Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Trace Element Samples
2.3. Assessment of Outcome
2.4. Assessment of Covariates
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
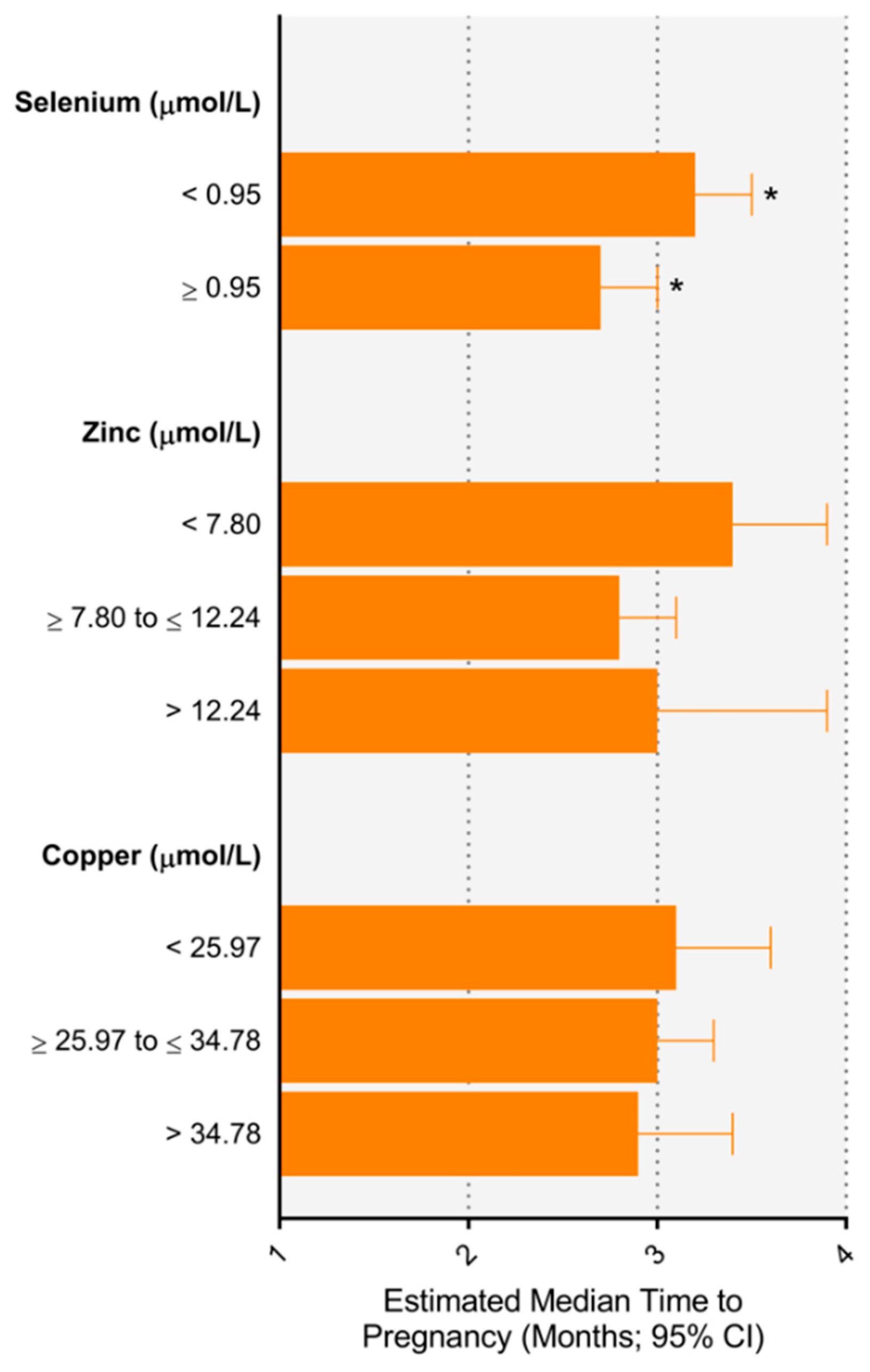
3.2. Relationship between Trace Elements and Time to Pregnancy
3.3. Relationship between Trace Element Concentrations and Subfertility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. 2015. Available online: http://www.who.int/reproductivehealth/topics/infertility/definitions/en/ (accessed on 6 May 2017).
