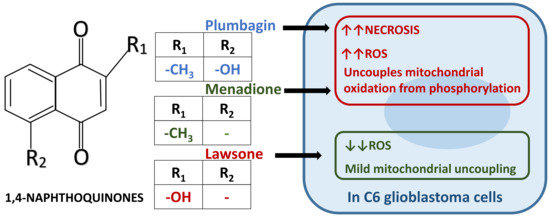
Comparison of the Effect of Native 1,4-Naphthoquinones Plumbagin, Menadione, and Lawsone on Viability, Redox Status, and Mitochondrial Functions of C6 Glioblastoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Line and Cell Culture
2.3. Cell Viability Assessment
2.4. Measurement of Intracellular ROS Concentration
2.5. Assessment of Mitochondrial Oxygen Consumption
2.6. Statistical Analysis
3. Results
3.1. The Effect of 1, 4-Naphthoquinones on C6 Cell Viability
3.2. The Effect of 1,4-Naphthoquinones on the Intracellular ROS Concentration in a C6 Cell Culture
3.3. Cytotoxic Effect of Plumbagin was Partially Prevented by Ascorbate
3.4. The Effect of 1,4-Naphthoquinones on the Mitochondrial Respiration Rate in C6 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Muscara, C.; Ferlazzo, G.; Costa, G.; Saija, A.; Cimino, F.; Speciale, A. Curcumin Potentiates the Antitumor Activity of Paclitaxel in Rat Glioma C6 Cells. Phytomedicine 2018, 55, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.I.; Mason, W.P. Temozolomide: The Evidence for its Therapeutic Efficacy in Malignant Astrocytomas. Core Evid. 2010, 4, 93–111. [Google Scholar] [PubMed]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First Results on Survival from a Large Phase 3 Clinical Trial of an Autologous Dendritic Cell Vaccine in Newly Diagnosed Glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.Y.; Wang, P.F.; Lin, H.Y.; Tang, C.Y.; Zhu, H.L.; Yang, Y.H. Naphthoquinones: A Continuing Source for Discovery of Therapeutic Antineoplastic Agents. Chem. Biol. Drug Des. 2018, 91, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Meskelevicius, D.; Sidlauskas, K.; Bagdonaviciute, R.; Liobikas, J.; Majiene, D. Juglone Exerts Cytotoxic, Anti-Proliferative and Anti-Invasive Effects on Glioblastoma Multiforme in a Cell Culture Model. Anticancer Agents Med. Chem. 2016, 16, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Sidlauskas, K.; Sidlauskiene, R.; Li, N.; Liobikas, J. 5-Hydroxy-1,4-Naphthalenedione Exerts Anticancer Effects on Glioma Cells through Interaction with the Mitochondrial Electron Transport Chain. Neurosci. Lett. 2017, 639, 207–214. [Google Scholar] [CrossRef]
- Li, B.; Gu, X.; Wu, M.; Zhao, Y.; Yang, J.; Feng, L.; Gou, J.; Chen, L.; Li, T.; Li, L.; et al. Plumbagin Inhibits the Proliferation of Nasopharyngeal Carcinoma 6-10B Cells by Upregulation of Reactive Oxygen Species. Anticancer Drugs 2018, 29, 890–897. [Google Scholar] [CrossRef]
- Huang, H.; Xie, H.; Pan, Y.; Zheng, K.; Xia, Y.; Chen, W. Plumbagin Triggers ER Stress-Mediated Apoptosis in Prostate Cancer Cells Via Induction of ROS. Cell. Physiol. Biochem. 2018, 45, 267–280. [Google Scholar] [CrossRef]
- Sakunrangsit, N.; Kalpongnukul, N.; Pisitkun, T.; Ketchart, W. Plumbagin Enhances Tamoxifen Sensitivity and Inhibits Tumor Invasion in Endocrine Resistant Breast Cancer through EMT Regulation. Phytother. Res. 2016, 30, 1968–1977. [Google Scholar] [CrossRef]
- Eldhose, B.; Gunawan, M.; Rahman, M.; Latha, M.S.; Notario, V. Plumbagin Reduces Human Colon Cancer Cell Survival by Inducing Cell Cycle Arrest and Mitochondria-Mediated Apoptosis. Int. J. Oncol. 2014, 45, 1913–1920. [Google Scholar] [CrossRef]
- Lamson, D.W.; Plaza, S.M. The Anticancer Effects of Vitamin K. Altern. Med. Rev. 2003, 8, 303–318. [Google Scholar] [PubMed]
- Hassan, G.S. Menadione. Profiles Drug Subst. Excip. Relat. Methodol. 2013, 38, 227–313. [Google Scholar] [PubMed]
