Gene Expression and Cardiometabolic Phenotypes of Vitamin D-Deficient Overweight and Obese Black Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Measurements
3. Statistical Analysis
RNA-seq Processing and Data Analysis
4. Results
4.1. Differentially Expressed Genes Associated with BMI
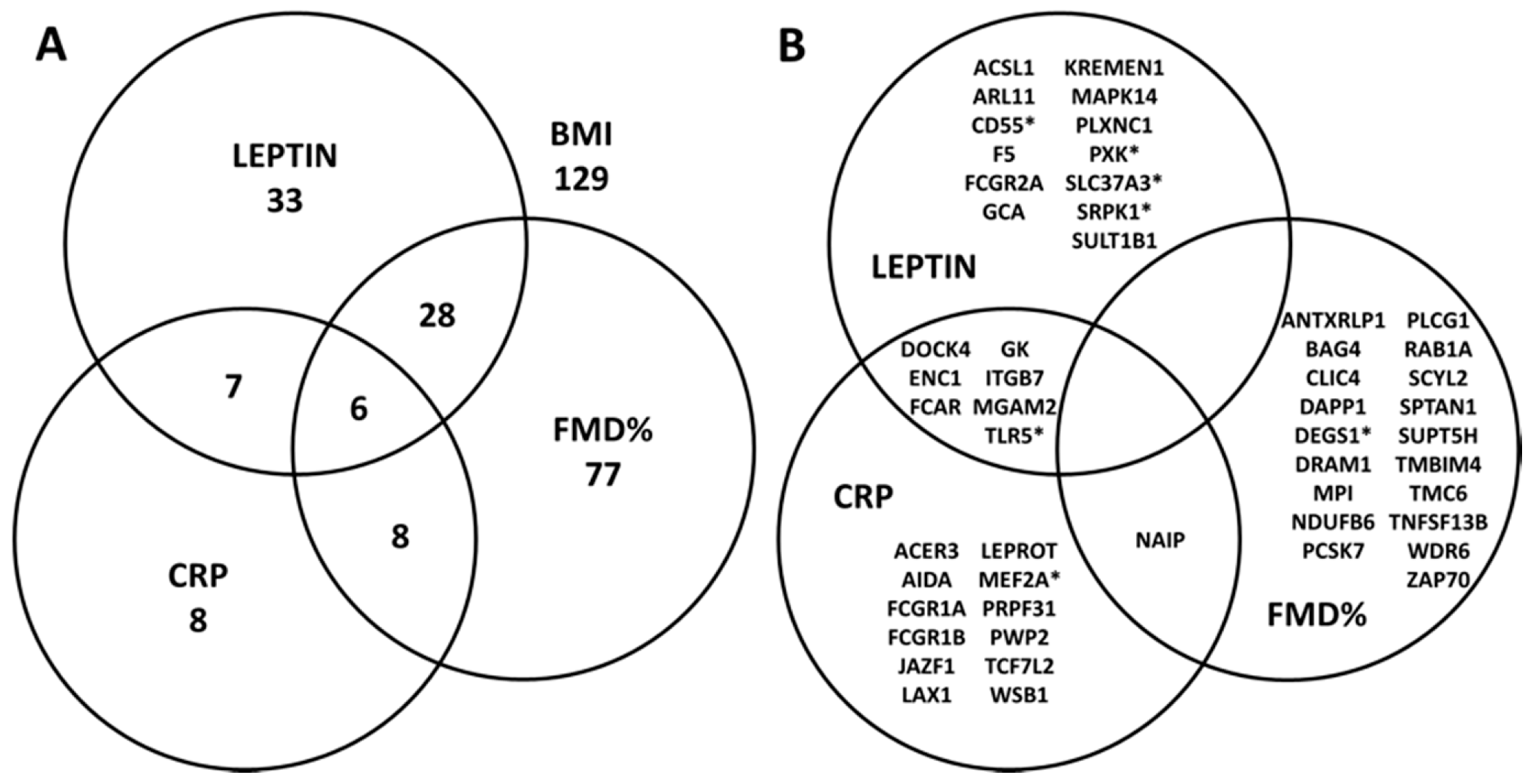
4.2. Association between Cardiometabolic Phenotypes and BMI-Related Genes
4.3. Canonical Pathways Associated with Significant Genes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohlgruber, A.; Lynch, L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Smotkin-Tangorra, M.; Purushothaman, R.; Gupta, A.; Nejati, G.; Anhalt, H.; Ten, S. Prevalence of vitamin D insufficiency in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2007, 20, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Turer, C.B.; Lin, H.; Flores, G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 2013, 131, e152–e161. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef]
- Grant, W.B.; Holick, M.F. Benefits and requirements of vitamin D for optimal health: A review. Altern. Med. Rev. 2005, 10, 94–111. [Google Scholar] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Jansson, P.A.; Perfilyev, A.; Volkov, P.; Pedersen, M.; Svensson, M.K.; Poulsen, P.; Ribel-Madsen, R.; Pedersen, N.L.; Almgren, P.; et al. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 2014, 63, 2962–2976. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, T.; Rinnankoski-Tuikka, R.; Rintala, M.; Seppanen-Laakso, T.; Pollanen, E.; Alen, M.; Sipila, S.; Kaprio, J.; Kovanen, V.; Rahkila, P.; et al. Differences in muscle and adipose tissue gene expression and cardio-metabolic risk factors in the members of physical activity discordant twin pairs. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L.; Tangpricha, V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Steele, J.; Fraher, L.; Barker, S.; Karmali, R.; O’Riordan, J.; Stanford, J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 1986, 57, 159–163. [Google Scholar] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.J.S. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Helming, L.; Bose, J.; Ehrchen, J.; Schiebe, S.; Frahm, T.; Geffers, R.; Probst-Kepper, M.; Balling, R.; Lengeling, A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood 2005, 106, 4351–4358. [Google Scholar] [CrossRef]
- Michel, H.; Olabopo, F.; Wang, L.; Nucci, A.; Greenspan, S.L.; Rajakumar, K. Determinants of 25-Hydroxyvitamin D Concentrations in Infants and Toddlers. Curr. Nutr. Food Sci. 2015, 11, 124–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.Y.; Bacha, F.; Tfayli, H.; Michaliszyn, S.F.; Yousuf, S.; Arslanian, S. Adipose Tissue Insulin Resistance in Youth on the Spectrum From Normal Weight to Obese and From Normal Glucose Tolerance to Impaired Glucose Tolerance to Type 2 Diabetes. Diabetes Care 2019, 42, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Michaliszyn, S.F.; Nasr, A.; Lee, S.; Tfayli, H.; Hannon, T.; Hughan, K.S.; Bacha, F.; Arslanian, S. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test Heralds Biomarkers of Type 2 Diabetes Risk in Obese Youth. Diabetes Care 2016, 39, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, I.D.; Griffin, P.; Michel, J.J.; Yano, H.; Gaffen, S.L.; Mueller, R.G.; Dvergsten, J.A.; Piganelli, J.D.; Rosenkranz, M.E.; Kietz, D.A.; et al. T Cell Receptor-Independent, CD31/IL-17A-Driven Inflammatory Axis Shapes Synovitis in Juvenile Idiopathic Arthritis. Front. Immunol. 2018, 9, 1802. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 25 May 2017).