- Buck Louis, G.M.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.M.; Lynch, C.D.; Gore-Langton, R.E.; Chen, Z.; Kim, S.; Caldwell, K.L.; Barr, D.B. Heavy metals and couple fecundity, the LIFE Study. Chemosphere 2012, 87, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Mendola, P.; Messer, L.C.; Rappazzo, K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil. Steril. 2008, 89, e81–e94. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Kim, K.; Kruger, P.C.; Parsons, P.J.; Arnason, J.G.; Steuerwald, A.J.; Fujimoto, V.Y. Associations between toxic metals in follicular fluid and in vitro fertilization (IVF) outcomes. J. Assist. Reprod. Genet. 2012, 29, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Cheng, B.H.; Lee, S.L.; Chuang, H.Y.; Yang, C.Y.; Sung, F.C.; Wu, T.N. Low blood lead concentration in association with infertility in women. Environ. Res. 2006, 101, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Coskun, S.; Mashhour, A.; Shinwari, N.; El-Doush, I.; Billedo, G.; Jaroudi, K.; Al-Shahrani, A.; Al-Kabra, M.; El Din Mohamed, G. Exposure to heavy metals (lead, cadmium and mercury) and its effect on the outcome of in-vitro fertilization treatment. Int. J. Hyg. Environ. Health 2008, 211, 560–579. [Google Scholar] [CrossRef] [PubMed]
- Younglai, E.V.; Holloway, A.C.; Foster, W.G. Environmental and occupational factors affecting fertility and IVF success. Hum. Reprod. Update 2005, 11, 43–57. [Google Scholar] [CrossRef]
- Hofstee, P.; McKeating, D.R.; Perkins, A.V.; Cuffe, J.S. Placental adaptations to micronutrient dysregulation in the programming of chronic disease. Clin. Exp. Pharmacol. Physiol. 2018, 45, 871–884. [Google Scholar] [CrossRef]
- Spencer, B.H.; Vanderlelie, J.J.; Perkins, A.V. Essentiality of Trace Element Micronutrition in Human Pregnancy: A Systematic Review. J. Pregnancy Child Health 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Ceko, M.J.; O’Leary, S.; Harris, H.H.; Hummitzsch, K.; Rodgers, R.J. Trace Elements in Ovaries: Measurement and Physiology. Biol. Reprod. 2016, 94, 86. [Google Scholar] [CrossRef]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Bloom, M.S.; Louis, G.M.; Sundaram, R.; Kostyniak, P.J.; Jain, J. Associations between blood metals and fecundity among women residing in New York State. Reprod. Toxicol. 2011, 31, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ingle, M.E.; Bloom, M.S.; Parsons, P.J.; Steuerwald, A.J.; Kruger, P.; Fujimoto, V.Y. Associations between IVF outcomes and essential trace elements measured in follicular fluid and urine: A pilot study. J. Assist. Reprod. Genet. 2017, 34, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, T.; Traub, A.I.; Robinson, S.Y.; McMaster, D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin. Chim. Acta 1995, 236, 173–180. [Google Scholar] [CrossRef]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Turgut, A.; Ozler, A.; Goruk, N.Y.; Tunc, S.Y.; Evliyaoglu, O.; Gul, T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1472–1478. [Google Scholar] [PubMed]
- Joffe, M.; Key, J.; Best, N.; Keiding, N.; Scheike, T.; Jensen, T.K. Studying time to pregnancy by use of a retrospective design. Am. J. Epidemiol. 2005, 162, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Joffe, M.; Villard, L.; Li, Z.; Plowman, R.; Vessey, M. Long-term recall of time-to-pregnancy. Fertil. Steril. 1993, 60, 99–104. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Bianco-Miotto, T.; Jankovic-Karasoulos, T.; Moran, L.J.; Wilson, R.L.; Leemaqz, S.Y.; Poston, L.; McCowan, L.; Kenny, L.C.; et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Hum. Reprod. 2018. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Smithers, L.G.; Bianco-Miotto, T.; Leemaqz, S.Y.; Andraweera, P.; Poston, L.; McCowan, L.M.; Kenny, L.C.; Myers, J.; et al. Metabolic syndrome and time to pregnancy: A retrospective study of nulliparous women. BJOG Int. J. Obstet. Gynaecol. 2019. [Google Scholar] [CrossRef]
- Grzeskowiak, L.E.; Smithers, L.G.; Grieger, J.A.; Bianco-Miotto, T.; Leemaqz, S.Y.; Clifton, V.L.; Poston, L.; McCowan, L.M.; Kenny, L.C.; Myers, J.; et al. Asthma treatment impacts time to pregnancy: Evidence from the international SCOPE study. Eur. Respir. J. 2018, 51, 1702035. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. 2018, 46, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Bath, S.C.; Westaway, J.; Williams, P.; Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W. Selenium status in U.K. pregnant women and its relationship with hypertensive conditions of pregnancy. Br. J. Nutr. 2015, 113, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.; McLeod, K.; Ransom, M.; Ongley, P. The New Zealand Socio-Economic Index of Occupational Status (NZSEI) (Research Report No. 2); Statistics New Zealand: Wellington, New Zealand, 1997. [Google Scholar]
- Galbraith, C.; Jenkin, G.; Davis, P.; Coope, P. New Zealand Socioeconomic Index 1996: User’s Guide; Statistics New Zealand: Wellington, New Zealand, 1996. [Google Scholar]
- Yaroch, A.L.; Tooze, J.; Thompson, F.E.; Blanck, H.M.; Thompson, O.M.; Colon-Ramos, U.; Shaikh, A.R.; McNutt, S.; Nebeling, L.C. Evaluation of three short dietary instruments to assess fruit and vegetable intake: The National Cancer Institute’s food attitudes and behaviors survey. J. Acad. Nutr. Diet. 2012, 112, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Department of Health and Aging. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2006.