- Bohl, L.; Guizzardi, S.; Rodriguez, V.; Hinrichsen, L.; Rozados, V.; Cremonezzi, D.; Tolosa de Talamoni, N.; Picotto, G. Combined Calcitriol and Menadione Reduces Experimental Murine Triple Negative Breast Tumor. Biomed. Pharmacother. 2017, 94, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Raghu, D.; Karunagaran, D. Menadione (Vitamin K3) Induces Apoptosis of Human Oral Cancer Cells and Reduces their Metastatic Potential by Modulating the Expression of Epithelial to Mesenchymal Transition Markers and Inhibiting Migration. Asian Pac. J. Cancer Prev. 2013, 14, 5461–5465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, R.; Dandawate, P.; Vyas, A.; Padhye, S.; Biersack, B.; Schobert, R.; Ahmad, A.; Sarkar, F.H. From Body Art to Anticancer Activities: Perspectives on Medicinal Properties of Henna. Curr. Drug Targets 2012, 13, 1777–1798. [Google Scholar] [CrossRef] [PubMed]
- Kamei, H.; Koide, T.; Kojima, T.; Hashimoto, Y.; Hasegawa, M. Inhibition of Cell Growth in Culture by Quinones. Cancer Biother. Radiopharm. 1998, 13, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Hamdoun, S.; Fleischer, E.; Klinger, A.; Efferth, T. Lawsone Derivatives Target the Wnt/beta-Catenin Signaling Pathway in Multidrug-Resistant Acute Lymphoblastic Leukemia Cells. Biochem. Pharmacol. 2017, 146, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.; Liany, L.D.; Correa, R.S.; Deflon, V.M.; Cominetti, M.R.; Batista, A.A. Selective Ru(II)/lawsone Complexes Inhibiting Tumor Cell Growth by Apoptosis. J. Inorg. Biochem. 2017, 176, 66–76. [Google Scholar] [CrossRef]
- Kubiliene, L.; Jekabsone, A.; Zilius, M.; Trumbeckaite, S.; Simanaviciute, D.; Gerbutaviciene, R.; Majiene, D. Comparison of Aqueous, Polyethylene Glycol-Aqueous and Ethanolic Propolis Extracts: Antioxidant and Mitochondria Modulating Properties. BMC Complement. Altern. Med. 2018, 18, 165. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-Resolution Respirometry: OXPHOS Protocols for Human Cells and Permeabilized Fibers from Small Biopsies of Human Muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Li, Y.C.; He, S.M.; He, Z.X.; Li, M.; Yang, Y.; Pang, J.X.; Zhang, X.; Chow, K.; Zhou, Q.; Duan, W.; et al. Plumbagin Induces Apoptotic and Autophagic Cell Death through Inhibition of the PI3K/Akt/mTOR Pathway in Human Non-Small Cell Lung Cancer Cells. Cancer Lett. 2014, 344, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Osada, S.; Tomita, H.; Tanaka, Y.; Tokuyama, Y.; Tanaka, H.; Sakashita, F.; Takahashi, T. The Utility of Vitamin K3 (Menadione) Against Pancreatic Cancer. Anticancer Res. 2008, 28, 45–50. [Google Scholar] [PubMed]
- Oztopcu, P.; Kabadere, S.; Mercangoz, A.; Uyar, R. Comparison of Vitamins K1, K2 and K3 Effects on Growth of Rat Glioma and Human Glioblastoma Multiforme Cells in Vitro. Acta Neurol. Belg. 2004, 104, 106–110. [Google Scholar] [PubMed]
- Wang, S.B.; Tao, Z.; Li, P. Lawsone Suppresses Azoxymethane Mediated Colon Cancer in Rats and Reduces Proliferation of DLD-1 Cells Via NF-kappaB Pathway. Biomed. Pharmacother. 2017, 89, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, T.; Ding, D.; Cai, T.; Jiang, C.; Li, H.; Yang, Q.; Chen, L. Biodegradation of 2-Hydroxyl-1,4 Naphthoquinone (Lawsone) by Pseudomonas Taiwanensis LH-3 Isolated from Activated Sludge. Sci. Rep. 2017, 7, 6795. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Valiveti, C.K.; Kumar, D.R.; Van Slambrouck, S.; Kesharwani, S.S.; Seefeldt, T.; Scaria, J.; Tummala, H.; Bhat, G.J. The Flavonoid Metabolite 2,4,6-Trihydroxybenzoic Acid is a CDK Inhibitor and an Anti-Proliferative Agent: A Potential Role in Cancer Prevention. Cancers 2019, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Powolny, A.A.; Singh, S.V. Plumbagin-Induced Apoptosis in Human Prostate Cancer Cells is Associated with Modulation of Cellular Redox Status and Generation of Reactive Oxygen Species. Pharm. Res. 2008, 25, 2171–2180. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, Y.K.; Sohn, D.S.; Lee, C.S. Menadione Induces the Formation of Reactive Oxygen Species and Depletion of GSH-Mediated Apoptosis and Inhibits the FAK-Mediated Cell Invasion. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 799–809. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, S.; Sharma, A. Antimutagenic and Antioxidant Properties of Plumbagin and Other Naphthoquinones. Mutat. Res. 2013, 755, 30–41. [Google Scholar] [CrossRef]
