8 | 0.8 ± 0.5 | 1.1 ± 0.8 | 2.4 ± 0.7 † # | 0.014 * | <0.001 * | <0.001 * |
| Fruit & vegetables 4 | 5.3 ± 3 | 8.5 ± 1.6 # | 4.4 ± 2.1 | 8.6 ± 2.1 # | <0.001 * | 0.41 | 0.535 |
| Variable | RDA (Mean ± SD) | 2RDA (Mean ± SD) | Effect (p) a | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Diet | Time × Diet | |
| Lipids & lipoproteins | |||||||
| T-C (mmol/L) | 4.7 ± 0.9 | 4.5 ± 1.2 | 4.6 ± 0.9 | 4.8 ± 1.2 | 0.82 | 0.877 | 0.055 |
| HDL-C (mmol/L) | 1.4 ± 0.5 | 1.4 ± 0.6 | 1.3 ± 0.3 | 1.3 ± 0.3 | 0.89 | 0.559 | 0.662 |
| LDL-C (mmol/L) | 3.0 ± 1 | 2.8 ± 1.1 | 3.0 ± 0.9 | 3.2 ± 1.3 | 0.7 | 0.668 | 0.049 * |
| TAG (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.8 | 1.1 ± 0.5 | 0.55 | 0.631 | 0.871 |
| Metabolic & inflammatory biomarkers | |||||||
| Glucose (mmol/L) | 5.7 ± 0.5 | 5.6 ± 0.5 | 5.7 ± 0.6 | 5.5 ± 0.4 | 0.020 * | 0.639 | 0.358 |
| Insulin (µU/mL) | 9.7 ± 3.9 | 9.6 ± 3.7 | 12.5 ± 10.7 | 9.9 ± 5.6 | 0.391 | 0.421 | 0.42 |
| HbA1c (mmol/mol) | 36.1 ± 5 | 36.8 ± 4.4 | 36.9 ± 3.7 | 36.9 ± 3.3 | 0.397 | 0.761 | 0.305 |
| HOMA-IR | 2.5 ± 1.2 | 2.4 ± 1.1 | 3.5 ± 3.5 | 2.4 ± 1.3 | 0.305 | 0.439 | 0.425 |
| Matsuda Index | 3.2 ± 1.7 | 3.2 ± 1.8 | 3.2 ± 1.9 | 3.5 ± 2.1 | 0.15 | 0.649 | 0.216 |
| CRP (mg/L) | 1.8 ± 2.1 | 2.1 ± 3.3 | 2 ± 3.1 | 2.3 ± 1.6 | 0.644 | 0.862 | 0.967 |
| Anthropometrics | |||||||
| Total body fat (kg) | 25.4 ± 11.5 | 23.9 ± 11.0 # | 23.5 ± 6.8 | 21.8 ± 6.8 # | <0.001 * | 0.534 | 0.954 |
| Android fat (%) | 38.4 ± 11 | 36.7 ± 11 # | 39.9 ± 8 | 36.9 ± 8.0 # | <0.001 * | 0.831 | 0.049 * |
| WC (cm) | 104.8 ± 15 | 99.6 ± 14.1 # | 101.5 ± 7.6 | 96 ± 7.5 # | <0.001 * | 0.377 | 0.765 |
| Systolic BP (mmHg) | 143 ± 14 | 144 ± 11 | 142 ± 18 | 141 ± 16 | 0.839 | 0.647 | 0.663 |
| Diastolic BP (mmHg) | 75 ± 7 | 74 ± 7 | 76 ± 9 | 75 ± 9 | 0.126 | 0.668 | 0.975 |
| Variable | RDA (Mean ± SD) | 2RDA (Mean ± SD) | Effect (p) a | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Diet | Time × Diet | |
| Renal biomarkers | |||||||
| Plasma Cr (µmol/L) | 90.8 ± 18.9 | 82 ± 16.1 # | 98.1 ± 11 | 97.4 ± 16.7 † | 0.010 * | 0.057 | 0.027 * |
| Urinary Cr (mmol/L) | 6.3 ± 2.9 | 5.7 ± 3.8 | 6.8 ± 2.1 | 6.3 ± 2.3 | 0.174 | 0.673 | 0.730 |
| Cr clearance (mL/min) | 128.1 ± 50.5 | 101 ± 37.1 # | 116.8 ± 25.2 | 116 ± 24.4 | 0.010 * | 0.882 | 0.013 * |
| Fractional renal excretion of TMAO (%) | 76.7 ± 68.7 | 173.2 ± 213.7 | 305.2 ± 288.3 † | 54.3 ± 49.4 † # | 0.095 | 0.619 | 0.002 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, S.M.; Milan, A.M.; Mitchell, C.J.; Gillies, N.A.; D’Souza, R.F.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; et al. Protein Intake at Twice the RDA in Older Men Increases Circulatory Concentrations of the Microbiome Metabolite Trimethylamine-N-Oxide (TMAO). Nutrients 2019, 11, 2207. https://doi.org/10.3390/nu11092207
Mitchell SM, Milan AM, Mitchell CJ, Gillies NA, D’Souza RF, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, et al. Protein Intake at Twice the RDA in Older Men Increases Circulatory Concentrations of the Microbiome Metabolite Trimethylamine-N-Oxide (TMAO). Nutrients. 2019; 11(9):2207. https://doi.org/10.3390/nu11092207
Chicago/Turabian StyleMitchell, Sarah M., Amber M. Milan, Cameron J. Mitchell, Nicola A. Gillies, Randall F. D’Souza, Nina Zeng, Farha Ramzan, Pankaja Sharma, Scott O. Knowles, Nicole C. Roy, and et al. 2019. "Protein Intake at Twice the RDA in Older Men Increases Circulatory Concentrations of the Microbiome Metabolite Trimethylamine-N-Oxide (TMAO)" Nutrients 11, no. 9: 2207. https://doi.org/10.3390/nu11092207
APA StyleMitchell, S. M., Milan, A. M., Mitchell, C. J., Gillies, N. A., D’Souza, R. F., Zeng, N., Ramzan, F., Sharma, P., Knowles, S. O., Roy, N. C., Sjödin, A., Wagner, K.-H., Zeisel, S. H., & Cameron-Smith, D. (2019). Protein Intake at Twice the RDA in Older Men Increases Circulatory Concentrations of the Microbiome Metabolite Trimethylamine-N-Oxide (TMAO). Nutrients, 11(9), 2207. https://doi.org/10.3390/nu11092207