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Mariath, A.B.; Bergamaschi, D.P.; Rondo, P.H.; Tanaka, A.C.; Hinnig Pde, F.; Abbade, J.F.; Diniz, S.G. The possible role of selenium status in adverse pregnancy outcomes. Br. J. Nutr. 2011, 105, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C. Dietary indicators for assessing the adequacy of population zinc intakes. Food Nutr. Bull. 2007, 28, S430–S453. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef]
- Ebisch, I.M.; Thomas, C.M.; Peters, W.H.; Braat, D.D.; Steegers-Theunissen, R.P. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Update 2007, 13, 163–174. [Google Scholar] [CrossRef]
- Keen, C.L.; Hanna, L.A.; Lanoue, L.; Uriu-Adams, J.Y.; Rucker, R.B.; Clegg, M.S. Developmental consequences of trace mineral deficiencies in rodents: Acute and long-term effects. J. Nutr. 2003, 133, 1477S–1480S. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.M.; McConnaughey, D.R.; Weinberg, C.R.; Wilcox, A.J.; Baird, D.D. Long-term Recall of Time to Pregnancy. Epidemiology 2016, 27, 705–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McAlpine, J.M.; McKeating, D.R.; Vincze, L.; Vanderlelie, J.J.; Perkins, A.V. Micronutrition status of pregnant women in South-East Queensland and their association with outcomes. Nutri. Metab. Insights 2019, in press. [Google Scholar]
- Olsen, J.; Bolumar, F.; Boldsen, J.; Bisanti, L. Does moderate alcohol intake reduce fecundability? A European multicenter study on infertility and subfecundity. European Study Group on Infertility and Subfecundity. Alcohol Clin. Exp. Res. 1997, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Killick, S.R. Negative lifestyle is associated with a significant reduction in fecundity. Fertil. Steril. 2004, 81, 384–392. [Google Scholar] [CrossRef] [PubMed]

| Maternal Characteristics | Fertile | Subfertile |
|---|---|---|
| <12 months | ≥12 months | |
| 894 (84.3%) | 166 (15.7%) | |
| Age (years), mean (SD) | 23.4 (5.0) | 25.4 (5.3) |
| Maternal age, ≥35 years, n (%) | 20 (2.2%) | 9 (5.4%) |
| Body mass index (kg/m2), mean (SD) | 26.7 (6.3) | 28.6 (7.3) |
| Socioeconomic index, mean (SD) | 27.5 (10.2) | 29.3 (11.0) |
| Ethnicity, n (%) | ||
| Caucasian | 822 (91.6) | 149 (89.8) |
| Other | 72 (8.1) | 17 (10.2) |
| Trace element concentration | ||
| Copper (µmol/L), mean (SD) | 30.3 (5.4) | 30.5 (6.1) |
| Zinc (µmol/L), mean (SD) | 9.4 (2.2) | 9.16 (2.7) |
| Selenium (µmol/L), mean (SD) | 73 (12) | 71 (11) |
| Frequency of sexual intercourse prior to pregnancy a | 18.1 (16.7) | 15.4 (16.1) |
| Pre-pregnancy alcohol intake, yes (n %) | 485 (54.3) | 69 (41.6) |
| Pre-pregnancy smoking, yes (n %) | 363 (40.6) | 63 (38.0) |
| Pre-pregnancy food group intake (n %) | ||
| Fast food, never | 58 (8.3) | 10 (6.9) |
| Fruit, ≥3/day | 63 (7.1) | 6 (3.6) |
| Green leafy vegetables, ≥1/day | 227 (25.4) | 45 (27.1) |
| Fish, ≥1/week | 327 (36.6) | 74 (44.6) |
| Multivitamin use in first trimester, yes (n %) | 531 (59.5) | 117 (70.5) |
