Impact of Carbohydrate Ingestion on Cognitive Flexibility and Cerebral Oxygenation during High-Intensity Intermittent Exercise: A Comparison between Maple Products and Usual Carbohydrate Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
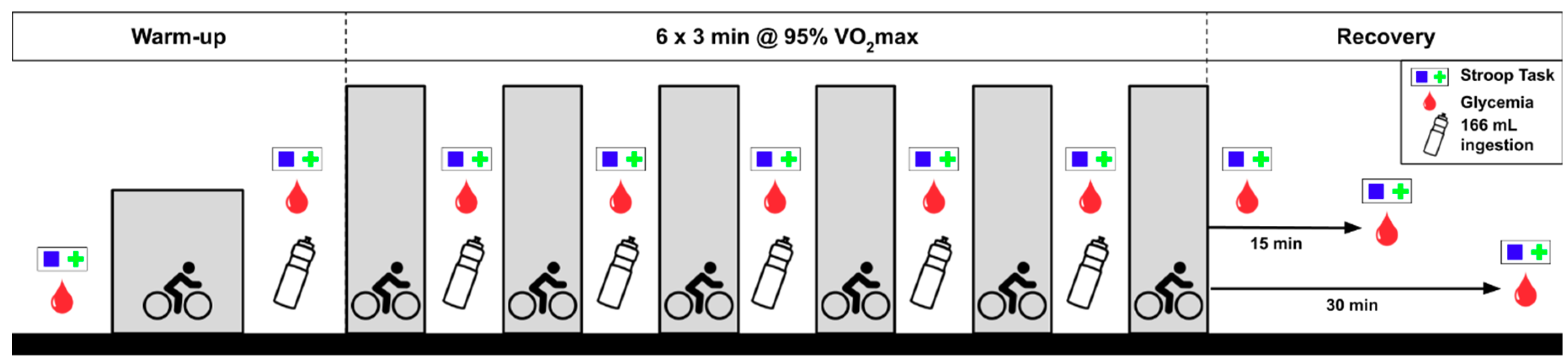
2.2. Experimental Protocol
2.3. Placebo and CHO Solutions
2.4. Measurements
2.4.1. Plasma Glucose Concentration
2.4.2. Peak Power Output and Maximal Oxygen Consumption
2.4.3. Cognitive Task
2.5. Cerebral Oxygenation
2.6. Statistical Analyses
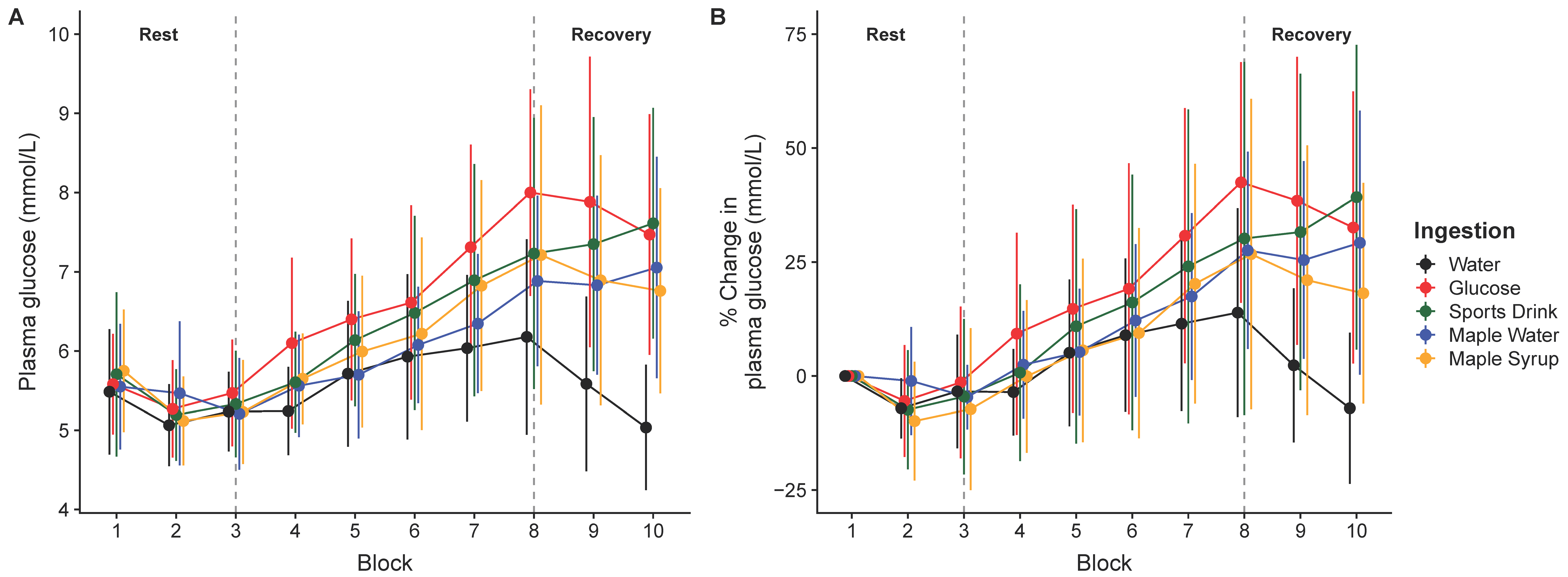
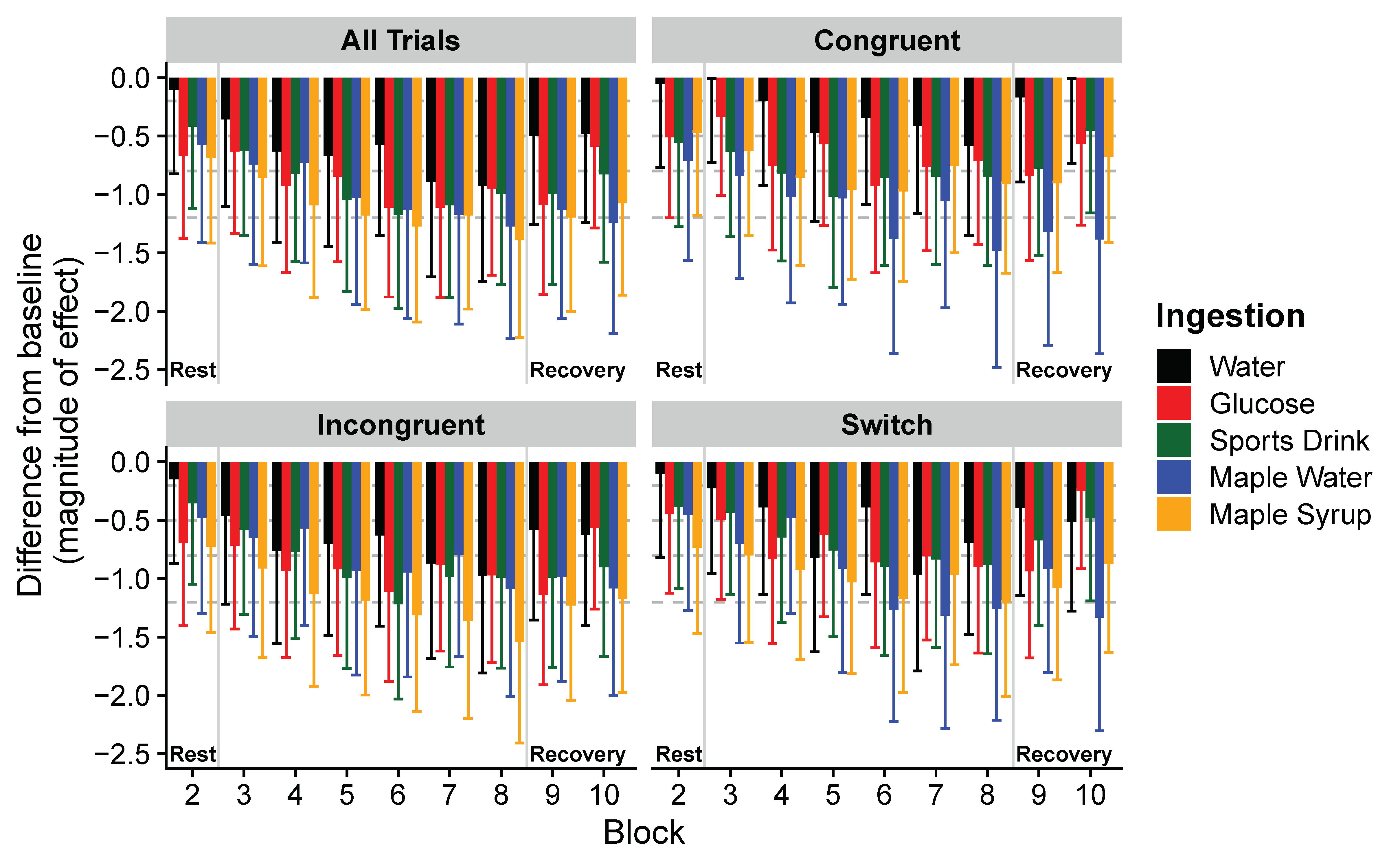
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baker, L.B.; Rollo, I.; Stein, K.W.; Jeukendrup, A.E. Acute Effects of Carbohydrate Supplementation on Intermittent Sports Performance. Nutrients 2015, 7, 5733–5763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karelis, A.D.; Smith, J.W.; Passe, D.H.; Peronnet, F. Carbohydrate administration and exercise performance: What are the potential mechanisms involved? Sports Med. 2010, 40, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R. Exercise, nutrition and the brain. Sports Med. 2014, 44, S47–S56. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Bridge, M.W.; Jones, D.A. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, K.; Roelands, B.; Knaepen, K.; Polfliet, M.; Stiens, J.; Meeusen, R. Effects of caffeine and maltodextrin mouth rinsing on P300, brain imaging, and cognitive performance. J. Appl. Physiol. 2015, 118, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Byblow, W.D.; Stinear, C.M.; Gant, N. Carbohydrate in the mouth enhances activation of brain circuitry involved in motor performance and sensory perception. Appetite 2014, 80, 212–219. [Google Scholar] [CrossRef]
- Baker, L.B.; Nuccio, R.P.; Jeukendrup, A.E. Acute effects of dietary constituents on motor skill and cognitive performance in athletes. Nutr. Rev. 2014, 72, 790–802. [Google Scholar] [CrossRef]
- Pomportes, L.; Brisswalter, J.; Casini, L.; Hays, A.; Davranche, K. Cognitive Performance Enhancement Induced by Caffeine, Carbohydrate and Guarana Mouth Rinsing during Submaximal Exercise. Nutrients 2017, 9, 589. [Google Scholar] [CrossRef]