- Krueger, F. Trim Galore! 2018. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 25 May 2017).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Rajakumar, K.; de las Heras, J.; Chen, T.C.; Lee, S.; Holick, M.F.; Arslanian, S.A. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J. Clin. Endocrinol. Metab. 2011, 96, 1560–1567. [Google Scholar] [CrossRef]
- Rajakumar, K.; de las Heras, J.; Lee, S.; Holick, M.F.; Arslanian, S.A. 25-hydroxyvitamin D concentrations and in vivo insulin sensitivity and beta-cell function relative to insulin sensitivity in black and white youth. Diabetes Care 2012, 35, 627–633. [Google Scholar] [CrossRef][Green Version]
- Knudson, J.D.; Dincer, U.D.; Zhang, C.; Swafford, A.N., Jr.; Koshida, R.; Picchi, A.; Focardi, M.; Dick, G.M.; Tune, J.D. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H48–H56. [Google Scholar] [CrossRef]
- Green, D.J.; Dawson, E.A.; Groenewoud, H.M.; Jones, H.; Thijssen, D.H. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension 2014, 63, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Okamoto, Y.; Rocha, V.Z.; Folco, E. Inflammation in atherosclerosis: Transition from theory to practice. Circ. J. 2010, 74, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. C-reactive protein and risks of future myocardial infarction and thrombotic stroke. Eur. Heart J. 1998, 19, 1–3. [Google Scholar] [PubMed]
- Visvikis-Siest, S.; Marteau, J.B.; Samara, A.; Berrahmoune, H.; Marie, B.; Pfister, M. Peripheral blood mononuclear cells (PBMCs): A possible model for studying cardiovascular biology systems. Clin. Chem. Lab. Med. 2007, 45, 1154–1168. [Google Scholar] [CrossRef]
- Silbiger, V.N.; Luchessi, A.D.; Hirata, R.D.; Lima-Neto, L.G.; Cavichioli, D.; Carracedo, A.; Brion, M.; Dopazo, J.; Garcia-Garcia, F.; dos Santos, E.S.; et al. Novel genes detected by transcriptional profiling from whole-blood cells in patients with early onset of acute coronary syndrome. Clin. Chim. Acta 2013, 421, 184–190. [Google Scholar] [CrossRef]
- Tanaka, J.; Imamura, M.; Kasai, M.; Masauzi, N.; Matsuura, A.; Ohizumi, H.; Morii, K.; Kiyama, Y.; Naohara, T.; Saitho, M.; et al. Cytokine gene expression in peripheral blood mononuclear cells during graft-versus-host disease after allogeneic bone marrow transplantation. Br. J. Haematol. 1993, 85, 558–565. [Google Scholar] [CrossRef]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef]
- Sanchez, J.; Pico, C.; Ahrens, W.; Foraita, R.; Fraterman, A.; Moreno, L.A.; Russo, P.; Siani, A.; Palou, A. Transcriptome analysis in blood cells from children reveals potential early biomarkers of metabolic alterations. Int. J. Obes. 2017, 41, 1481–1488. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.; Li, N.; Yu, H.; Si, J.; Wang, J. Identification of key genes and pathways associated with obesity in children. Exp. Ther. Med. 2017, 14, 1065–1073. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | N = 41 |