- Wang, S.X.; Wang, J.; Shao, J.B.; Tang, W.N.; Zhong, J.Q. Plumbagin Mediates Cardioprotection Against Myocardial Ischemia/Reperfusion Injury through Nrf-2 Signaling. Med. Sci. Monit. 2016, 22, 1250–1257. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Luo, X.; Chen, Z.; Liang, H. Antioxidant Activities of Plumbagin and its Cu (II) Complex. Bioinorg. Chem. Appl. 2011, 2011, 898726. [Google Scholar] [CrossRef] [PubMed]
- Mikhaeil, B.R.; Badria, F.A.; Maatooq, G.T.; Amer, M.M. Antioxidant and Immunomodulatory Constituents of Henna Leaves. Z. Naturforsch. C 2004, 59, 468–476. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Sarvate, S.D.; Oatis, J.E.; Jollow, D.J. Role of Oxidant Stress in Lawsone-Induced Hemolytic Anemia. Toxicol. Sci. 2004, 82, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Berghot, M.A.; Kandeel, E.M. Synthesis, Antioxidant and Cytotoxic Activities of Novel Naphthoquinone Derivatives from 2,3-Dihydro-2,3-Epoxy-1,4-Naphthoquinone. Med. Chem. 2014, 4, 381–388. [Google Scholar]
- Awad, B.; Bradford, P.G. Nutrition and Cancer Prevention, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 411–435. [Google Scholar]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure-Activity Relationships for Antioxidant Activities of a Series of Flavonoids in a Liposomal System. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Kapur, A.; Barroilhet, L.; Patankar, M.S. Oxidative Phosphorylation: A Target for Novel Therapeutic Strategies Against Ovarian Cancer. Cancers 2018, 10, 337. [Google Scholar] [CrossRef]




| Treatment | µM | VL | VDNF | VADP |
|---|---|---|---|---|
| Control | 27.4 ± 5.2 | 58.4 ± 6.4 | ||
| Control + DNF | 86.5 ± 7.7 | |||
| Plumbagin | 0.5 | 34.1 ± 5.6 | 62.9 ± 4.8 | |
| 1 | 39.8 ± 5.5 * | 70.0 ± 5.4 * | ||
| 2 | 62.7 ± 3.9 * | 77.9 ± 5.3 * | ||
| 3 | 84.5 ± 4.7 * | 89.6 ± 7.2 * | ||
| Menadione | 0.5 | 31.7 ± 3.1 | 60.8 ± 7.2 | |
| 1 | 37.8 ± 4.8 * | 77.2 ± 6.8 * | ||
| 2 | 50.9 ± 5.7 * | 90.7 ± 8.4 * | ||
| 3 | 84.1 ± 6.4 * | 89.5 ± 7.8 * | ||
| Lawsone | 10 | 30.3 ± 5.2 | 60.6 ± 4.8 | |
| 20 | 34.9 ± 4.7 | 63.1 ± 5.2 | ||
| 50 | 43.6 ± 3.9 * | 65.4 ± 3.9 | ||
| 100 | 52.5 ± 4.3 * | 68.1 ± 4.5 * | ||
| 200 | 61.3 ± 5.7 * | 73.9 ± 5.3 * | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majiene, D.; Kuseliauskyte, J.; Stimbirys, A.; Jekabsone, A. Comparison of the Effect of Native 1,4-Naphthoquinones Plumbagin, Menadione, and Lawsone on Viability, Redox Status, and Mitochondrial Functions of C6 Glioblastoma Cells. Nutrients 2019, 11, 1294. https://doi.org/10.3390/nu11061294
Majiene D, Kuseliauskyte J, Stimbirys A, Jekabsone A. Comparison of the Effect of Native 1,4-Naphthoquinones Plumbagin, Menadione, and Lawsone on Viability, Redox Status, and Mitochondrial Functions of C6 Glioblastoma Cells. Nutrients. 2019; 11(6):1294. https://doi.org/10.3390/nu11061294
Chicago/Turabian StyleMajiene, Daiva, Jolita Kuseliauskyte, Arturas Stimbirys, and Aiste Jekabsone. 2019. "Comparison of the Effect of Native 1,4-Naphthoquinones Plumbagin, Menadione, and Lawsone on Viability, Redox Status, and Mitochondrial Functions of C6 Glioblastoma Cells" Nutrients 11, no. 6: 1294. https://doi.org/10.3390/nu11061294