- Parrott, M.D.; Greenwood, C.E. Dietary influences on cognitive function with aging: From high-fat diets to healthful eating. Ann. N. Y. Acad. Sci. 2007, 1114, 389–397. [Google Scholar] [CrossRef]
- Benton, D.; Owens, D.S.; Parker, P.Y. Blood glucose influences memory and attention in young adults. Neuropsychologia 1994, 32, 595–607. [Google Scholar] [CrossRef]
- Donohoe, R.T.; Benton, D. Cognitive functioning is susceptible to the level of blood glucose. Psychopharmacology 1999, 145, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, R.T.; Benton, D. Declining Blood Glucose Levels after a Cognitively Demanding Task Predict Subsequent Memory. Nutr. Neurosci. 1999, 2, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Sunram-Lea, S.I.; Greer, J.; Elliott, J.; Kennedy, D.O. Glucose enhancement of memory depends on initial thirst. Appetite 2009, 53, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Sunram-Lea, S.I.; Foster, J.K.; Durlach, P.; Perez, C. Investigation into the significance of task difficulty and divided allocation of resources on the glucose memory facilitation effect. Psychopharmacology 2002, 160, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Messier, C. Glucose improvement of memory: A review. Eur. J. Pharmacol. 2004, 490, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Desjardins-Crepeau, L.; Tournier, I.; Desjardins, M.; Lesage, F.; Greenwood, C.E.; Bherer, L. Near-infrared imaging of the effects of glucose ingestion and regulation on prefrontal activation during dual-task execution in healthy fasting older adults. Behav. Brain Res. 2012, 232, 137–147. [Google Scholar] [CrossRef]
- Meeusen, R.; Decroix, L. Nutritional Supplements and the Brain. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 200–211. [Google Scholar] [CrossRef]
- Collardeau, M.; Brisswalter, J.; Vercruyssen, F.; Audiffren, M.; Goubault, C. Single and choice reaction time during prolonged exercise in trained subjects: Influence of carbohydrate availability. Eur. J. Appl. Physiol. 2001, 86, 150–156. [Google Scholar] [CrossRef]
- Bottoms, L.; Sinclair, J.; Taylor, K.; Polman, R.; Fewtrell, D. The effects of carbohydrate ingestion on the badminton serve after fatiguing exercise. J. Sports Sci. 2012, 30, 285–293. [Google Scholar] [CrossRef]