| Multivitamin containing copper (n %) | 234 (26.2%) | 73 (44.0%) |
| Multivitamin containing selenium (n %) | 16 (1.8%) | 3 (1.8%) |
| Multivitamin containing zinc (n %) | 458 (51.2%) | 104 (62.7%) |
| Paternal characteristics (n = 930) | ||
| Age (years), mean (SD) | 26.7 (6.5) | 28.7 (6.1) |
| Body mass index (kg/m2), mean (SD) | 26.9 (5.1) | 28.1 (5.5) |
| Missing, n = 99 |
| Trace Element | Concentration (μmol/L) a | N | % | Unadjusted Time Ratio | Adjusted Time Ratio (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Selenium | <0.95 | 634 | 59.8% | 1.10 (0.96–1.26) | 1.14 (0.99–1.30) | 1.17 (1.00–1.37) | 1.19 (1.01–1.40) |
| ≥0.95 | 426 | 40.2% | 1 | 1 | 1 | 1 | |
| Zinc | <7.80 | 237 | 22.4% | 1.24 (1.05–1.46) | 1.19 (1.01–1.41) | 1.17 (0.98–1.40) | 1.20 (0.99–1.44) |
| ≥7.80 to ≤12.24 | 734 | 69.3% | 1 | 1 | 1 | 1 | |
| >12.24 | 88 | 8.3% | 1.14 (0.89–1.46) | 1.17 (0.92–1.49) | 1.08 (0.80–1.47) | 1.05 (0.77–1.44) | |
| Copper | <25.97 | 202 | 1.03 (0.87–1.22) | 1.05 (0.89–1.24) | 1.04 (0.86–1.25) | 1.04 (0.86–1.26) | |
| ≥25.97 to ≤34.78 | 506 | 1 | 1 | 1 | 1 | ||
| >34.78 | 186 | 1.03 (0.87–1.23) | 0.98 (0.82–1.17) | 0.94 (0.76–1.16) | 0.97 (0.78–1.21) | ||
| Trace Element | Concentration (μmol/L) a | N | n (%) | Unadjusted RR | Model 1 b | Model 2 c | Model 3 d |
|---|---|---|---|---|---|---|---|
| Selenium | <0.95 | 634 | 113 (17.8) | 1.43 (1.06–1.94) | 1.52 (1.13–2.05) | 1.44 (1.04–1.98) | 1.46 (1.06–2.03) |
| ≥0.95 | 426 | 53 (12.4) | 1 | 1 | 1 | 1 | |
| Zinc | <7.80 | 237 | 44 (18.6) | 1.25 (0.91–1.72) | 1.13 (0.82–1.56) | 1.04 (0.74–1.45) | 1.07 (0.76–1.50) |
| ≥7.80 to ≤12.24 | 734 | 109 (14.9) | 1 | 1 | 1 | 1 | |
| >12.24 | 88 | 13 (14.8) | 0.99 (0.58–1.69) | 1.07 (0.63–1.81) | 0.88 (0.46–1.68) | 0.0.76 (0.38–1.53) | |
| Copper | <25.97 | 202 | 38 (15.8) | 1.02 (0.72–1.44) | 1.10 (0.77–1.58) | 0.97 (0.67–1.40) | 1.01 (0.69–1.47) |
| ≥25.97 to ≤34.78 | 506 | 93 (15.5) | 1 | 1 | 1 | 1 | |
| >34.78 | 186 | 35 (15.8) | 1.02 (0.71–1.46) | 0.88 (0.61–1.25) | 0.79 (0.53–1.18) | 0.83 (0.55–1.25) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. https://doi.org/10.3390/nu11071609
Grieger JA, Grzeskowiak LE, Wilson RL, Bianco-Miotto T, Leemaqz SY, Jankovic-Karasoulos T, Perkins AV, Norman RJ, Dekker GA, Roberts CT. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients. 2019; 11(7):1609. https://doi.org/10.3390/nu11071609
Chicago/Turabian StyleGrieger, Jessica A., Luke E. Grzeskowiak, Rebecca L. Wilson, Tina Bianco-Miotto, Shalem Y. Leemaqz, Tanja Jankovic-Karasoulos, Anthony V. Perkins, Robert J. Norman, Gus A. Dekker, and Claire T. Roberts. 2019. "Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility" Nutrients 11, no. 7: 1609. https://doi.org/10.3390/nu11071609
APA StyleGrieger, J. A., Grzeskowiak, L. E., Wilson, R. L., Bianco-Miotto, T., Leemaqz, S. Y., Jankovic-Karasoulos, T., Perkins, A. V., Norman, R. J., Dekker, G. A., & Roberts, C. T. (2019). Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients, 11(7), 1609. https://doi.org/10.3390/nu11071609