|---|---|
| Demographic | |
| Female | 26 (63) |
| Non-Hispanic | 40 (98) |
| Age, yrs | 13.2 ± 2.0 |
| Anthropometrics | |
| Weight, kg | 80.6 ± 19.3 |
| Height, cm | 163.3 ± 10.9 |
| BMI, kg/m2 | 30.0 ± 5.7 |
| BMI percentile | 95.8 ± 4.0 |
| Waist circumference, cm | 89.9 ± 14.4 |
| Waist-to-height ratio | 0.55 ± 0.09 |
| Percent total body fat | 32.4 ± 8.4 |
| Weight classification | |
| Overweight (BMI 85th to < 95th %tile) | 16 (39) |
| Obese (BMI ≥ 95th %tile) | 25 (61) |
| Pubertal status, Tanner Stage | |
| I | 2 (5) |
| II | 3 (7) |
| III | 6 (15) |
| IV | 17 (41) |
| V | 13 (32) |
| Laboratory data | |
| 25(OH)D, ng/mL | 13.7 ± 4.1 |
| PTH, pg/mL | 48.1 ± 20.5 |
| Total cholesterol, mg/dL | 152.9 ± 24.6 |
| LDL cholesterol, mg/dL | 91.3 ± 23.9 |
| HDL cholesterol, mg/dL | 46.5 ± 9.8 |
| Triglycerides, mg/dL | 75.3 ± 25.1 |
| Non-HDL cholesterol, mg/dL | 106.4 ± 25.1 |
| Triglyceride-HDL-ratio | 1.7 ± 0.7 |
| Leptin, ng/mL | 18.4 ± 9.9 |
| Adiponectin, ng/mL | 13.2 ± 10 |
| CRP, pg/mL | 3028 ± 5546 |
| Interleukin-6, pg/mL | 6.9 ± 13.1 |
| Vascular health data | |
| Baseline brachial artery diameter, cm | 0.32 ± 0.05 |
| FMD% | 7.32 ± 5.4 |
| PWV, m/sec | 4.7 ± 0.7 |
| AIx@75bpm | 2.83 ± 11.2 |
| Central systolic BP, mm Hg | 98.1 ± 9.1 |
| Central diastolic BP, mm Hg | 68.3 ± 7.6 |
| Systemic systolic BP, mm Hg | 115.1 ± 11.1 |
| Systemic diastolic BP, mm Hg | 67.5 ± 7.3 |
| Gene | Name | Category | FDR * |
|---|---|---|---|
| LILRA5 | leukocyte immunoglobulin like receptor A5 | immune function (pro-inflammatory) | 1.37 × 10−3 |
| ANXA3 | annexin A3 | general cell growth/signaling vascular effects (anti-coagulation) | 2.73 × 10−3 |
| PXK | PX domain containing serine/threonine kinase like | integumentary effector | 2.73 × 10−3 |
| S100A12 | S100 calcium binding protein A12 | general cell growth/differentiation innate immune sensor (innate sensor, anti-bacterial) | 2.73 × 10−3 |
| SLC37A3 | solute carrier family 37 member 3 | potential metabolic effector (regulator of adipose tissue) | 2.73 × 10−3 |
| TLR5 | toll like receptor 5 | innate immune signaling | 2.73 × 10−3 |
| EXOSC10 | exosome component 10 | general cellular effector (RNA degradation) immune function (Ig class-switching, Ig extracellular trafficking) | 8.22 × 10−3 |
| S100A9 | S100 calcium binding protein A9 | general cell growth/differentiation innate immune sensor (anti-bacterial/fungal) | 8.22 × 10−3 |
| WDR46 | WD repeat domain 46 | general cellular function (nucleolar scaffolding, granule localization) | 8.22 × 10−3 |
| DEGS1 | delta 4-desaturase, sphingolipid 1 | metabolic effector (fatty acid desaturation) | 1.02 × 10−2 |
| FCER1G | Fc receptor for IgE | immune function (hypersensitivity) | 1.05 × 10−2 |
| MEF2A | myocyte enhancer factor 2A | muscular effector | 1.05 × 10−2 |
| NDUFB2 | NADH:ubiquinone oxidoreductase subunit B2 | general cellular energy production (electron transport system) | 1.05 × 10−2 |