- Pomportes, L.; Brisswalter, J.; Hays, A.; Davranche, K. Effects of Carbohydrate, Caffeine and Guarana on Cognitive Performance, Perceived Exertion and Shooting Performance in High Level Athletes. Int. J. Sports Physiol. Perform. 2018, 9, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Falco, C.M.; Slade, S.S. Carbohydrate administration during a day of sustained aerobic activity improves vigilance, as assessed by a novel ambulatory monitoring device, and mood. Am. J. Clin. Nutr. 2002, 76, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomportes, L.; Davranche, K.; Brisswalter, I.; Hays, A.; Brisswalter, J. Heart rate variability and cognitive function following a multi-vitamin and mineral supplementation with added guarana (Paullinia cupana). Nutrients 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, P.; Pilon, G.; Dumais, V.; Dion, C.; Dubois, M.-J.; Dubeé, P.; Desjardins, Y.; Marette, A. Comparative analysis of maple syrup to other natural sweeteners and evaluation of their metabolic responses in healthy rats. J. Funct. Foods 2014, 11, 460–471. [Google Scholar] [CrossRef]
- Ma, H.; DaSilva, N.A.; Liu, W.; Nahar, P.P.; Wei, Z.; Liu, Y.; Pham, P.T.; Crews, R.; Vattem, D.A.; Slitt, A.L.; et al. Effects of a standardized phenolic-enriched maple syrup extract on β-amyloid aggregation, neuroinflammation in microglial and neuronal cells and β-amyloid induced neurotoxicity in Caenorhabditis elegans. Neurochem. Res. 2016, 41, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Parker, P.Y.; Benton, D. Blood glucose levels selectively influence memory for word lists dichotically presented to the right ear. Neuropsychologia 1995, 33, 843–854. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, O.; Billaut, F.; Raymond, F.; Benraiss, A.; Theurot, D.; Bosquet, L.; Fraser, S.; Tremblay, J. Effect of Acute Intermittent Exercise on Cognitive Flexibility: the Role of Exercise Intensity. J. Cogn. Enhan. 2018, 2, 146–156. [Google Scholar] [CrossRef]
- Delignieères, D. La perception de l’effort et de la difficulté. In Cognition Et Performance; Famose, J.P., Ed.; INSEP Publications: Paris, France, 1993; pp. 183–218. [Google Scholar]
- Delignières, D.; Brisswalter, J.; Legros, P. Influence of physical exercise on choice reaction time in sport experts: The mediating role of resource allocation. J. Hum. Mov. Studies 1994, 27, 173–188. [Google Scholar]
- Davranche, K.; Audiffren, M. Facilitating effects of exercise on information processing. J. Sports Sci. 2004, 22, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Mandrick, K.; Peysakhovich, V.; Remy, F.; Lepron, E.; Causse, M. Neural and psychophysiological correlates of human performance under stress and high mental workload. Biol. Psychol. 2016, 121, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, O.; Gauthier, C.J.; Fraser, S.A.; Desjardins-Crepeau, L.; Desjardins, M.; Mekary, S.; Lesage, F.; Hoge, R.D.; Pouliot, P.; Bherer, L. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front. Hum. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Lague-Beauvais, M.; Brunet, J.; Gagnon, L.; Lesage, F.; Bherer, L. A fNIRS investigation of switching and inhibition during the modified Stroop task in younger and older adults. Neuroimage 2013, 64, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Labelle, V.; Bosquet, L.; Mekary, S.; Bherer, L. Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain Cogn. 2013, 81, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Labelle, V.; Bosquet, L.; Mekary, S.; Vu, T.T.; Smilovitch, M.; Bherer, L. Fitness level moderates executive control disruption during exercise regardless of age. J. Sport Exerc. Psychol. 2014, 36, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Mekari, S.; Fraser, S.; Bosquet, L.; Bonnery, C.; Labelle, V.; Pouliot, P.; Lesage, F.; Bherer, L. The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. Eur. J. Appl. Physiol. 2015, 115, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 2004, 21, 99–111. [Google Scholar] [CrossRef]
- Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, Australia, 1988. [Google Scholar]
- Williams, C.; Rollo, I. Carbohydrate Nutrition and Team Sport Performance. Sports Med. 2015, 45, S13–S22. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Ogoh, S.; Hashimoto, T. Repeated high-intensity interval exercise shortens the positive effect on executive function during post-exercise recovery in healthy young males. Physiol. Behav. 