| UBE2F | ubiquitin conjugating enzyme E2 F (putative) | general cellular function (cell cycle, protein folding) | 1.05 × 10−2 |
| HCAR2 | hydroxycarboxylic acid receptor 2 | innate immune function (neutrophil apoptosis activator) | 1.15 × 10−2 |
| PFKL | phosphofructokinase, liver type | general cellular energy production (glycolysis in liver) | 1.15 × 10−2 |
| SRPK1 | SRSF protein kinase 1 | general cell transcriptional regulation | 1.15 × 10−2 |
| CD55 | cluster of differentiation 55 | immune function (regulator of complement-driven cellular damage) | 1.19 × 10−2 |
| IL4R | interleukin 4 receptor | immune cell signaling | 1.19 × 10−2 |
| LAMTOR5 | late endosomal/lysosomal adaptor, MAPK and MTOR activator | endosome formation, intracellular signaling | 1.19 × 10−2 |
| Pathways | p-Value |
|---|---|
| Inflammatory Signaling | |
| Phagosome Formation | 1.38 × 10−7 |
| Chronic Inflammatory Syndrome | 1.62 × 10−6 |
| IL-10 Signaling | 3.16 × 10−6 |
| NF-κB Signaling | 2.69 × 10−5 |
| TREM1 Signaling | 5.50 × 10−5 |
| Altered T-Cell & B-Cell Signaling | 1.74 × 10−4 |
| Role of PKR in Interferon Induction | 1.86 × 10−4 |
| Role of NFAT in Regulation of the Immune Response | 7.76 × 10−4 |
| Inflammasome Pathway | 2.14 × 10−3 |
| PPARα/RXRα Activation | 2.57 × 10−3 |
| p38 MAPK Signaling | 4.90 × 10−3 |
| IL-6 Signaling | 2.63 × 10−2 |
| Role of JAK family kinases in IL-6-type Cytokine Signaling | 4.17 × 10−2 |
| Immune Cell Function | |
| Toll-like Receptor Signaling | 6.61 × 10−6 |
| Role of Macrophages, Fibroblasts & Endothelial Cells | 1.62 × 10−5 |
| Dendritic Cell Maturation | 4.79 × 10−5 |
| Communication between Innate & Adaptive Immune Cells | 2.29 × 10−4 |
| Th1 & Th2 Activation Pathway | 3.02 × 10−3 |
| Cardiovascular Effect | |
| Cardiac Hypertrophy Signaling | 1.26 × 10−2 |
| iNOS Signaling | 2.09 × 10−2 |
| Metabolic Functions | |
| Phospholipase C Signaling | 3.39 × 10−4 |
| Glycolysis I | 4.47 × 10−2 |
| Cell Survival/Death | |
| TWEAK Signaling | 1.05 × 10−2 |
| Apoptosis Signaling | 3.02 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajakumar, K.; Yan, Q.; Khalid, A.T.; Feingold, E.; Vallejo, A.N.; Demirci, F.Y.; Kamboh, M.I. Gene Expression and Cardiometabolic Phenotypes of Vitamin D-Deficient Overweight and Obese Black Children. Nutrients 2019, 11, 2016. https://doi.org/10.3390/nu11092016
Rajakumar K, Yan Q, Khalid AT, Feingold E, Vallejo AN, Demirci FY, Kamboh MI. Gene Expression and Cardiometabolic Phenotypes of Vitamin D-Deficient Overweight and Obese Black Children. Nutrients. 2019; 11(9):2016. https://doi.org/10.3390/nu11092016
Chicago/Turabian StyleRajakumar, Kumaravel, Qi Yan, Arshad T. Khalid, Eleanor Feingold, Abbe N. Vallejo, F. Yesim Demirci, and M. Ilyas Kamboh. 2019. "Gene Expression and Cardiometabolic Phenotypes of Vitamin D-Deficient Overweight and Obese Black Children" Nutrients 11, no. 9: 2016. https://doi.org/10.3390/nu11092016
APA StyleRajakumar, K., Yan, Q., Khalid, A. T., Feingold, E., Vallejo, A. N., Demirci, F. Y., & Kamboh, M. I. (2019). Gene Expression and Cardiometabolic Phenotypes of Vitamin D-Deficient Overweight and Obese Black Children. Nutrients, 11(9), 2016. https://doi.org/10.3390/nu11092016