2016, 160, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billaut, F.; Basset, F.A.; Giacomoni, M.; Lemaitre, F.; Tricot, V.; Falgairette, G. Effect of high-intensity intermittent cycling sprints on neuromuscular activity. Int. J. Sports Med. 2006, 27, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.W.; Pearcey, G.E.P.; Buckle, N.C.M.; Power, K.E.; Button, D.C. Neuromuscular fatigue during repeated sprint exercise: Underlying physiology and methodological considerations. Appl. Physiol. Nutr. Metab. 2018, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.; Watt, A.P.; Liukkonen, J. Differences in ball sports athletes speed discrimination skills before and after exercise induced fatigue. J. Sports Sci. Med. 2009, 8, 259–264. [Google Scholar] [PubMed]



| Water | Glucose | Sports Drink | Maple Water | Maple Syrup | |
|---|---|---|---|---|---|
| n | 16 | 19 | 18 | 14 | 18 |
| Age (year) | 24.4 ± 4.6 | 30.2 ± 7.6 | 31.1 ± 6.1 | 32.7 ± 7.8 | 25.7 ± 5.0 |
| Mass (kg) | 71.6 ± 7.9 | 76.1 ± 11.6 | 74.7 ± 8.4 | 74.3 ± 9.7 | 69.6 ± 8.9 |
| Height (m) | 1.79 ± 0.05 | 1.81 ± 0.07 | 1.80 ± 0.10 | 1.78 ± 0.08 | 1.77 ± 0.07 |
| VO2max (mL·kg−1·min−1) | 59.9 ± 7.8 | 56.8 ± 7.2 | 57.6 ± 7.2 | 58.7 ± 9.1 | 63.4 ± 7.1 |
| Maximal power output (W) | 301 ± 51 | 302 ± 51 | 314 ± 39 | 313 ± 38 | 309 ± 47 |
| Plasma glucose concentration (mmol·L−1) | |||||
| Pre-ingestion | 4.99 ± 0.49 | 5.19 ± 0.56 | 5.33 ± 0.60 | 5.21 ± 0.38 | 5.07 ± 0.48 |
| 120 min post-ingestion | 5.76 ± 0.97 | 5.53 ± 1.01 | 5.55 ± 0.95 | 5.59 ± 0.67 | 5.32 ± 1.06 |
| Ingestion | p Value | Block 1 | Block 2 | Block 3 | Block 4 | Block 5 | Block 6 | Block 7 | Block 8 | Block 9 | Block 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All trials (ms) | Water | C: 0.22 | 1017 ± 298 | 990 ± 282 | 922 ± 237 | 841 ± 252 * | 832 ± 252 * | 847 ± 286 * | 785 ± 209 * | 787 ± 178 * | 885 ± 226 | 890 ± 227 |
| Glucose | 1116 ± 284 | 952 ± 195 * | 967 ± 172 * | 899 ± 161 * | 897 ± 224 * | 856 ± 161 * | 834 ± 210 * | 858 ± 253 * | 853 ± 181 * | 928 ± 348 * | ||
| Sports drink | T < 0.0001 | 1156 ± 380 | 1026 ± 225 | 956 ± 240 * | 909 ± 180 * | 847 ± 160 * | 815 ± 141 * | 817 ± 209 * | 856 ± 184 * | 847 ± 211 * | 897 ± 220 * | |
| Maple water | 1284 ± 405 | 1082 ± 276 * | 1020 ± 284 * | 1030 ± 269 * | 941 ± 221 * | 916 ± 195 * | 883 ± 245 * | 868 ± 201 * | 918 ± 191 * | 873 ± 216 * | ||
| Maple syrup | I: 0.25 | 1092 ± 332 | 896 ± 226 * | 857 ± 194 * | 791 ± 195 * | 764 ± 201 * | 746 ± 182 * | 767 ± 195 * | 721 ± 171 * | 764 ± 190 * | 791 ± 206 * | |
| Incongruent trials (ms) | Water | C: 0.13 | 1026 ± 318 | 983 ± 289 | 896 ± 237 | 808 ± 242 * | 816 ± 272 * | 829 ± 301 * | 781 ± 230 * | 765 ± 193 * | 861 ± 238 * | 853 ± 221 * |
| Glucose | 1126 ± 286 | 955 ± 199 * | 954 ± 178 * | 902 ± 176 * | 888 ± 226 * | 860 ± 173 * | 899 ± 218 * | 854 ± 268 * | 846 ± 192 * | 928 ± 405 * | ||
| Sports drink | T < 0.0001 | 1157 ± 405 | 1039 ± 254 | 957 ± 260 * | 906 ± 210 * | 845 ± 169 * | 787 ± 124 * | 835 ± 213 * | 840 ± 190 * | 835 ± 206 * | 869 ± 191 * | |
| Maple water | 1260 ± 442 | 1080 ± 281 * | 1010 ± 297 * | 1039 ± 313 * | 924 ± 230 * | 924 ± 214 * | 937 ± 349 * | 868 ± 231 * | 911 ± 218 * | 864 ± 246 * | ||
| Maple syrup | I: 0.48 | 1073 ± 305 | 876 ± 228 * | 841 ± 185 * | 779 ± 198 * | 757 ± 210 * | 734 ± 190 * | 725 ± 184 * | 695 ± 154 * | 747 ± 209 * | 760 ± 214 * | |
| Congruent trials (ms) | Water | C: 0.50 | 993 ± 283 | 1007 ± 325 | 992 ± 301 | 933 ± 341 | 872 ± 221 | 895 ± 290 | 879 ± 268 | 846 ± 213 | 950 ± 243 | 990 ± 347 |
| Glucose | 1087 ± 313 | 944 ± 244 | 1002 ± 184 | 891 ± 183 * | 924 ± 254 * | 846 ± 183 * | 878 ± 221 * | 870 ± 292 * | 873 ± 172 * | 928 ± 244 | ||
| Sports Drink | T < 0.0001 | 1154 ± 366 | 990 ± 195 | 954 ± 253 * | 913 ± 187 * | 852 ± 194 * | 887 ± 240 * | 895 ± 224 * | 897 ± 210 * | 884 ± 320 * | 981 ± 398 | |
| Maple water | 1334 ± 381 | 1087 ± 301 * | 1039 ± 305 * | 1012 ± 217 * | 976 ± 293 * | 894 ± 221 * | 1001 ± 215 * | 875 ± 198 * | 933 ± 177 * | 900 ± 209 * | ||
| Maple syrup | I: 0.20 | 1143 ± 479 | 953 ± 306 * | 902 ± 248 * | 824 ± 212 * | 784 ± 212 * | 778 ± 212 * | 850 ± 253 * | 790 ± 252 * | 811 ± 189 * | 885 ± 237 * | |
| Switching trials (ms) | Water | C: 0.40 | 1035 ± 302 | 1008 ± 276 | 972 ± 264 | 916 ± 306 | 819 ± 206 * | 914 ± 321 | 794 ± 172 * | 842 ± 247 * | 928 ± 232 | 890 ± 252 |
| Glucose | 1124 ± 303 | 998 ± 263 | 1000 ± 184 | 918 ± 171 * | 944 ± 269 * | 912 ± 164 * | 899 ± 252 * | 873 ± 246 * | 876 ± 213 * | 1035 ± 414 * | ||
| Sports drink | T < 0.0001 | 1160 ± 451 | 1028 ± 187 | 1002 ± 251 | 933 ± 199 * | 890 ± 217 * | 850 ± 177 * | 865 ± 204 * | 853 ± 179 * | 910 ± 265 * | 974 ± 306 * | |
| Maple water | 1282 ± 381 | 1131 ± 266 | 1032 ± 320 * | 1111 ± 327 | 971 ± 280 * | 893 ± 190 * | 874 ± 198 * | 888 ± 207 * | 995 ± 207 * | 887 ± 152 * | ||
| Maple syrup | I: 0.07 | 1190 ± 459 | 916 ± 252 * | 898 ± 225 * | 849 ± 231 * | 811 ± 229 * | 770 ± 196 * | 827 ± 253 * | 763 ± 184 * | 802 ± 200 * | 854 ± 278 * | |
| Accuracy (%) | Water | C: 0.43 | 0.97 ± 0.03 | 0.95 ± 0.04 | 0.95 ± 0.04 | 0.94 ± 0.05 | 0.95 ± 0.05 | 0.93 ± 0.09 | 0.94 ± 0.08 | 0.91 ± 0.07 * | 0.95 ± 0.09 | 0.92 ± 0.11 |
| Glucose | 0.96 ± 0.05 | 0.96 ± 0.05 | 0.97 ± 0.04 | 0.96 ± 0.05 | 0.95 ± 0.05 | 0.95 ± 0.06 | 0.96 ± 0.06 | 0.94 ± 0.08 | 0.95 ± 0.08 | 0.94 ± 0.09 | ||
| Sports drink | T: 0.03 | 0.93 ± 0.09 | 0.94 ± 0.04 | 0.95 ± 0.07 | 0.92 ± 0.08 | 0.93 ± 0.06 | 0.98 ± 0.08 | 0.90 ± 0.11 | 0.90 ± 0.11 | 0.92 ± 0.12 | 0.91 ± 0.12 | |
| Maple water | 0.96 ± 0.03 | 0.96 ± 0.02 | 0.97 ± 0.04 | 0.95 ± 0.04 | 0.95 ± 0.06 | 0.95 ± 0.06 | 0.95 ± 0.08 | 0.94 ± 0.09 | 0.94 ± 0.11 | 0.95 ± 0.11 | ||
| Maple syrup | I: 0.90 | 0.96 ± 0.04 | 0.95 ± 0.05 | 0.96 ± 0.04 | 0.95 ± 0.05 | 0.94 ± 0.07 | 0.94 ± 0.07 | 0.94 ± 0.07 | 0.95 ± 0.06 | 0.96 ± 0.06 | 0.96 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuy, O.; Tremblay, J. Impact of Carbohydrate Ingestion on Cognitive Flexibility and Cerebral Oxygenation during High-Intensity Intermittent Exercise: A Comparison between Maple Products and Usual Carbohydrate Solutions. Nutrients 2019, 11, 2019. https://doi.org/10.3390/nu11092019
Dupuy O, Tremblay J. Impact of Carbohydrate Ingestion on Cognitive Flexibility and Cerebral Oxygenation during High-Intensity Intermittent Exercise: A Comparison between Maple Products and Usual Carbohydrate Solutions. Nutrients. 2019; 11(9):2019. https://doi.org/10.3390/nu11092019
Chicago/Turabian StyleDupuy, Olivier, and Jonathan Tremblay. 2019. "Impact of Carbohydrate Ingestion on Cognitive Flexibility and Cerebral Oxygenation during High-Intensity Intermittent Exercise: A Comparison between Maple Products and Usual Carbohydrate Solutions" Nutrients 11, no. 9: 2019. https://doi.org/10.3390/nu11092019





